Abstract
An in vitro study was conducted to determine the apoptosis induced by tamoxifen (TAM) and TAM-loaded solid lipid nanoparticles (SLNs) in breast cancer cell lines, MCF-7 and MDA-MB231 cells. The effect of free drug and drug-loaded SLN on the cell lines was characterised by cell morphology and cell cycle distribution using phase contrast microscopy, nuclear morphology and flow cytometry, respectively. The results showed that TAM-loaded SLNs have an equally efficient cytotoxic activity against MCF-7 and MDA-MB231 cells, compared to free TAM, and the half maximal inhibitory concentration (IC50) of TAM-loaded SLNs was generally lower than that of free TAM. In the presence of TAM and TAM-loaded SLN, the viability of the both cells diminishes and the cancer cells lose their normal morphological characteristics, detaches, aggregates and later develops apoptotic bodies. Flow cytometry analysis showed that TAM-loaded SLN like the free TAM caused a dose- and time-dependent apoptosis without cell cycle arrest of human breast cancer cells. Therefore, TAM-loaded SLN has great potential in human medicine for the treatment of breast cancers.
1. Background
Cell death basically can occur in two ways. The first is through the necrosis pathway, where traumatic injuries cause cell, in particular cell enlarges, bursts and liberate its intracellular components into the surrounding environment. The second pathway is programmed cell death or apoptosis, which is a molecular signalling cascade, inducing a disturbance in the organisation and package of the cell causing death.[Citation1,Citation2] Other mode of cell death has also been suggested, for example mitotic cell death, which plays an important role in cell death caused by ionising radiation.[Citation3]
Breast cancer is the most common malignancy (18% of all malignancies) in women worldwide and its occurrence is slowly increasing.[Citation4] Like many cancers, breast cancer appears to be a result of high genetic damage that caused uncontrolled cellular proliferation and unusual apoptosis. These phenomena activate proto-oncogenes and inactivate tumour suppressor genes. These events can be activated by exposure of living cells to environmental, physical, chemical and/or biological carcinogens.[Citation5] The antiestrogen molecule tamoxifen (TAM) is a strong, hydrophobic endocrine drug widely used for treating breast cancers and high risk patients.[Citation6,Citation7] Depending on the dose and the tissues targeted, the function of TAM can be estrogenic or antiestrogenic. The dose-dependent side effects of TAM include liver cancer, increased blood clotting and ocular adverse effects, such as retinopathy and corneal opacities.[Citation8] These findings suggest that small doses given through colloidal delivery systems would be useful for long-term treatment of breast cancers.
Nanoparticulate delivery systems in the form of nanospheres like poly-caprolactone nanoparticles were used for TAM encapsulation. The basis of this formulation is to obtain the necessary dose of drug at tumour location for a known period of time and reducing adverse effects on normal organs in the body.[Citation9] We have previously reported the effect of TAM-loaded solid lipid nanoparticles (SLNs) on the LA7 cell-induced rat mammary tumour gland. The result showed that TAM-loaded SLNs have similar effect on the tumour as free TAM, which promotes apoptosis in the rat mammary gland tumour. The efficacy of free-TAM and TAM-loaded SLNs was similar. However, the TAM-loaded SLNs showed a more prolonged effect suggesting that incorporation of TAM in SLNs is suitable for delayed drug release in the chemotherapy of breast cancers, while decreasing the hepatotoxic effects.[Citation10–Citation11] In this study, the objective was to determine the apoptotic effect of TAM-loaded SLNs on breast cancer cell lines, MCF-7 and MDA-MB231 cells in vitro. The responses of breast cancer cell lines were determined by cell morphology, apoptosis and cell cycle distribution.
