Abstract
A novel method of covalently grafting solid or hollow microcapsules on cellulosic textiles was developed by surface functionalisation of capsules with reactive triazine moieties. Demonstrative silica microspheres, with 454 nm average particle size, were developed by electrospraying silica sol prepared via sol–gel process. The chemical–physical properties of submicron particles were characterised by Fourier transform infrared spectroscopy (FTIR), thermogravimetry (TGA) and scanning (SEM) and transmission electron microscopy (TEM). As a potential process towards functional textiles, the micro/nanosphere surface was modified with chlorotriazine ligands in a two-step procedure to react with the hydroxyl groups in cellulose under mild conditions. The first amine functionalisation was demonstrated by evaluation with FTIR and silicon-29 nuclear magnetic resonance (29Si-NMR), as well as using TGA. Subsequent chemical functionalisation of an amine–silica surface with triazine moieties was analysed by FTIR, proton solid-state NMR spectroscopy (1H-NMR) and TEM coupled to an X-ray detector. Finally, the covalent bonding of silica nanoparticles to cellulose was performed under simulated dying conditions and it was evaluated by SEM in combination to energy dispersive X-ray spectroscopy.
1. Introduction
Enhancement of the competitiveness of the traditional textile industry greatly depends on the rapid response to the customer requirements. The new generation of wearable articles is expanding into the promising field of multifunctional textiles, characterised by added value properties and interactive features.[Citation1] In this scenario, microencapsulation becomes an outstanding technology for effectively imparting new properties and related added value on textiles. Progressively, textile and clothing companies are experimenting with this microtechnology to produce more attractive and functional articles.[Citation2] Therefore, commercial applications of microencapsulation continue to grow from being a cost-effective technique to control and prolong the target functionality of innovative fabrics. Thus, the literature reports a good description of a series of applications where microencapsulation is demonstrating effectiveness in therapeutic/health care or cosmetic textiles [Citation3–8] and in other comfort garments by insect repelling or thermal control with phase change materials, among others.[Citation9–11]
Considering the microencapsulation a technology focused on creating micro-packaged materials by coating solid, liquid, or gaseous core components, there are numerous microencapsulation processes of potential interest in the textile industry disclosed in the literature.[Citation12–14] Those existing techniques can be classified as chemical, physic–chemical and physic–mechanical methods, but their design or selection must evaluate involved requirements from fabrication to final target functionality usable at industrial scale.[Citation15,16]
Among reported methods to create microparticles and capsules, formation and breaking-up of micro/nanojets by electrospraying is proved to be a reliable technology to fabricate narrow-size particles and capsules in the nano/micrometre range with limited agglomeration and high yields.[Citation17–19] An outstanding step forward in electrospraying encapsulation, reported by the authors, is based on coaxial electrospraying of two immiscible liquids using electrodynamic forces for injecting them at appropriate flow rates through two concentric needles.[Citation20,21] Thus, the possibility of easily controlling geometrical parameters of capsules by tailoring flow rates and physical properties of the liquids by this configuration attracts research towards versatile micro/nanocapsules and core–shell fibres.[Citation22–27]
Generally, microcapsules are applied by coating processes like spraying, padding or exhausting, where capsules are dispersed in a polymer binder (water-soluble polymers, latexes, amino-aldehyde resins, etc.) coated onto the fabric.[Citation28,Citation29] However, hindrance to easy access to the functional substance from resin coating promotes other direct bonding alternatives of hollow or solid capsules onto the fibres towards enhanced accessibility. Thus, apart from potential ionic bonding through ionic interaction between the surface potential of fibres and ionic functional groups on the microsphere surface, covalent bonding stands out as an emerging highest option. For example, the functionalisation with carboxylic acids as suitable crosslinking agents able to react with hydroxyl groups of cellulose allows forming stable ester bonds.[Citation30–32]
In this research, a method of covalently bonding solid, hollow or core–shell submicron capsules onto cellulosic fibres applying well-established methods for grafting reactive dyes is presented. This method involves chemical functionalisation of capsules with highly reactive triazine moieties. Continuous reactive dying of cotton is based on chromophore groups linked to functional groups capable of forming covalent bonds with cellulose. Those dyes are easily applied and provide good fastness properties from covalent grafting onto the fibres. In particular, the dichlorotriazine-based dyes are well-known reactive dyes with high reactivity and fixation to cellulose at moderate temperature dying. These dyes mainly react with the hydroxyl groups in cellulosic fibres by nucleophilic substitution reaction of reactive chlorine atoms in an aqueous alkaline medium to generate cellulosate anions.[Citation33–35] Therefore, functionalising microspheres with dichlorotriazinyl reactive groups, the authors demonstrate a versatile method for grafting microcapsules onto a cotton fabric under conventional reactive dying conditions.
