Abstract
This experimental study evaluated the influence of the presence of lipopolysaccharide (LPS), a common bacterial endotoxin, on the nitric acid oxidised multiwalled carbon nanotubes (Ox-MWCNT) during the assessment of in vitro toxicity to splenocytes, an immune system cell isolated from BALB/c mice. The concentration of LPS was determined by Limulus Amebocyte assay and this endotoxin was removed from Ox-MWCNT by using three cycles of autoclave. Splenocytes were cultured in RPMI 1640 media with 1.0, 5.0 or 10 ng/mL of Ox-MWCNT. The results showed that the presence of LPS on Ox-MWCNT did not affect the growth of splenocytes in vitro. However, the absence of LPS decreased the splenocytes viability significantly.
1. Introduction
Carbon nanotubes (CNTs) are a new allotrope of carbon with particular properties that can guarantee an effective activity when applied as drug carriers, vaccine adjuvants and biosensors.[Citation1] Nanomaterials are typically defined as engineered structures with at least one dimension of 100 nm or less, though from the perspective of biology these definitions may be too narrow.[Citation2]
Ultrafine particles such as nanomaterials can cross cellular membranes by nonphagocytic mechanisms,[Citation3] which may facilitate their access to sites within cells and tissues were not previously found, further contributing to their toxicity. A number of studies have addressed the potential of CNTs to cause pulmonary fibrosis, the scarring of lung tissue caused by an increase in fibroblasts and their collagen deposits.[Citation4] Treatment of CNTs associated with lipopolysaccharide (LPS) showed marked neutrophils accumulation into the lung parenchyma, IL-1β and TNF-α production.[Citation5]
The endotoxin LPS is one of the most important bacterial products involved in the progression of inflammation in mammalians. This biomolecule can bind to receptors on the surface of macrophages and monocytes named toll-like receptor 4 (TLR4), the activation of these receptors induces an intracellular signaling route mediated by Myeloid Differentiation Primary Response 88 (MyD88), Janus Kinase (JAK) quinases and Signal Transducer and Activation of Transcription (STATS) that lead to activation of the transcription factor Nuclear Factor Kappa β (NFKβ), the regulator of the expression of inflammatory cytokine such as IL-1β and TNF-α.[Citation6]
On the other hand, CNTs can strongly adsorb proteins and other molecules (e.g. LPS), which can lead to intrinsic cell responses to CNTs.[Citation2] The combination of CNTs with LPS increased the cellularity in blood and lymphoid organs, after intrathecal instillation in rats.[Citation7] In addition, the combination of LPS with functionalised multiwalled CNTs (MWCNTs) increased the levels of platelet-derived growth factor (PDGF) in mice after 24 h of exposition; PDGF induces pulmonary fibrosis.[Citation8]
Different methods used to synthesise and functionalise CNTs can lead to nanotubes with different physicochemical properties (e.g. size distribution, morphology, surface chemistry, surface area, surface charge, structural defects and impurities) with important impacts on their biological and toxicological properties.[Citation9–11] Nitric acid oxidation of CNTs is a common method employed during purification and functionalisation of CNTs in toxicological studies. This chemical process introduces a mixture of oxygenated groups on the CNT surface as well as oxidative debris which improve their water dispersibility and stability. Besides, the metallic impurities (e.g. iron catalyst) are also eliminated during this oxidative process.[Citation12,13]
Then, the main goal of this study was to quantify the levels of LPS in nitric acid oxidised MWCNTs (termed Ox-MWCNT), remove the LPS and determine if the presence/absence of this endotoxin could influence the growth and survival of murine splenocytes in culture media containing the Ox-MWCNT.
2. Experimental
2.1. Preparation and characterisation of oxidised multiwalled carbon nanotubes
Industrial grade MWCNTs were commercially obtained from CNT Co. Ltd. (Incheon, Korea). First, the as-prepared MWCNTs (1.0 g) were treated using classical reflux technique with nitric acid (9.0 mol L−1) at 150 ºC for 6 h. After cooling down to room temperature, the oxidised nanotubes were vacuum-filtered through a 0.2-μm polytetrafluoroethylene (PTFE) membrane and washed with deionised water until the filtrate reach a neutral pH. The oxidised MWCNTs were dried in vacuum system for 24 h. This Ox-MWCNT sample is termed as Ox-MWCNT.
