ABSTRACT
Liposomes are attractive encapsulation systems that provide enhanced stability of encapsulated materials against a range of environmental, enzymatic, and chemical stresses, including the presence of enzymes or reactive chemicals, and exposure to extreme pH, temperature, and ionic strength changes. Liposomes have been widely used in the pharmaceutical and food industries because of their biocompatibility, biodegradability, absence of toxicity, small size, and ability to carry a wide variety of bioactive compounds due to the amphiphilicity of the phospholipid encapsulating material. In the food industry, liposomes have recently been used to deliver different functional compounds in food systems. In this paper, the food application of liposomes and nanoliposomes as emerging carrier vehicles of vitamins, enzymes, food antimicrobials, essential oils, and polyphenols is discussed in detail.
1. Introduction
In recent years, the food industry has required the addition of functional compounds in products.[Citation1] Functional compounds are used to control flavour, colour, texture, or preservation properties, and also for a health-related function. However, these compounds are usually highly susceptible to environmental, processing, and/or gastrointestinal conditions, and therefore, encapsulation may offer possible benefits for effective protection of those.[Citation2]
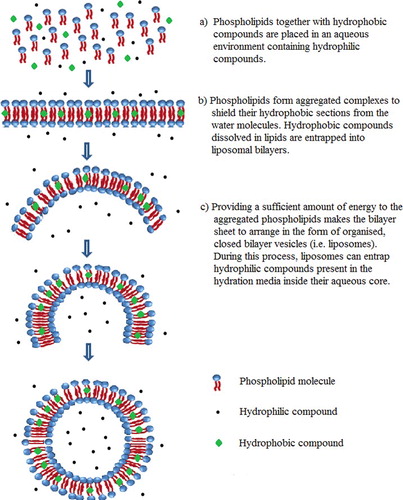
Liposomes and nanoliposomes are attractive encapsulation systems for the delivery of both lipophilic and hydrophilic functional compounds. They are spherical-shell structures consisting of a phospholipid bilayer (or more such bilayers) enclosing a liquid core. Placing phospholipids into an aqueous medium results in their associating with each other and forming a bilayer sheet arrangement to shield their hydrophobic sections from the water molecules while still maintaining contact with the aqueous phase via the hydrophilic head groups. The unfavourable interaction between the fatty acids and the water will be completely eliminated by folding the edges of bilayer sheet together, through inputting a sufficient amount of energy to the aggregated phospholipids, which makes the bilayer sheet to arrange in the form of organised, closed bilayer vesicles (i.e. liposomes) (). Because of the orientation of polar lipids in water, liposomes can entrap both hydrophilic solutes present in the hydration medium and lipophilic molecules dissolved in lipids ().[Citation3–5]
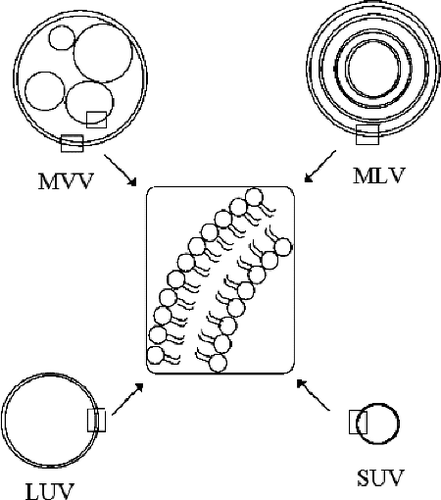
Based on their lamellarity, liposomes can be classified as unilamellar vesicles (ULV), oligolamellar vesicles (OLV), and multilamellar vesicles (MLV). Another type of liposomes known as multivesicular vesicles (MVV) include some small non-concentric vesicles entrapped within a single lipid bilayer. Vesicles can also be categorised by their size, as small unilamellar vesicles (SUV), large unilamellar vesicles (LUV), and giant unilamellar vesicles (GUV) (; ).[Citation6,Citation7]
Table 1. Classification of liposomes.[Citation4,Citation5,Citation9,Citation39,Citation157–161]
There are many different strategies for the preparation of liposomes and nanoliposomes.[Citation4,Citation8] MLVs form readily when bilayer-forming polar lipids are dispersed in aqueous media under mild agitation. In order to produce LUVs and SUVs, substantial energy inputs, e.g. in the form of sonication, homogenisation, heating, etc., are required to disrupt MLV and MVV structures and force the formation of ULVs.[Citation9] Methods for preparing LUVs and SUVs can be classified based on the input of mechanical energy in the system, e.g. ultrasonication, microfluidisation, high-pressure homogenisation, and extrusion. Non-mechanical methods include reverse-phase evaporation, removal of detergents, solvent (ether or ethanol) injection, and heating methods.