2. Materials and methods
2.1. Silica microspheres by electrospraying
Hollow and plain silica submicron particles were manufactured by the sol–gel electrospraying process from the acid catalysis of tetraethoxysilane (TEOS) in ethanol/water. A solution from TEOS (98% from Sigma-Aldrich) (52 g) in water (6.75 g), ethanol (99.5% from Panreac) (11.5 g) and hydrochloric acid (HCl 37% v/v from Acros Organics) (0.125 g) was stirred in a sealed flask at room temperature (23 ºC) for 10 minutes and warmed at 80 ºC for one hour. Afterwards, the solution was cooled down to 60 ºC and allowed to settle for 48 hours to slowly evaporate the remaining ethanol.
A 20 coaxial-nozzle multielectrospray device (0.4 mm inner diameter and collector spaced at 15 cm) was designed and fabricated by Yflow for the production of the particles. The working flow range was between 0.3 and 0.5 ml per hour with a throughput of 0.3 g of microparticles per injected millilitre.
2.2. Characterisation of microspheres
Once manufactured, Si–O–Si linkages and hydroxyl groups of microspheres were characterised by Fourier transformed infrared spectroscopy (FTIR) (Nicolet Nexus 670 spectrometer) from 4000 to 400 cm−1. Morphological properties were evaluated by scanning electron microscopy (SEM) (EVO50 from Zeiss) at high vacuum using a secondary electron detector and by transmission electron microscopy (TEM) (TECNAI G2 S-TWIN from FEI) in transmission and scanning transmission (STEM) mode. Thermogravimetry (TGA) (TGA DSC1 LF from Mettler) analysis from 23 to 800 ºC at 10 ºC min−1 heating rate under a nitrogen atmosphere as well as differential scanning calorimetry (DSC) experiments (DSC 1/500 from Mettler) from 0 to 500 ºC at 10 ºC min−1 heating rate under a nitrogen atmosphere was used to determine thermal properties of silica.
2.3. Surface chemical modification
In the first stage, microspheres were functionalised with amine groups using an aminosilane (aminopropyltrietoxysilane, hereafter referred to as APTS) based on inter- or intra-molecular catalysis of the reaction between silane molecules and surface silanol groups by amine moieties to form siloxane bonds.[Citation36,37]
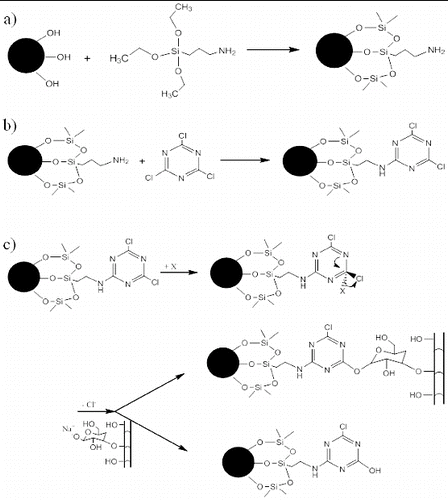
Functionalisation with amine groups ((a)) was performed using standard reaction conditions as described in the literature,[Citation37,38] by dispersing 400 mg of silica in 50 ml of methanol (99.8% from Merck) and adding 2 ml of APTS (assay ≥ 98% from Sigma-Aldrich) under vigorous stirring for 24 hours at room temperature. After centrifugation, the solid residue was washed and centrifuged again with methanol, and the amine-functionalised powder microspheres were vacuum dried at room temperature.