2. Methods
2.1. Materials
Hydrogenated palm oil (Softisan 154 or S154) was a gift from Condea (Witten, Germany). Hydrogenated soybean lecithin (LipoidS100-3, containing 90% phosphatidylcholine, including 12%–16% palmitic acid, 83%–88% stearic acid, oleic acid and isomers, and linoleic acid] was a gift from Lipoid (Ludwigshafen, Germany). Thimerosal, mercury((o-carboxyphenyl)thio)ethyl sodium salt and sorbitol, (2S,3R,4R,5R)-hexane-1,2,3,4,5,6-hexol were purchased from Sigma (Kuala Lampur, Malaysia). Oleyl alcohol (octadecenol or cis-9-octadecen-1-ol) was purchased from Fluka. Tamoxifen, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), fetal bovine serum and RPMI-1640 (Roswell Park Memorial Institute) medium were obtained from Sigma‑Aldrich (Kuala Lampur, Malaysia). Bidistilled water was used.
2.2. Preparation of tamoxifen-loaded SLNs
Tamoxifen-loaded SLNs were prepared using the high-pressure homogenisation technique. A mixture of S154 and Lipoid S100 at a ratio of 70:30 was ground in a ceramic crucible. The mixture was then heated to 65 °C–70 °C while being stirred with a polytetrafluoroethylene (PTFE)-coated magnet until a clear-yellowish lipid matrix (LM) solution was obtained. A solution containing 1 mL oleyl alcohol, 0.005 g thimerosal, 4.75 g sorbitol and 89.25 mL bidistilled water (all w/w) at the same temperature was added to 5 g of LM. A pre-emulsion of SLN was obtained using the homogenizer (Ultra Turrax, Ika, Staufen, Germany) at 13,000 rpm for 10 min and high-pressure homogenizer (EmulsiFlex-C50 CSA10, Avestin, Ottawa, Canada) at 1000 bar, 20 cycles and 60 °C. The lipophilic drug TAM (1 mg) was dissolved in oleyl alcohol and mixed with 5 mg of SLN pre-emulsion using the Ultra Turrax homogenizer at 13,000 rpm for 10 min. This mixture was then incubated overnight at 50 °C–60 °C, stirred periodically with a PTFE-coated magnet at 500 rpm, and finally exposed to air to solidify. Tamoxifen-loaded SLNs were characterised in vitro for particle size, particle-size distribution and zeta potential using a high-performance particle sizer (HPP5001, Malvern Instruments, Worcestershire, UK) and analyzer (Zeta sizer; ZEN-2600, Malvern Instruments, Worcestershire, UK) in triplicate.
2.3. Cell line
Breast cancer cell lines, MCF‑7 and MDA-MB231, were kindly offered by Dr Teo Guan Young (Institute Bioscience, UPM). Cells were maintained at 37 °C in a humidified atmosphere of 5% CO2 in RPMI medium supplemented with 10% fetal bovine serum, 100 μg/mL streptomycin and 100 IU/mL penicillin. The confluent cells in the 25 cm3 cell culture flask were washed with phosphate buffered saline (PBS) and detached by trypsin. A haemocytometer was used to determine the concentration of the cells in the flask. One hundred microlitres of culture medium containing approximately 105 cells/mL were seeded in a six-well plate and allowed to adhere. After 24 h, the old media was removed and the attached cells were washed with PBS (pH 7.2). The cells were treated with TAM solution in dimethyl sulfoxide (DMSO), TAM-loaded SLN and SLN (dispersed in DMSO) for 24, 48 and 72 h. The culture medium was supplemented with 12 and 15 µg/mL TAM and TAM-loaded SLN for MCF-7 cells and with 16, 18 and 20 µg/mL TAM and TAM-loaded SLN for MDA-MB231. The control wells received 0.1% DMSO as vehicle. The viability of the breast cancer cells in presence of TAM and TAM-loaded SLN was assessed by the MTT assay.
2.4. Fixation of the cells
Following treatment of the cells at defined time (24, 48 and 72 h), all adhering and floating cells from either control or treated wells were harvested. The cell pellet was obtained by centrifugation at 100 g for 10 min and the supernatant discarded. The pellet was then washed twice with cold PBS. The cell pellet was then resuspended in 500 μL of PBS. The resuspended cell was subsequently fixed with 500 μL of 70% ethanol drop-wisely. The fixed cell was kept at −20 °C for 2 h up to two weeks.