Liposomes were initially developed for medical purposes [Citation9] and then for cosmetics.[Citation10] They have been used for delivery of vaccines, hormones, enzymes, and vitamins into the body.[Citation11] Possessing a number of benefits, including possibility of large-scale production, targetability,[Citation4,Citation7] possibility of manufacturing by using safe natural ingredients, such as egg, soy, or milk,[Citation12] biocompatibility, small size, ability to carry a wide variety of bioactive compounds,[Citation13] as well as health benefits of liposomal ingredients such as phospholipids and sphingolipids for human,[Citation14–18] liposomes are beneficial delivery systems amenable to utilisation in the food sector. Liposome entrapment may enhance stability of encapsulated materials against a range of environmental, enzymatic, and chemical stresses, including the presence of enzymes or reactive chemicals and exposure to extreme pH, temperature, and high ion concentrations.[Citation19] There are many potential applications of liposomes as delivery systems in the food sector. The objective of this review is to discuss the application of liposomes for entrapping the main food bioactive compounds (vitamins, enzymes, antimicrobial compounds, essential oils, phenolic compounds) in terms of their characteristics and efficacies in food or model systems (–).
Table 2. Summary of preparation methods and some characteristics of vitamin-loaded liposomes.
Table 3. Summary of preparation methods and some characteristics of enzyme-loaded liposomes.
Table 4. Summary of preparation methods and some characteristics of antimicrobial-loaded liposomes.
Table 5. Summary of preparation methods and some characteristics of essential oils and phenolic-compounds-loaded liposomes.
2. Application of liposomes for delivery of food functional compounds
2.1. Vitamin carrying liposomes
Many vitamins are not stable in food systems and are susceptible to degradation, the rate and extent of which depend on chemical structure of vitamins, properties of the food matrix, processing conditions, and distribution/storage environment. Some of the critical parameters are temperature, oxygen, light, moisture, pH, and time.[Citation20] For instance, vitamin E is easily oxidised in the air.[Citation21] Also, vitamin C (ascorbic acid) is unstable and can be easily oxidised in the presence of oxygen, humidity, high temperatures, and heavy metals during the storage and processing.[Citation22] Vitamin C is also known to become less stable as the pH approaches neutral.[Citation23] It has been shown that classical approaches, such as exclusion of oxygen, lowering the temperature, and changing the pH, are not always adequate and applicable. Therefore, extensive studies have been carried out, such as encapsulation into liposomes,[Citation22–25] lipid nanoparticles,[Citation26,Citation27] and/or polymeric nanoparticles,[Citation28,Citation29] to overcome to the instability of vitamins in food systems.