Figure 1. Reaction steps for grafting microspheres/capsules onto cellulosic fibres: (a) functionalisation of silica microspheres with amine groups; (b) linkage of cyanuric chloride molecule to amine-functionalised silica microspheres; and (c) competing nucleophilic substitution reaction of triazine functionalisation.
Amine functionalisation was evaluated by FTIR and silicon-29 nuclear magnetic resonance (29Si-NMR) (400 WB Plus model from Bruker) to identify the silanol groups (Si–OH) remnant on the surface. The Si-29 spectra were performed, with a 4 mm cross polarisation magic angle spinning (CP-MAS) probe rotating the samples at 10 kHz. Furthermore, the functionalisation percentage was determined by TGA.
Subsequently, the chemical functionalisation of the amine–silica surface with triazine moieties was performed by diluting 1.84 g of 2,4,6-trichloro-1,3,5-triazine (cyanuric chloride, 99% from Sigma-Aldrich) in 150 ml of hexane (95% from Labscan) and adding 13.2 mg of amine-functionalised silica under reflux conditions for 24 hours ((b)). The resulting solution, thus, was washed by centrifugation with hexane, and the obtained powder was dried under vacuum at room temperature.
The linkage of triazine molecules to amino-functionalised silica was analysed by FTIR, proton solid-state NMR spectroscopy (1H-NMR) of amino- and azine-functionalised microspheres (400 WB Plus from Bruker) rotating the samples at 60 kHz on a 1.3 mm probe, and TEM coupled to an X-ray detector (TECNAI G2 S-TWIN coupled to DPP-II from EDAX).
2.4. Covalent grafting on cellulosic textile
As triazine ring structures are used in certain reactive dyes, the presence of surface dichlorotriazine groups on micro/nanospheres provides suitable bonding capabilities to cellulose fibres under an alkaline medium at moderated temperatures (typical dyeing conditions for bifunctional dichlorotriazine dyes). However, the simultaneous competitive hydrolysis reaction has to be considered since the number of functionalised spheres able to link covalently to cellulose fibres is lowered when the hydrolysis reaction occurs ((c)).
In particular, simulating dying conditions, a 10 wt% of triazine-functionalised microspheres, based on the weight of cellulose, were dispersed in 50 ml of water at 50 ºC together with a raw cotton fabric (150 g m−2 plain weave cotton fabric with a thickness of 0.24 mm supplied by AITEX). After 15 minutes, 50 ml of a 5.1 M NaCl dilution, for favouring adsorption, was added to the microsphere dispersion to react for 30 minutes at 50 ºC and the pH was stabilised at 8–9 by adding an NaOH solution to react for another 45 minutes. Subsequently, the fabric was removed and vacuum dried at room temperature. The covalent bonding of silica spheres to cellulose was evaluated by SEM in combination to energy dispersive X-ray spectroscopy (EDX).
3. Results and discussion
3.1. Electrosprayed silica microspheres
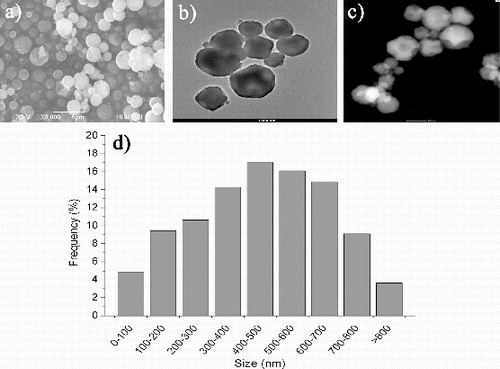
From the morphological point of view, SEM, TEM and STEM ((a)–(c), respectively) demonstrate the overall spherical morphology, with irregularities of the particle surface, as well as reveal the size distribution (TEM) and a 454 nm average particle size ((d)).