2.5. DNA labelling
The most commonly used dye for DNA content/cell cycle analysis is propidium iodide (PrI). The PrI stains whole cells and isolated nuclei. The PrI intercalates into the major groove of double-stranded DNA and produces a highly fluorescent adduct that can be excited at 488 nm with a broad emission centred around 600 nm. Since PrI can also bind to double-stranded RNA, it is necessary to treat the cells with RNase for optimal DNA resolution. The excitation of PrI at 488 nm facilitates its use on the bench top cytometer.[Citation12,Citation13] The fixed cells were washed three times with PBS (pH 7.2) to remove the ethanol. The cell pellet was obtained by centrifugation at 100 g for 10 min and the supernatant discarded. The pellet was resuspended in 950 μL PBS and stained with 40 μL PrI (50 mg/mL) in the presence of 10 μL RNase (50 mg/mL) (Sigma, St Louis, MO, USA) for 30 min at 37 °C in dark. Propidium iodide staining is for the identification of apoptotic cells and the distribution of the cell population in the cell cycle phases.
2.6. Flow cytometry
The antiproliferative and apoptotic effects of free TAM and TAM-loaded SLN on the MCF-7 and MDA-MB231 cells were examined using a flow cytometer (Cyan ADP, Dako, Denmark) and the histograms were analysed by Summit V4.3 software.
2.7. Cell morphology
The morphology of treated MCF-7 and MDA-MB231 cells with TAM and TAM-loaded SLN for 48 h were viewed and captured using light microscopy (Nikon ECLIPSE TS 100, Japan) at 10× magnification using NIS-Elements D2.30 Image Software.
2.8. Nuclear morphology
The MCF-7 and MDA-MB23 cells were treated with TAM and TAM-loaded SLN at a concentration equal to the half maximal inhibitory concentration (IC50), obtained via MTT assay. The cells were then fixed as described earlier. Staining was performed according to the method described by Hishikava et al.[Citation14] The fixed cells were washed with PBS (pH 7.2) and resuspended in 1 mL PBS (pH 7.2) containing 60 μg/mL DNA-specific dye (Hoechst 33258) for 30 min at room temperature in the dark. The cells were then washed three times with PBS (pH 7.2) and placed on chamber slides (Labtek II Chamber Slide, Nunc) until excess PBS dried out. Examination of cells was done under an epifluorescence microscope (Leica Microsystems CMS GMbH, Wetzlar, Germany) equipped with a standard Hoechst optical filter set. Measurement of fluorescence was expressed in arbitrary units. Apoptotic cells were defined on the basis of nuclear morphology changes such as chromatin condensation, fragmentation (bead-like formation) and formation of degraded cells as apoptotic bodies.
2.9. Statistical analysis
The data obtained were subjected to statistical analysis. The differences in mean values among the groups were expressed as mean ± standard deviation. All data were subjected to one-way analysis of variance followed by post hoc multiple comparison and Duncan test after verification of the normal distribution of the data. SPSS version 15.0 (SPSS 2006) was used to perform all statistical tests and p-value less than 0.05 was considered significant.
3. Results and discussion
In this study, the average size of TAM -loaded SLNs (251.65 ± 33.02) was significantly larger than that of the free SLNs (152.87 ± 9.91), and the surfaces of TAM-loaded SLNs carried a positive charge (+10.16 ± 0.22). This may be assigned to the fact that drug is either adsorbed to particle surface or entangled in aliphatic chains of triglycerides.[Citation8]
The IC50 of TAM, TAM-loaded SLN and free TAM SLN for MDA-MB231 cells, estrogen-receptor (ER)-negative or ER-independent was higher than for MCF-7 cells, ER-positive or ER-dependent (). The mechanisms of ER-independent, TAM-induced apoptosis may be through the inhibition of protein kinase C.
Table 1. The IC50 of TAM, TAM-loaded SLN and SLN formulations on MCF-7 and MDA-MB231cells after 24, 48 and 72 h.