Liposomal entrapment appears to be a promising solution to protect water-soluble and lipid-soluble vitamins from degradation. Stabilisation of vitamin C by encapsulation in liposomes has been studied by Kirby,[Citation23] who reported more than 50% of liposomal vitamin C surviving after 50 days at 4 °C and about 15% at room temperature. By contrast, free vitamin C disappeared completely from solution after 18 days at 4 °C and only 6 days at room temperature. The authors also found little effect of the external pH on the rate of encapsulated vitamin loss after 40 days.[Citation23] Also, retaining 69%–80% of the preservation rate of vitamin C incorporated into lipid bilayers after 24 hours was reported by Yang [Citation22] compared to only 35% in free vitamin solution. By storing under 4 °C for 60 days, only 10% decrease of preservation rate was shown in liposomal vitamin C, compared to about 60% for pure vitamin solution.[Citation22] Increased half-life of liposomal vitamin C (up to 300-fold) in model systems and also its decreased rate of oxidation in apple juice (by one to two orders of magnitude), relative to that of free vitamin, were found by Wechtersbach;[Citation25] however, liposomal stabilisation of vitamin was less noticeable in fermented milk product as another food model system.[Citation25] The latter was attributed to the integration of the lipids present in the product into the membrane, thus increasing liposome permeability and reducing its efficacy, or the activity of bacterial and milk lipoprotein lipase that could hydrolyse membrane phospholipid.[Citation30]
Incorporating liposoluble vitamins in liposomes, Tesoriere [Citation31] reported extended half-life of the ULV-encapsulated vitamins A and E. Also, Banville [Citation32] reported increased recovery of vitamin D from cheeses containing liposomal vitamin D (<62%), compared to the commercially prepared vitamin (<43%) and a solubilised form of vitamin D in cream (<40%), indicating the ability of liposomes to protect vitamin from being degraded. According to Lee,[Citation24] free aqueous vitamin A was completely degraded within two days at pH 7.0 and 4 °C in the light, compared to only 20% degradation of the vitamin A incorporated into the MLVs after eight days. Similarly, the same degradation rate of bare vitamin A in the buffer system was reported by Ko and Lee,[Citation33] while over 60% of the initial vitamin in nanoliposomes was kept until eight days under dark and UV exposure. The authors also reported rapid degradation of vitamin as temperature increased from 4 to 50 °C.[Citation33]
2.1.1. Characterisation of liposomes encapsulating vitamins
2.1.1.1. Encapsulation efficiency (EE)
Previous reports of EE of vitamins entrapped in liposomes have been shown in . Hydrophobic substances can be efficiently incorporated into the phospholipid bilayers of liposomes, especially if the vesicle is formed by multiple bilayers like multilamellar liposomes.[Citation34]
According to , higher EE% of about 80%–99% was reported for liposoluble vitamins, compared to lower amounts of 2%–57% for water-soluble compounds. Various factors can influence EE of the compounds encapsulated in liposomes. The EE could differ for a vitamin prepared by different liposomal constituents and ratios. For example, Wechtersbach [Citation25] obtained about 2%–8% EE of vitamin C, which was much lower than 86% EE reported by Marsanasco,[Citation35] 39%–57% by Kirby,[Citation23] and 41%–47% by Yang.[Citation22] The relatively low EE observed by Wechtersbach [Citation25] was related to the low phospholipid/buffer ratio used in the liposome preparation. The ratio of phosphatidylcholine (PC) and cholesterol and the amount of core materials are other important factors affecting EE. Increased EE of retinol was reported by increasing cholesterol and β-sitosterol content in the liposome structure.[Citation36,Citation37] Also, comparing different ratios of PC and cholesterol, Liu and Park [Citation38] obtained the highest loading efficiencies of vitamin E at 40:60 and 60:40 ratios. With regard to core material content, decreased loading efficiency of vitamin E (up to 45%) was reported by increasing vitamin E content in liposomal formulation from 20 to 100 mg (per 100 mg PC and cholesterol (40:60 ratio)).[Citation38] The method of liposome preparation can also influence EE% for the same compound. For example, high degrees of encapsulation have been obtained by freeze drying and dehydration/rehydration cycles ().