Figure 2. (a) SEM, (b) TEM, (c) STEM images of silica submicron particles and (d) particle size distribution of synthesised silica particles.
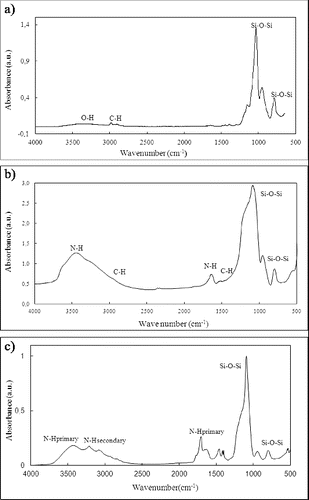
From FTIR analysis, asymmetric and symmetric stretching of the Si–O–Si linkage appears at 1050 and 790 cm−1, respectively ((a)). Furthermore, the broad band corresponding to the O–H stretch from silanol groups goes up at 3400 cm−1 together with a band at 2950 cm−1 from the C–H linkage of silica without reacting.
Figure 3. FTIR spectrums of different reaction steps: (a) silica submicron spheres manufactured by electrospraying; (b) amine-functionalised silica microspheres; and (c) functionalised silica modified with cyanuric chloride.
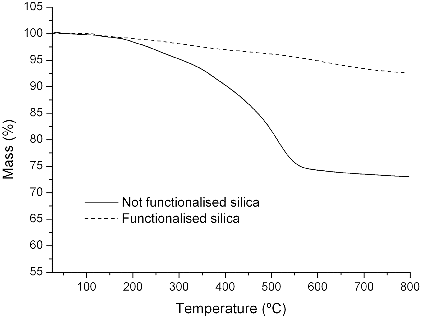
As shown in , after drying the particles in heated (50 ºC) vacuum desiccators for 24 hours and, subsequently, performing an isothermal pre-heating at 100 ºC for 5 minutes, the thermal degradation of electrosprayed silica spheres is characterised by one main step starting above 170 ºC from breakage of OH linkages of silanol groups (25% loss in mass). This result, together with FTIR analysis, corroborates the presence of surface reactive hydroxyl groups for further chemical functionalisation.
3.2. Surface modification
As observed in FTIR spectroscopy ((b)), the amine surface functionalisation of silica microspheres was confirmed by the presence of the stretching and bending vibration bands from amine groups at 3440 cm−1 and 1520 cm−1, respectively. Furthermore, bands at 2900 cm−1 and 1471 cm−1 from stretching and bending vibrations of propyl groups from APTS are identified. The bands at 1036 cm−1 and 786 cm−1 again correspond to the Si–O linkage.
The functionalisation of silica was further analysed by 29Si-NMR. Thus, surface remnant silanol groups were identified considering a potential classification according to their nature and type of association ((a)). Therefore, isolated silanol groups are characterised by a free OH group located far enough from neighbouring OH groups to prevent hydrogen bonding. Vicinal groups are silicon sites with three bridging oxygens (SiO–) and one OH group forming hydrogen bonding with neighbouring atoms. Geminal silanols are silanediol groups, i.e. characterised by two free surface hydroxyl groups, and siloxane bridges (non-isolated silanol groups) are characterised by absence of free hydroxyl groups.[Citation39]
Figure 5. NMR spectra of the first and second steps of the reaction: (a) surface Si–OH sites on silica; (b) 29Si-NMR analysis of amine functionalised and non-functionalised silica microspheres; and (c) 1H-NMR analysis of effective modification with 2,4,6-trichloro-1,3,5-triazine.