Size distribution of the Ox-MWCNT sample was estimated by using field-emission gun scanning electron microscopy (FEG-SEM) (FEI NanoLab200) and transmission electron microscopy (TEM) (Carl Zeiss Libra-120) techniques. The specific surface area was evaluated with the BET method (Nova Win Quantachrome equipment). The zeta potential was determined in Milli-Q water (in triplicate) by Zetasizer nano-ZS (Malvern Instruments). The ratio between disorder-induced and tangential mode was obtained from Raman spectrum (measured in a TS-150 spectrometer from WITEC), which was excited with 532 nm laser line. The qualitative elementary analysis was obtained using energy dispersive X-ray spectroscopy (EDS) (JEOL 660-LV). Decomposition temperature was determined using thermal measurements (SDT Q600 TA Instruments) and final metallic residue was measured using an analytical microbalance (AD-6 Perkin-Elmer), following the protocol recommended by National Institute of Standards and Technology (NIST).[Citation14]
2.2. Mice and splenocytes isolation
BALB/C mice were purchased from Universidade Federal de Santa Maria, RS, Brazil. Mice were housed at Centro Universitário Franciscano animal facility with ad libitum access to food and water. Female of 8–12 weeks old were used for all experiments. Cellular isolation was done as previously described.[Citation15] Briefly, animals were euthanised by CO2 inhalation and splenocytes were isolated. After lysis of erythrocytes with cell lysis buffer (8.26 g ammonium chloride; 1 g potassium bicarbonate; 0.037 g EDTA (ethylenediamine tetraacetic acid)/L), cells were stained with Trypan blue and the percentage of dead cells (blue) and alive cells (shine) were determinate in Neubauer chamber (Trypan blue exclusion). A number of 2 × 105 alive cells were cultured in 24-well plate or in 96-well plate dependent on the method used to evaluate the cell viability. All the cultures were made with Roswell Park Memorial Institute (RPMI 1640 – Gibco, Carlsbad, CA, USA) containing 10% fetal bovine serum (FBS – Gibco, Carlsbad, CA, USA), Gentamicin 80 mg/L (Novafarma, Anápolis, Brazil), Fungisone – Anfotericin B, 5 mg/L (Bristol Myers Squibb, New York, NY, USA), 2 mM L-Glutamine (Sigma, St. Louis, MO, USA).
2.3. Cell viability assays
We used two different methods to determine the splenocytes viability in vitro. First, the viability was evaluated by Trypan blue exclusion after 24 and 48 h of incubation in vitro with different concentrations of Ox-MWCNT (1.0, 5.0 and 10 ng/mL). The second method used to determine the splenocyte viability was the 3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide (MTT) assay (Gibco). Briefly, 2 × 105 cells per well were cultured in 96-well plate in RPMI medium supplemented with Ox-MWCNT for 4 days, after this period MTT reagent was added and incubated for more four hours. The supernatant was harvested and the precipitated diluted in 100 μL of dimethyl sulphoxide (DMSO) and the absorbance analysed at 570 nm. The mitogen Phytohemagglutinin (PHA) (Gibco) was used as positive control of the assay, since lymphocytes proliferate in presence of this mitogen.
2.4. LPS quantification and decontamination
The level of LPS in the Ox-MWCNT was determinate using the Limulus Amebocyte lysate (LAL) Pyrogent Plus Single Test vials (Lonza). This assay is based on catalytic activity of the LPS to the proenzyme in the LAL. The activation of coagulase hydrolyzes specific bonds within a clotting protein (coagulogen) also present in LAL. Once hydrolysed the resultant coagulin self-associated and forms a gelatinous clot. The sensitivity of the assay is 0.125 EU/mL (Endotoxin Unit). In this study, samples beyond 0.5 EU/mL of LPS were considered positives. For decontamination, the Ox-MWCNT were treated by two different methods for removing LPS from Ox-MWCNT, the conventional treatment by heating for two hours in oven (180 ºC) (method 1) and an alternative method using three cycles of autoclave (180 °C/15 min) (method 2). Samples were rehydrated with LPS-free water.