The IC50 value of TAM for protein kinase C inhibition is 4–10 times the concentration for ER inhibition in ER-positive cells. Therefore, the dose of tamoxifen for treatment of patients with ER-positive breast cancer would have to be increased over the usual 20 mg per day used. High dose of tamoxifen might decrease the therapeutic index by increasing toxicity.[Citation15] In our study, the IC50 of TAM-loaded SLN on the breast cancer cell lines was generally lower than that for free TAM. This indicates that TAM cytotoxicity may be the result of improved drug internalisation through encapsulation into SLN matrix and endocytosis.[Citation16] A previous study showed a similar finding, where there was reduced MCF-7 cell viability in the presence of TAM-loaded SLN.[Citation6] It seems that improved cytotoxicity of incorporated drug is not dependent on the composition of the SLN. In fact, it was reported that the IC50 value of drug-loaded SLN composed of different materials was lower than that of free drug solution.[Citation17]
The IC50 of SLN was time dependent and different between the two breast cancer cell lines tested. The IC50 values suggest that SLN has low cytotoxicity to the MDA-MB231 and the MCF-7 cells. The low cytotoxicity of the SLN can be attributed to the lecithin [Citation18] and components of the aqueous phase used, especially the nonionic emulsifier.[Citation19,Citation20] However, the cytotoxicity of the SLN in this study is comparable to the nanoparticulate systems consisting of polylactic acid/glycolic acid or polycyanocrylate nanoparticles.[Citation21]
3.1. Morphological changes of the breast cancer cells treated with TAM, TAM-loaded SLN and SLN
Apoptotic cell death can be recognised under phase contrast and fluorescence inverted microscope after staining. This is the most practical method to identify cell morphological changes attributed to apoptotic cell death.
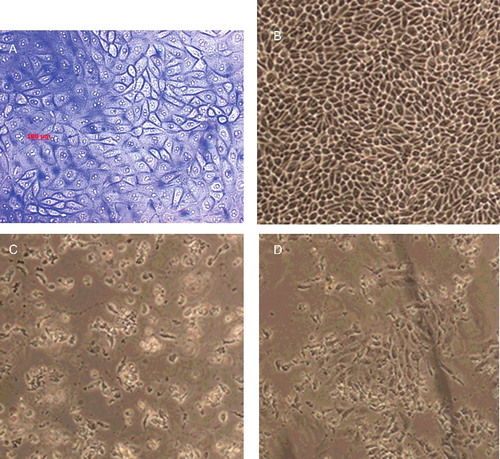
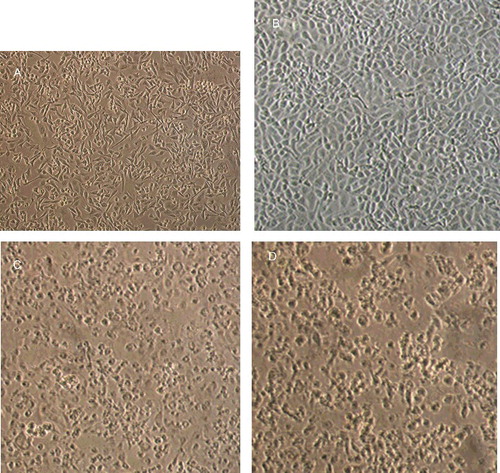
3.2. Phase contrast microscopy
Morphological features of untreated and treated breast cancer cells were examined using phase contrast light microscopy ( and ). The normal untreated MCF-7 and MDA-MB-231 cells normally appeared healthy and exhibiting epithelial-like features and forming a monolayer on the surface of the culture flask. TAM and TAM-loaded SLN treatments at concentrations equal to IC50 caused detachment of both breast cancer cells and loss of colony formation ability. These cells appeared rounded up and lose contact with neighbouring cells. The cells treated with unloaded SLN, however, did not show morphological change. This indicates that SLN is suitable to be used as drug-delivery system. In the presence of TAM and TAM-loaded SLN, the viability of the both cells diminished and the cancer cells lost their normal morphological characteristics, detached and aggregated, and later developed apoptotic bodies. The detachment of cells in the presence of free TAM and TAM-loaded SLN suggests that TAM is cytotoxic, even when incorporated in the SLN. The results from this study showed that SLN is a suitable carrier for TAM in a drug-delivery system for the treatment of breast cancers.