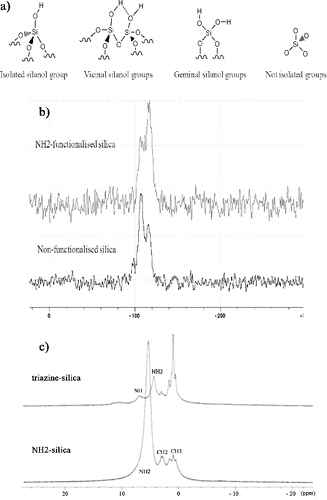
According to NMR analysis ((b)), a change in proportion of surface silanol linkages occurs during functionalisation, as well as chemical displacements shift to higher values. If compared, original silica microspheres are characterised by three differential displacements based on existing silanol groups, one corresponding to silanediol groups at 98 ppm, a sharp one at 107 ppm from isolated silanols and another peak corresponding to silicon atoms without Si–OH groups. However, amine-functionalised silica shows notable variations in the ratio of silanol groups and drop of proportion of hydroxyl groups from APTS linking to oxygen atoms. An absence of germinal silanols is thus observed by disappearing the displacements at 98 ppm, as well as a drop of the peak corresponding to isolated silanol groups at 107 ppm, which demonstrates a reduction in the proportion of silicon atoms linked to hydroxyl groups from chemical functionalisation. Furthermore, a considerable increase of signal at 116 ppm can be observed due to higher proportion of silicon atoms without surface Si–OH sites.
On the other hand, TGA allowed detecting variations in mass loss from functionalisation compared to electrosprayed silica (). Once thoroughly dried by the same described procedure, the sharp degradation of silanol groups at 150 ºC is minimised and substituted by a continuous mass loss from remnant silanols and alkyl moieties from APTS.
Once demonstrated the amine sites on the modified silica surface, the subsequent functionalisation with chlorotriazine ligands was confirmed by FTIR analysis ((c)). The presence of a secondary amine group can be observed by the stretching vibration band at 3200 cm−1, although the presence of the stretching and bending vibration bands at 2450 cm−1 from primary amine groups demonstrated remnant non-reacted silica.
Solid-state 1H-NMR confirms the infrared analysis by comparing amine- and triazine-functionalised silica solid capsules and identifying the formation of secondary amines and the possible remaining primary amines after modification ((c)). The intensity of the principal peak at 5.3 ppm, corresponding to the N‒H bond in primary amine groups for amine-functionalised silica, notably decreases, as well as shifts to lower values, in the case of triazine-modified microspheres, where the formation of secondary amines is demonstrated by the related peak at 6.9 ppm. The rest of peaks are assigned to alkyl groups from APTS.
3.3. Covalent grafting on cellulosic fabric
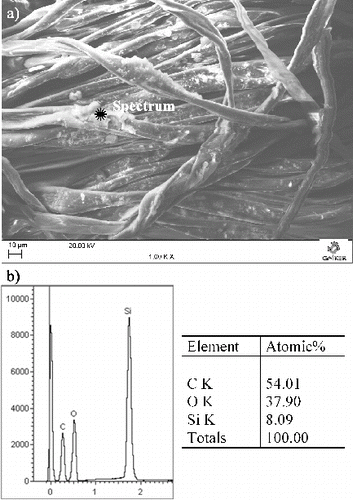
The presence of grafted capsules after reaction and rinsing operations with water at 50 ºC for 30 minutes, under stirring to erase sodium and chloride derivatives from reactants, was assessed by SEM coupled to an X-ray detector. Before evaluation, the samples were subjected to direct compressed air blowing (vapour pressure: 449 kPa at 20 °C) for 10 seconds as parallel assessment of covalent linking on cotton fibres. shows cotton fabric grafted by silica activated with triazine and the chemical composition of covalently bonded spheres in a position as an example.