2.5. Statistical analysis
Data were expressed as mean ± SEM. Statistical analysis was performed using student's t-test and two-way ANOVA. Values of P < 0.05 were considered statistically significant.
3. Results and discussion
3.1. Characterisation of oxidised multiwalled carbon nanotubes studied
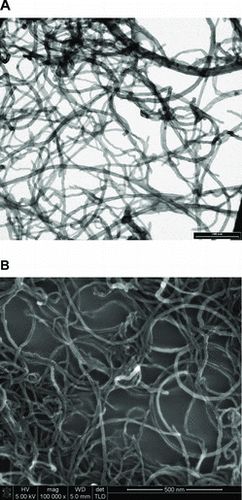
Considering the critical influence of the CNT physicochemical properties on toxicity results, we have developed an integrated characterisation of the nitric acid Ox-MWCNT studied (). TEM and scanning electron microscopy (FEG-SEM) analysis were performed to estimate the range of size distribution of Ox-MWCNT. The diameter distribution was found to vary from 10 to 40 nm and tube length was found to be less than 5 microns. shows the representative electron microscopy images of the Ox-MWCNT studied here.
Table 1. Physicochemical properties of the oxidised multiwalled carbon nanotubes (Ox-MWCNT).
3.2. Unsterile Ox-MWCNT does not impair the splenocyte in vitro
The effects of CNTs on mammalian cells are very controversial, some studies have showed that the treatment of cells with CNTs can induce cell death or proliferation inhibition, while others claim that CNTs have a minimal toxicity in cultures.[Citation16–18] Thus, initially we determined if Ox-MWCNT could influence the viability of murine splenocytes at low and high doses. The Ox-MWCNT in any dose tested did not change significantly the magnitude of cell viability (∼75%) in the first 24 h of incubation compared to cells treated with saline (control group) (A). It was observed that after 48 h the viability in the group treated 1.0 ng/mL was slightly less than controlled with saline and it seems to be restored in higher concentrations of Ox-MWCNT; however, it is not statistically significant.
Figure 2. Surviving of splenocytes after culture with Ox-MWCNT. Splenocytes were isolated from spleen and 2 × 105 cells cultured with different concentrations of Ox-MWCNT diluted in RPMI media supplemented with 10% FBS. The viability was analysed 24 and 48 h after in Trypan blue exclusion method and 96 h in MTT assay: (A) cell viability determined with Trypan blue; (B) viability determined through MTT assay. *P < 0.05.
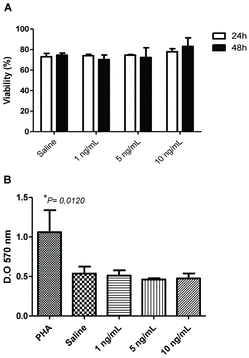
Due to the fact that the splenocytes viability was unchanged with Ox-MWCNT, we investigated this phenomenon using an MTT assay, which method is more reliable to determine the cell viability. Cells treated with all concentrations of Ox-MWCNT did not change the basal viability (B). Significant increasing in cell viability was observed only in cells stimulated with the mitogen PHA, used as a positive control for cells response in our assay. These data indicated that the presence of Ox-MWCNT in culture of murine splenocytes does not impair or increase the cell viability of splenocytes, independent of the dose used. Sun and collaborators also showed that CNTs did not affect significantly the proliferation or apoptosis of cell lines of lymphocytes in vitro.[Citation16] However, they did not apply any specific method for decontamination and quantification of LPS. Culture of macrophages with CNTs led to a limited toxicity and no effect on proliferation, even in doses of 30 μg/mL.[Citation2]
3.3. Ox-MWCNT with LPS can protect splenocytes from cytotoxicity
CNTs can be contaminated with particles from different sources as metals (e.g. iron, nickel, cobalt) used during the process of synthesise or from environmental source as microbes and its products.[Citation18,19] Indeed LPS from Gram negative bacteria is a potential contaminant of CNTs and its effects involve the modulation of cell proliferation. Considering this possibility and the controversial results regarding the effects of CNT in splenocytes and lymphocytes, this study investigated the influence of the LPS presented in the Ox-MWCNT sample in survival and viability of murine splenocytes. Initially, we compared the efficacy of the method 1 and 2 for removing LPS from Ox-MWCNT. The level of LPS in unsterile Ox-MWCNT or treated only with one cycle of autoclave was higher than 0.5 EU/mL (A). Sterilisation by autoclave 1 cycle was not enough to remove LPS from Ox-MWCNT. On the other hand, when Ox-MWCNT was treated by the methods 1 and 2, the LPS was completely removed (<0.5 EU/mL). However, autoclaved samples were more promptly rehydrated and diluted in the media for culture.