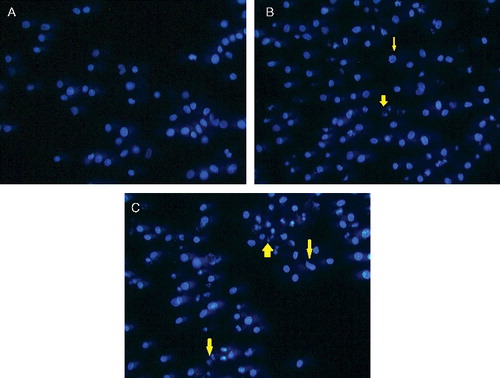
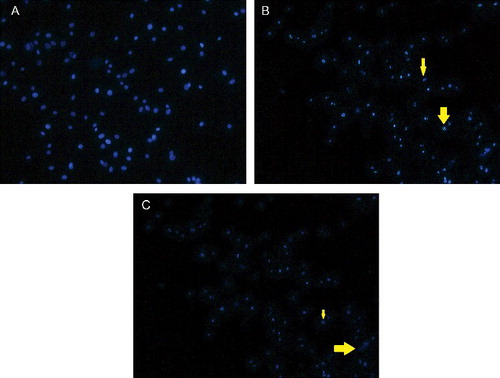
3.3. Nuclear morphology
Cell death is either by physiological or pathological means. Physiological cell death is distinguished by apoptotic morphology, including chromatin condensation, membrane blebbing, internucleosomal degradation of DNA and apoptotic body formation. Pathological cell death or necrosis is associated with cellular swelling and collapse, without severe damage to nuclei or breakdown of the DNA. In apoptosis, several cellular and molecular biological features, including cell shrinkage and DNA fragmentation, are exhibited.[Citation22] To characterise the cell death induced by TAM and TAM-loaded SLN, the nuclear morphology of dying cells was examined under Hoechst dye 33258 staining ( and ). The Hoechst dye 33258 is a bis-benzimide derivative and a fluorescent DNA-binding agent. This dye is useful for cell cycle analysis because it can be used in low concentrations, and thus minimising the problem of toxicity. According to Latt and Stetten,[Citation23] the Hoechst dye binds to AT-rich regions of the DNA and when excited with an ultraviolet light produces bright fluorescence at 465 nm. In this study, TAM and TAM-loaded SLN induced death of MCF-7 and MDA-MB231 cells by apoptosis. This was evident by the typical apoptotic changes showing clear condensation of cell nuclei, nuclear fragmentation and apoptotic bodies.
3.4. Cell cycle distribution
Apoptosis plays an important role in maintaining cellular homeostasis and a useful ally in cancer therapies.[Citation24,Citation25] In this study, cell cycle analyses showed that TAM and TAM-loaded SLN at IC50 concentrations are able to induce apoptosis in ER-positive MCF-7 and ER-negative MDA-MB231 breast cancer cells ( and ).
Table 2. Effect of TAM and TAM-loaded SLN at concentration 12 µg/mL on MCF-7 cell cycle distribution.
Table 3. Effect of TAM and TAM-loaded SLN at concentration 16 µg/mL on MDA-MB231 cell cycle distribution.
The cytotoxic effect of TAM probably involves more than one pathway. One of the pathways may be estrogen receptor independent. In this pathway, TAM increased the levels of transforming growth factor-β1 (TGF-β1), a pleiotropic cytokine that regulates the proliferation and functional activity of a wide range of cell types.[Citation26] The second pathway is ER dependent, and this involves TAM binding to the ER to form a TAM–ER complex that competitively inhibits the binding of estrogen to its receptor. The TAM–ER complex binds to an estrogen responsive element (ERE) that contains estrogen sensitive genes. As a result, transcription of estrogen sensitive genes is attenuated. Thus, TAM arrests the cell cycle in the G1 phase, thereby decreasing cell proliferation [Citation27] and promoting apoptosis.[Citation9]
The estrogen receptor-mediated antiestrogenic effect of TAM on breast cancers could lead to either cytostatic or cytotoxic effects. This effect of TAM is concentration dependent. Nanomole concentrations of TAM only cause growth arrest, whereas at micromole concentrations, the drug induces cell death.[Citation28–Citation30]
In our study, TAM and TAM-loaded SLN at concentration 12 µg/mL induced significant apoptosis (p < 0.05) in the MCF-7 cells in a time- and concentration-dependent manner. These results are in agreement with those obtained by [Citation4]. It seems that the MCF-7 cell is more sensitive than the MDA-MB231 cell to TAM and TAM-loaded SLN treatment. The cytotoxicity of TAM-loaded SLN on MCF-7 cell at 24 and 48 h was similar to that produce by TAM (p > 0.05). Therefore, encapsulation of TAM in SLN did not reduce antitumoural activity of TAM. As shown in , the TAM and TAM-loaded SLN at 16 µg/mL for 24 h induced significant (p < 0.05) increase in number of cells in the sub-G0/G1 phase suggests apoptosis of the MDA-MB231 cells. Both treatments also significantly increase the number of cells in the G0/G1 phase (R3). These values are in comparison with the control at the same time period. It seems 16 µg/mL TAM-loaded SLN like TAM induced apoptosis of MDA-MB231 through cell cycle arrest in G0/G1 phase (R3).