4. Conclusions
This work shows a versatile process of covalent grafting of solid, hollow or core–shell submicron capsules onto cellulosic textiles by conventional dyeing processes. After manufacturing demonstrative silica solid submicron capsules by electrospraying, the surface functionalisation with cyanuric chloride moieties, similar to triazine ring structures in reactive dyes, allowed their covalent bonding to cotton fibres via nuchleophilic substitution reaction with hydroxyl groups in cellulosic fibres. Therefore, considering that the wide range of applications of the presented procedure is based on the properties of grafted solid or hollow capsules, the properties of potential active ingredients and the nature, and design of the textile substrate, among others, this work widens functionalisation opportunities for the textile industry. In this scenario, further research and development work in optimised covalent bonding could be based, for example, on conventional pre-treatments dealing with textile dyeing (desizing, boiling, etc.) or adjusting dyeing conditions. Besides, the integration of chain extenders with optimal hydrophilic character for easier adsorption and for diminishing steric hindrances for further penetration between fibres could be helpful tools for industrially effective process.
Acknowledgements
This work was supported by the Centre for Industrial Technological Development (CDTI) – Spanish Ministry of Science and Innovation, under the project INFINITEX [CEN-20091014] – Research on the New and Smart Features Implemented in Textiles, within CENIT Programme.
References
- Pan N, Sun G. Functional textiles for improved performance, protection and health. Cambridge (MA): Woodhead Publishing; 2012.
- Nelson G. Application of microencapsulation in textiles. Int J Pharm. 2002;242:55–62.
- Zhang Y, Rochefort D. Characterisation and applications of microcapsules obtained by interfacial polycondensation. J Microencapsul. 2012;29(7):636–649.
- Martí M, Martínez V, Rubio L, Coderch L, Parra JL. Biofunctional textiles prepared with liposomes: in vivo and in vitro assessment. J Microencapsul. 2011;28(8):799–806.
- Ocepek B, Boh B, Šumiga B, Tavčer P. Printing of antimicrobial microcapsules on textiles. Color Technol. 2012;128:95–102.
- Cheng SY, Yuen CWM, Kan CW, Cheuk KKL. Development of cosmetic textiles using microencapsulation technology. Res J Text Apparel. 2008;12:41–51.
- Rodrigues SN, Fernández I, Martins IM, Mata VG, Barreiro F, Rodrigues AE. Microencapsulation of limonene for textile application. Ind Eng Chem Res. 2008;47:4142–4147.
- Ghosh SK. Functional coatings: by polymer microencapsulation. Weinheim: Wiley-VCH Verlag GmbH; 2006.
- Mondal S. Phase change materials for smart textiles – an overview. Appl Therm Eng. 2008;28:1536–1550.
- Suthaphot N, Chulakup S, Chonsakorn S, Mongkholrattanasit R. Application of aromatherapy on cotton fabric by microcapsules. Paper presented at: RMUTP International Conference: Textiles & Fashion; 2012 July 3–4; Bangkok, Thailand.
- Specos M, García JJ, Tornesello J, Marino P, Della Vecchia M, Defain Tesoriero MV, Hermida LG. Microencapsulated citronella oil for mosquito repellent finishing of cotton textiles. Trans Roy Soc Trop Med Hyg. 2010;104:653–658.
- Yoshizawa H. Trends in microencapsulation research. Kona. 2004;22:23–31.
- Obeidat WN. Recent patents review in microencapsulation of pharmaceuticals using the emulsion solvent removal methods. Recent Pat Drug Deliv Formul. 2009;3:178–192.
- Sliwka W. Microencapsulation. Angew Chem Int Ed. 1975;14:539–550.
- Benita S. Microencapsulation: methods and industrial applications. Boca Raton (FL): CRC Press; 2006.
- Ciriminna R, Sciortino M, Alonzo G, Schrijver A, Pagliaro M. From molecules to systems: sol-gel microencapsulation in silica-based materials. Chem Rev. 2011;111:765–789.
- Bocanegra R, Gaonkar AG, Barrero A, Loscertales IG, Pechack D, Marquez M. Production of cocoa butter microcapsules using an electrospray process. J Food Sci. 2005;70:492–497.
- Bock N, Woodruff MA, Hutnacher DW, Dargaville TR. Electrospraying, a reproducible method for production of polymeric microspheres for biomedical applications. Polymers. 2011;3:131–149.