2.1.1.2. Stability of liposomes encapsulating vitamins
Liposome stability is a complex issue, and consists of physical, chemical, and biological stabilities. The physical instability of liposomes may be caused by phase transitions or merging bilayer structures which results in loss of entrapped components and increase in particle size distributions.[Citation3] The chemical instability in liposomes primarily indicates hydrolysis and oxidation of lipids.[Citation39] The stability of liposomes encapsulating bioactive compounds has been generally evaluated in terms of liposome size, EE%, and zeta potential measurements. The zeta potential is an index of the magnitude of the repulsive interaction between colloidal particles and is used to assess the stability of a vesicular suspension. In particles with low zeta potential, there is only a little repulsion force and the particles will eventually aggregate, resulting in suspension instability.[Citation40]
Yang [Citation22] noted that vitamin C nanoliposomes, prepared by combining film evaporation method with dynamic high-pressure microfluidisation, exhibited storage stability at 37 °C for 24 hours and at 4 °C for 60 days. Also, Wechtersbach [Citation25] evaluating the influence of heating on leakage of vitamin C out of liposomes, found less stability of aged liposomes than freshly prepared ones. Also, dipalmitoylphosphatidylcholine (DPPC)/cholesterol liposomes retained the majority of entrapped vitamin C during short thermal treatment at 72 °C, compared to DPPC liposomes. While lower stabilisation efficacy of cholesterol was observed at room temperature.[Citation25] This is in accordance with the fact that cholesterol increases the fluidity of a PC bilayer below the transition temperature, and above the transition temperature it has a stabilising effect.[Citation41]
Marsanasco [Citation35] reported that EE of freshly prepared liposomes encapsulating vitamin C (86%) reached 24%–38% after 72-hour dialysis depending on liposomal formulation.
Vitamins encapsulated in liposomes have also been shown to affect lipid bilayer stability. For example, lower oxidation of liposomes composed of PC, PC: stearic acid, and PC: calcium stearate (CaS) containing vitamin C, vitamin E, or both, than vitamin-free formulations was reported by Marsanasco,[Citation35] with less anti-oxidative impact of vitamin E in PC: CaS systems. This effect was attributed to formation of compact areas of the membrane by PCs and Ca2+ binding, resulting in an increased lipid packing, which might limit the mobility and, therefore, the antioxidant action of the vitamin E.
2.2. Enzyme carrying liposomes
Liposome technology has been widely developed for enzyme encapsulation in food and nutrition, most notably for acceleration of cheese ripening ().[Citation42–46] Ripening time varies widely according to the cheese variety from four weeks for soft cheeses up to three years for very hard varieties. Accelerated cheese ripening would provide both economic and technical advantages such as savings in refrigeration costs and capital, as well as minimising weight losses and microbiological risks.[Citation46] However, by direct addition of ripening accelerating enzymes to milk, a large portion of the enzymes are lost in the whey stream, leading to the increased product cost.[Citation47] Also, addition of free enzymes to the cheese milk causes early proteolysis, resulting in unfavourable flavour and texture defects, as well as reduced cheese yields. Poor enzyme distribution has also been reported by direct addition of the enzymes to curd.[Citation46,Citation47] To overcome these problems, an alternative approach is to encapsulate enzymes to protect them from the outside environment or for controlled release. Liposomal entrapment of enzymes offers advantages for cheese applications such as being prepared from ingredients naturally present in cheese, protecting casein from early hydrolysis during cheese production, well partitioning in the curd,[Citation48] and being prepared on industrial scales with food-grade properties, for example, by Mozafari method.[Citation4,Citation49]