Figure 3. Effects of LPS in cytotoxicity of Ox-MWCNT forsplenocytes in vitro: (A) after sterilisation process Ox-MWCNT was washed with LPS-Free water and the residual water used to quantify the LPS. Samples beyond 0.5 EU/mL were considered positive. Splenocytes were isolated from spleen and 2 × 105 cells cultured with 10 ng/mL of Ox-MWCNT diluted in RPMI media supplemented with 10% FBS. The viability was analyzed after 48 h in Trypan blue exclusion method and 96 h for in MTT assay; (B) cell viability determined with Trypan blue; (C) viability determined through MTT assay. *P < 0.05.
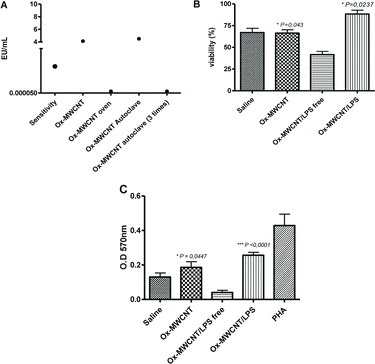
Next, we determine if the absence of LPS had any effect on cell viability of spleen cells. As observed in the previous experiment, unsterile Ox-MWCNT did not improve or reduce the cell viability (B). However, when we incubated the splenocytes with Ox-MWCNT LPS-free the cell viability significantly decreased compared with saline or unsterile nanotubes. When Ox-MWCNT (1 EU/mL) was treated with LPS and added to the culture, the cell viability, the cell viability was recovered and even more than treatment with saline or unsterile CNTs. The same pattern was observed on MTT assay, the LPS-free Ox-MWCNT significantly impaired the viability of splenocytes and it was restored and improved after addition of LPS (C).
3. Conclusion
The use of CNTs as drug carriers or adjuvant for vaccines and biosensors is attracting much interest from scientific community. However, the biocompatibility and toxicity of nanomaterials in vitro and in vivo need to be investigated; especially because there are controversial results related to the source of the CNTs. Our results suggest that the nitric acid oxidised multiwalled carbon nanotubes studied, designated Ox-MWCNT, can interact with LPS integrating the properties of this endotoxin and this interaction can rescue splenocytes from the death. That combination has also a positive aspect because Ox-MWCNT can sequestrate LPS from the media and prevent the binding of this endotoxin in other cell receptors.
Acknowledgments
The authors acknowledge the Brazilian agencies CNPq, CAPES and FAPERGS for financial support. D.S.T. Martinez and O.L. Alves thank INCT-Inomat and Brazilian nanotoxicology network (Cigenanotox/MCTI).
References
- Prato M, Kostarelos K, Bianco A. Functionalized carbon nanotubes in drug design and discovery. Acc Chem Res. 2008;41(1):60–68.
- Dutta D, Sundaram SK, Teeguarden JG, Riley BJ, Fifield LS, Jacobs JM, Addleman SR, Kaysen GA, Moudgil BM, Weber TJ. Adsorbed proteins influence the biological activity and molecular targeting of nanomaterials. Toxicol Sci. 2007;100(1):303–315.
- Geiser M, Rothen-Rutishauser B, Kapp N, Schurch S, Kreyling W, Schulz H, Semmler M, Im Hof V, Heyder J, Gehr P. Ultrafine particles cross cellular membranes by nonphagocytic mechanisms in lungs and in cultured cells. Environ Health Perspect. 2005;113(11):1555–1560.
- Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214(2):199–210.
- Inoue K, Takano H, Koike E, Yanagisawa R, Sakurai M, Tasaka S, Ishizaka A, Shimada A. Effects of pulmonary exposure to carbon nanotubes on lung and systemic inflammation with coagulatory disturbance induced by lipopolysaccharide in mice. Exp Biol Med (Maywood). 2008;233(12):1583–1590.
- Palsson-McDermott EM, O’Neill LA. Signal transduction by the lipopolysaccharide receptor, toll-like receptor-4. Immunology. 2004;113(2):153–162.
- Inoue K, Takano H, Yanagisawa R, Hirano S, Kobayashi T, Fujitani Y, Shimada A, Yoshikawa T. Effects of inhaled nanoparticles on acute lung injury induced by lipopolysaccharide in mice. Toxicology. 2007;238(2–3):99–110.
- Cesta MF, Ryman-Rasmussen JP, Wallace DG, Masinde T, Hurlburt G, Taylor AJ, Bonner JC. Bacterial lipopolysaccharide enhances PDGF signaling and pulmonary fibrosis in rats exposed to carbon nanotubes. Am J Respir Cell Mol Biol. 2010;43(2):142–151.
- Gutierrez-Praena D, Pichardo S, Sanchez E, Grilo A, Camean AM, Jos A. Influence of carboxylic acid functionalization on the cytotoxic effects induced by single wall carbon nanotubes on human endothelial cells (HUVEC). Toxicol In Vitr. 2011;25(8):1883–1888.
- Villagarcia H, Dervishi E, de Silva K, Biris AS, Khodakovskaya MV. Surface chemistry of carbon nanotubes impacts the growth and expression of water channel protein in tomato plants. Small. 2012;8(15):2328–2334.
- Bussy C, Pinault M, Cambedouzou J, Landry MJ, Jegou P, Mayne-L’hermite M, Launois P, Boczkowski J, Lanone S. Critical role of surface chemical modifications induced by length shortening on multi-walled carbon nanotubes-induced toxicity. Part Fibre Toxicol. 2012;9(46):1–15.
- Andrade NF, Martinez DST, Paula AJ, Silveira JV, Alves OL, Souza Filho AG. Temperature effects on the nitric acid oxidation of industrial grade multiwall carbon nanotubes. J Nanoparticle Res. 2013;15(6):1761–1772.
- Marques RRN, Machado BF, Faria JL, Silva AMT. Controlled generation of oxygen functionalities on the surface of single-walled carbon nanotubes by HNO3 hydrothermal oxidation. Carbon. 2010;48:1515–1523.
- Freiman S, Hooker S, Migler K, Arepalli S. Messurent issues in single wall carbon nanotubes. Washington (DC): National Institute of Standards and Technology; 2008. p. 78.
- Rodrigues L, Nandakumar S, Bonorino C, Rouse BT, Kumaraguru U. IL-21 and IL-15 cytokine DNA augments HSV specific effector and memory CD8+ T cell response. Mol Immunol. 2009;46(7):1494–1504.
- Song M, Zeng L, Yuan S, Yin J, Wang H, Jiang G. Study of cytotoxic effects of single-walled carbon nanotubes functionalized with different chemical groups on human MCF7 cells. Chemosphere. 2013;92(5):576–582.
- Shimizu K, Uchiyama A, Yamashita M, Hirose A, Nishimura T, Oku N. Biomembrane damage caused by exposure to multi-walled carbon nanotubes. J Toxicol Sci. 2013;38(1):7–12.
- Dumortier H, Lacotte S, Pastorin G, Marega R, Wu W, Bonifazi D, Briand JP, Prato M, Muller S, Bianco A. Functionalized carbon nanotubes are non-cytotoxic and preserve the functionality of primary immune cells. Nano Lett. 2006;6(7):1522–1528.
- Sun Z, Liu Z, Meng J, Duan J, Xie S, Lu X, Zhu Z, Wang C, Chen S, Xu H. Carbon nanotubes enhance cytotoxicity mediated by human lymphocytes in vitro. PLoS One. 2011;6(6):e21073.