Incubation of the MCF-7 cell with higher concentrations (15 µg/mL) of TAM and TAM-loaded SLN significantly (p < 0.05) increased the sub-G0/G1 phase, which represents increase in apoptotic activity (). Therefore, TAM and TAM-loaded SLN at micromole concentrations produced a time- and dose-dependent apoptotic effect on MCF-7 cells without cell cycle arrest.
Table 4. Effect of TAM and TAM-loaded SLN at concentration 15 µg/mL on MCF-7 cell cycle distribution.
In this study, the TAM- and TAM-loaded SLN treated MCF-7 cells in G0/G1 decreased, while those in sub-G0/G1 phase increased and no cycle arrest was observed at 72 h.
With longer incubation (48 and 72 h) of the MDA-MB231 cells with TAM and TAM-loaded SLN, the same concentrations produced significantly different effect on cell cycle distribution. The percentage of the S+G2/M population of MDA-MB231 cells treated with TAM which correlated with the cell proliferation phase is significant (p < 0.05) lesser than the cells treated with TAM-loaded SLN at 48 h. This may be attributed to the cytostatic effect of TAM-loaded SLN. Since the population of the MDA-MB231 cells treated with TAM in sub-G0/G1 phase that represent the apoptotic cells was significantly (p < 0.05) higher than those treated with TAM-loaded SLN, the conclusion that can be made is that the cytotoxicity of TAM is more prevalent than its cytostatic effect. Thus, encapsulation of TAM into SLN leads to high cytostatic effect on MDA-MB231 cells during in vitro incubation periods of 48 h.
Tamoxifen-loaded SLN decreased the proliferation of MDA-MB23 cells, and this effect appeared to be due to growth inhibition rather than cell death. On the basis of these results, it can be concluded that while TAM governs the morphological changes of the cells and exert in vitro cytostatic activity. Its cytotoxic effect is evident only when the drug is applied in the free form for at least after 48 and 72 h. According to Chawla and Amiji,[Citation9] TAM blocks the cell cycle in the G1 phase, thereby decreasing cell proliferation and increasing apoptosis. This finding is also reflected in this study.
Incubation of the MDA-MB231 cells with TAM at a concentration of 18 µg/mL for 24 h produced significantly (p < 0.05) greater cytotoxicity than the same concentration of TAM-loaded SLN ().
Table 5. Effect of TAM and TAM-loaded SLN at concentration 18 µg/mL on MDA-MB231 cell cycle distribution.
Treatment of the MDA-MB231 cells with the 20 µg/mL TAM-loaded SLN produced a devastating effect on the cell distribution (). At 24 h, the TAM-loaded SLN treated cells produced significant (p < 0.05) increases in population in the apoptotic phase (sub-G0/G1) while still preserving its cytostatic effect.
Table 6. Effect of TAM and TAM-loaded SLN at concentration 20 µg/mL on MDA-MB231 cell cycle distribution.