Most studies have used MLV-type liposomes in dairy products, since SUVs have shown limited potential due to their poor encapsulation efficiencies.[Citation48] In one of the early studies on encapsulating cheese-ripening enzymes inside liposomes, Law and King [Citation50] reported accelerated proteolysis after a delayed time interval using MLV-entrapped proteolytic enzymes. Then, by developing other procedures of liposome preparation, accelerated ripening of Cheddar cheese was observed through the action of Neutrase entrapped in dehydration–rehydration (DR) vesicles,[Citation51] trypsin-containing microfluidised liposomes,[Citation42] and also encapsulated bacterial or fungal proteinases.[Citation46] Moreover, acceleration in Manchego cheese ripening was reported by addition of liposome-entrapped proteinase from Bacillus subtilis,[Citation43,Citation44] cyprosins,[Citation43,Citation45] and chymosin [Citation43] to cheese milk. In liposomal enzyme-assisted cheese ripening process, the entrapped enzyme is gradually released, allowing catalysing degradation and modification reactions in the cheese matrix during the ripening period.[Citation52] The rate of enzyme release from liposomes incorporated into cheese could be affected by some factors such as cheese fat content (0%–20%) and the pH (4.9–5.5).[Citation53]
Liposomal entrapment of lipases to improve the cheese manufacture has also been investigated. Addition of moderate quantities of liposome-entrapped lipase (0.5 U/g) in Cheddar cheese increased the production of free fatty acids and improved flavour profile. However, higher concentrations (1.0 lipase U/g milk fat) led to the development of a soapy off-flavour after 60–90 days of ripening.[Citation54] Liposomes entrapping β-galactosidase have also been used in dairy products to induce slow digestion of lactose, aiding the digestion of dairy foods by the lactose intolerants and preventing flavour change of food which happens by lactose hydrolysis.[Citation55–57]
2.2.1. Characteristics of liposomes encapsulating enzymes
2.2.1.1. Encapsulation efficiency
Entrapment values of enzymes in lipid bilayers can be calculated as the percentage of entrapped protein or on the basis of proteolytic activity, which the former is reported to be slightly higher than the latter.[Citation46] Entrapment efficiencies of liposome-entrapped proteinases were in the range of 10%–36% () depending on the type of enzyme, liposome preparation method, and liposome formulation. For example, Kirby,[Citation51] entrapping Neutrase in liposomes obtained by different methods, reported higher EE for DR vesicles (26%–34%) than that obtained by reverse-phase evaporation (RE) (5%–14%), thin-film hydration (2%–8%), and sonicated vesicles (1%) (). In another study, Matsuzaki [Citation55] reported 10%–20% loss of β-galactosidase specific activity during the DR procedure, compared to no significant loss in specific activity of enzyme entrapped in RE vesicles.[Citation55]
Change in the specific activity and, therefore, EE% of enzymes due to the encapsulation procedure could also vary between the different enzymes encapsulated with the same method. For example, lower EE of ∼12% for cyprosins,[Citation43,Citation45] 13% for trypsin,[Citation42] and 14.5% for chymosin [Citation43] were obtained by using DR vesicles compared to the higher levels of ∼28% for proteinase from B. subtilis,[Citation43,Citation44] 26%–34% for Neutrase,[Citation51] 24%–34% and 18% for β-galactosidase.[Citation55,Citation57] Other factors can also influence the EE obtained with the same method. For example, EE in DR vesicles increased with decreasing solute concentration.[Citation58] Also, Matsuzaki [Citation55] reported 10% decrease in EE of β-galactosidase by increasing cholesterol/lecithin ratio from 0:1 to 4:1.
2.2.1.2. Stability of liposomes encapsulating enzymes
There are several scientific reports on stability of enzyme entrapping liposomes in different media. Matsuzaki [Citation55] showed that β-galactosidase entrapping vesicles retained their stability at low pH conditions (pH = 3.0 for 60 minutes at 37 °C). Enzymatic activity of vesicles was also retained after one month at 5 °C under nitrogen. It was indicated that the resistance of encapsulated enzyme to acid depends on the lecithin:cholesterol molar ratio of the vesicles, which was the highest for molar ratio of 1:3 (lecithin:cholesterol). However, at the same molar ratio, enzyme activity decreased with decreasing pH (3.7–2.7).[Citation55] Also, Rao [Citation57] observed that enzymatic activity of β-galactosidase was retained over 20 days in refrigerator at neutral pH, while it was significantly decreased with exposure to low pH conditions (pH = 2.0–4.0), indicating the effectiveness of liposomes to stabilise the enzyme during storage, but it can rapidly release entrapped material upon exposure to the high acidic conditions of the digestive system. In another study, Chawan [Citation56] reported that liposomes containing either bacterial or fungal β-galactosidase (from E. coli and Aspergillus flavus, respectively) were stable in buffer as well as freshly pasteurised milk for up to 20 days at 4 °C. Lactose hydrolysis was negligible in milk containing liposomes with bacterial β-galactosidase, while significant (25%) hydrolysis of lactose was observed in milk containing liposomes with fungal β-galactosidase during 20 days of storage at 4 °C.