- Park CH, Lee J. Electrosprayed polymer particles: effect of the solvent properties. J Appl Polym Sci. 2009;114:430–437.
- Loscertales IG, Barrero A, Guerrero I, Cortijo R, Marquez M, Gañan-Calvo AM. Micro/nano encapsulation via electrified coaxial liquid jets. Science. 2002;295:1695–1698.
- López-Herrera JM, Barrero A, López A, Loscertales IG, Márquez M. Coaxial jets generated from electrified Taylor cones. Scaling laws. J Aerosol Sci. 2003;34:535–552.
- Loscertales IG, Barrero A, Márquez M, Spretz R, Velarde-Ortiz R, Larsen G. Electrically forced coaxial nanojets for one-step hollow nanofiber design. J Am Chem Soc. 2004;126:5376–5377.
- Hwang YK, Jeong U, Cho EC. Production of uniform-sized polymer core–shell microcapsules by coaxial electrospraying. Langmuir. 2008;24:2446–2451.
- Chang M, Stride E, Edirisinghe M. Controlling the thickness of hollow polymeric microspheres prepared by electrohydrodynamic atomization. J R Soc Interface. 2010;7:451–460.
- Kim W, Kim SS. Multishell encapsulation using a triple coaxial electrospray system. Anal Chem. 2010;82:4644–4647.
- Kim W, Kim SS. Synthesis of biodegradable triple-layered capsules using a triaxial electrospray method. Polymer. 2011;52:3325–3336.
- Nguyen TTT, Ghosh C, Hwang S, Chanunpanich N, Park JS. Porous core/sheath composite nanofibers fabricated by coaxial electrospinning as a potential mat for drug release system. Int J Pharm. 2012;439:296–306.
- Smith WC. Smart textile coatings and laminates. Cambridge (MA): Woodhead Publishing; 2010.
- Badulescu R, Vivod V, Jausovec D, Voncina B. Grafting of ethylcellulose microcapsules onto cotton fibers. Carbohydr Polym. 2008;71:85–91.
- Voncina B, Majcen Le Marechal A. Grafting of cotton with β-cyclodextrin via poly(carboxylic acid). J Appl Polym Sci. 2005;96:1323–1328.
- Voncina B, Kreft O, Kokol V, Chen WT. Encapsulation of rosemary oil in ethylcellulose microcapsules. Mater Res Soc Symp. 2009;1:13–19.
- Yang CQ, Yiping L, Lickfield G. Chemical analysis of 1,2,3,4-butanetetracarboxylic acid. Text Res J. 2002;72:817–824.
- Shore J. Cellulosics dyeing. Cambridge (MA): Woodhead Publishing; 1995.
- Taylor JA, Pasha K, Phillips DAS. The dyeing of cotton with hetero bi-functional reactive dyes containing both a monochlorotriazinyl and a chloroacetylamino reactive group. Dyes Pigments. 2001;51:141–152.
- Klancnik M. Kinetics of hydrolysis of halogeno-s-triazine reactive dyes as a function of temperature. Chem Biochem Eng Q. 2008;22:81–88.
- Smith EA, Chen W. How to prevent the loss of surface functionality derived from aminosilanes. Langmuir. 2008;24:12405–12409.
- Kanan SM, Tze WTY, Tripp CP. Method to double the surface concentration and control the orientation of adsorbed (3-aminopropyl)dimethylethoxysilane on silica powders and glass slides. Langmuir. 2002;18:6623–6627.
- Ping W, Jian-qing Z, Zhi-jie J, Yun-chun L, Shu-mei L. Preparation of magnetic iron/mesoporous silica composites spheres and their use in protein immobilization. Trans Nonferrous Met Soc China. 2009;19:605–610.
- Bergna HE, Roberts WO. Colloidal silica: fundamentals and applications. Boca Raton (FL): Taylor & Francis Group; 2006.