4. Conclusion
Using phase contrast microscopy it was shown that following TAM and TAM-loaded SLN treatments at concentrations equal to IC50, caused detachment of both breast cancer cells, loss of colony formation ability, and development of apoptotic bodies. In this study, apoptosis induced by TAM and TAM-loaded SLN was further evaluated using Hoechst dye 33258 staining. The studies revealed typical apoptosis phenomenon, i.e. clear condensation of cell nuclei, nuclear fragmentation and formation of apoptotic bodies.
Cell cycle analyses showed that TAM and TAM-loaded SLN at micromole concentrations are able to induce apoptosis in the MCF-7 and MDA-MB231 breast cancer cells. The study showed that TAM and TAM-loaded SLN at defined concentrations significantly (p < 0.05) increased the G0/G1 phase and produced a time- and dose-dependent apoptosis in the MCF-7 cell line without cycle arrest. The percentage of the S+G2/M population of MDA-MB231 cells treated with TAM which correlated with the cell proliferation phase is significantly (p < 0.05) lesser than the cells treated with TAM-loaded SLN at 48 h, which may be attributed to the cytostatic effect of TAM-loaded SLN. Since the population of the MDA-MB231 cells treated with TAM in sub-G0/G1 phase that represent the apoptotic cells was significantly (p < 0.05) higher than those treated with TAM-loaded SLN, the conclusion that can be made is that the cytotoxicity of free TAM is prevalent over its cytostatic effect. Thus, encapsulation of TAM into SLN leads to the higher cytostatic effect toward MDA-MB231 cells during in vitro incubation periods of 48 h.
Acknowledgments
The authors would like to thank Condea and Lipoid for their kind support with materials, and the Universiti Putra Malaysia for financial support and facilities. Roghayeh Abbasalipourkabir drafted the manuscript, Aref Salehzadeh helped discuss the data analysis and Rasedee Abdullah organised the final manuscript. All authors read and approved the final manuscript.
Disclosure statement
The authors declare that they have no competing interests.
References
- Fadok VA. Clearance: the last and often forgotten stage of apoptosis. J Mammary Gland Biol Neoplasia. 1999;4:203–211.
- Messmer UK, Pfeilschifter J. New insights into the mechanism for clearance of apoptotic cells. Bio Essays. 2000;22:878–881.
- Steel GG. The case against apoptosis. Acta Oncologica. 2001;40:968–975.
- Salami S, Karami-Tehrani F. Biochemical studies of apoptosis induced by tamoxifen in estrogen receptor positive and negative breast cancer cell lines. Clin Biochem. 2003;36:247–253.
- Russo J, Russo IH. Mechanisms involved in carcinogenesis of the breast. In: Pasqualini JR, editor. Breast cancer. New York, NY: Taylor & Francis; 2002. p. 1–2.
- Fontana G, Maniscalco L, Schillaci D, Cavallaro G. Solid lipid nanoparticles containing tamoxifen characterization and in vitro antitumoral activity. Drug Deliv. 2005;12:385–392.
- Hashem FM, Nasr M, Khairy A. In vitro cytotoxicity and bioavailability of solid lipid nanoparticles containing tamoxifen citrate. Pharm Dev Technol. 2014;19(7):824–832.
- Memisoglu-Bilensoy E, Vural I, Bochot A, Renoir JM, Ducheneb D, AtillaHincal A. Tamoxifen citrate loaded amphiphilic β-cyclodextrin nanoparticles: in vitro characterization and cytotoxicity. J Controlled Release. 2005;104:489–496.
- Chawla JS, Amiji MM. Cellular uptake and concentrations of tamoxifen upon administration in poly(-caprolactone) nanoparticles. Am Assoc Pharm Scientists J. 2003;5:28–34.
- Abbasalipourkabir R, Salehzadeh A, Rasedee Abdullah. Antitumor activity of tamoxifen loaded solid lipid nanoparticles on induced mammary tumor gland in Sprague-Dawley rats. Afr J Biotech. 2010;9(43):7337–7345.
- Tran TH, Ramasamy T, Cho HJ, Kim YI, Poudel BK, Choi HG, Yong CS, Kim JO. Formulation and optimization of raloxifen-loaded solid lipid nanoparticles to enhance oral bioavailability. J Nanosci Nanotechnol. 2014;14(7):4820–4831.
- Crissman HA, Steinkamp JA. Rapid simultaneous measurement of DNA, protein and cell volume in single cells from large mammalian cell populations. J Cell Biol. 1973;59:766–771.
- Krishan A. Rapid flow cytofluorometric analysis of cell cycle by propidium iodide staining. J Cell Biol. 1975;6:188–193.
- Hishikava K, Nakaki T, Fujii T. Transformation growth factor-b1 induces apoptosis via connective tissue growth factor in human aortic smooth muscle cells. Eur J Pharmacol. 1999;385:287–290.
- Gelman EP. Tamoxifen induction of apoptosis in estrogen receptor-negative cancers: new tricks for an old dog? J Natl Cancer Inst. 1996;88:224–226.
- Serpe L, Laurora S, Pizzimenti S, Ugazio E, Ponti R, Canaparo R, Briatore F, Barrera G, Gasco MR, Bernengo MG, Eandi M, Zara GP. Cholesteryl butyrate solid lipid nanoparticles as a butyric acid pro-drug: effects on cell proliferation, cell-cycle distribution and c-myc expression in human leukemic cells. Anti-Cancer Drug. 2004;15:525–536.
- Yuan H, Miao J, Du YZ, You J, Hu FQ, Su Z. Cellular uptake of solid lipid nanoparticles and cytotoxicity of encapsulated paclitaxel in A549 cancer cells. Int J Pharm. 2008;348:137–145.
- Schubert MA, Müller-Goymann CC. Characterization of surface-modified solid lipid nanoparticles (SLN): influence of lecithin and nonionic emulsifier. Eur J Pharm Biopharm. 2005;61:77–86.
- Schöler N, Zimmermann E, Katzfey U, Hahn H, Müller RH, Liesenfeld O. Preserved solid lipid nanoparticles (SLN) at low concentrations do cause neither direct nor indirect cytotoxic effects in peritoneal macrophages. Int J Pharm. 2000;196:235–239.
- Schöler N, Olbrich C, Tabatt K, Müller RH, Hahn H, Liesenfeld O. Surfactant but not the size of solid lipid nanoparticles (SLN) influences viability and cytokine production of macrophages. Int J Pharm. 2001;221:57–67.
- Müller RH, Maassen S, Schwarts C, Mehnert W. Solid lipid nanoparticles (SLN) as potential carrier for human use: interaction with human granulocytes. J Control Release. 1997;47:261–269.
- Yu T, Lee J, Lee YG, Byeon SE, Kim MH, Sohn EH, Lee YJ, Lee SG, Youl J. In vitro and in vivo anti-inflammatory effects of ethanol extract from Acer tegmentosum. J Ethnopharmacol. 2010;128(1):139–147.
- Latt SA, Stetten G. Spectral studies on 33258 Hoechst and related bisbenzimidazole dyes useful for fluorescent detection of deoxyribonucleic acid synthesis. J Histochem Cytochem. 1976;24:24–33.
- Green DR, Reed JC. Mitochondria and Apoptosis. Science. 1998;281:1309–1312.
- Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407:770–776.
- Tavassoli M, Soltaninia J, Rudnicka J, Mashanyare D, Johnson N, Gäken J. Tamoxifen inhibits the growth of head and neck cancer cells and sensitizes these cells to cisplatin induced-apoptosis: role of TGF-b1. Carcinogenesis. 2002;23:1569–1575.
- Clemons M, Danson S, Howell A. Tamoxifen (nolvadex): a review. Cancer Treat Rev. 2002;28:165–180.
- Budtz PE. Role of proliferation and apoptosis in net growth rates of human breast cancer cells (MCF-7) treated with oestradiol and/or tamoxifen. Cell Prolif. 1999;32:289–302.
- Perry RR, Kang Y, Greaves B. Effects of tamoxifen on growth and apoptosis of estrogen-dependent and -independent human breast cancer cells. Ann Surg Oncol. 1995;2:238–245.
- Martin G, Melito G, Rivera E. Effect of tamoxifen on intraperitoneal N-nitroso-N-methylurea induced tumors. Cancer Lett. 1996;100:227–234.