Evaluating stability of proteinases used for acceleration of cheese ripening, 75% retention of liposomal B. subtilis proteinase [Citation43,Citation44] and 73% of liposomal cyprosins [Citation43,Citation45] in the curd were reported during Manchego cheese making, which were lower than the 83%–91.6% reported for DR vesicles in Cheddar cheese making,[Citation51] but higher than the 60% retention of MLVs [Citation59] or the 24%–42% retention of RE vesicles in Saint-Paulin cheese making,[Citation60] and the 42% retention of DR vesicles in Taleggio cheese making.[Citation61]
2.3. Antimicrobial carrying liposomes
Antimicrobial polypeptides such as nisin and lysozyme are known to be inhibitory towards the growth of gram-positive pathogens such as Listeria monocytogenes, Staphylococcus aureus, and Bacillus spp,[Citation62] which control undesirable bacteria in food products such as cheeses and extend their shelf-life. However, direct addition of nisin or other antimicrobials to food products has several limitations. For example, significant loss of antimicrobial activity of nisin has been reported by direct addition of nisin in milk because of adhesion to milk fat globules.[Citation63] Also, direct addition of an antibiotic to the cheese curd would inactivate the starter culture required during the early stages of cheese production,[Citation64] and would not permit a homogeneous distribution of antimicrobial in the cheese matrix.[Citation65] In addition, divalent cations associated with bacterial cell wall surfaces prevent the cationic polypeptide nisin to interact with the bacterial pathogens, hence reducing its activity.[Citation66] Addition of nisinogenic strains with starter cultures may also affect the growth and acid and aroma production of starter cultures and reduce the overall quality of the final product.[Citation67] On the other hand, food antimicrobials may be affected by food processing conditions, storage temperature,[Citation66,Citation68,Citation69] and also food matrix,[Citation69] when they are added directly to food product.
Therefore, entrapment of nisin and other antimicrobials in encapsulating systems such as liposomes may offer a potential solution to overcome some of these limitations resulting in antimicrobial formulations better suited for use in foods such as cheeses.[Citation19] During cheese ripening, liposomes and micro-organisms accumulate in the same compartments in the cheese matrix,[Citation64] and this raises the possibility that liposomes could be used to deliver antimicrobial agents directly to the sites at which micro-organisms are present in foodstuffs. Such targeting would significantly reduce the overall concentration of antimicrobial agents required and enable the use of natural agents.[Citation4]
In one of the studies on the encapsulation of food antimicrobials, liposomes were used to entrap lysozyme and/or nisin in an effort to prevent the spoilage of various cheeses.[Citation70] Using liposomes, increased antilisterial activity of pediocin AcH was also observed in beef tallow and muscle slurries as well as dairy-based (non-fat dry milk, butterfat) slurries ().[Citation71,Citation72] Benech [Citation73] observed a decrease in Listeria innocua counts by 1.5–3.0 log units over a six-month ripening period, through the addition of liposome encapsulated nisin Z (a variant of nisin) into a cheese-milk solution and 90% of the nisin Z initial activity was still retained at the end of the period. Also, ≥2 logs inhibition of L. monocytogenes was reported by Were [Citation74] for nisin encapsulated in PC and PC:cholesterol liposomes, with lesser inhibitory effect of liposome-entrapped lysozyme to test strains (). Nisin incorporated in nanoliposomes, prepared from distearoylphosphatidylcholine (DSPC) and distearoylphosphatidylglycerol (DSPG),[Citation75] was also shown to be able to effectively inhibit the growth of L. monocytogenes strains inoculated into liposome-containing UHT-processed milk samples for 48 hours at 25 °C, regardless of milk fat content.
2.3.1. Characteristics of liposomes encapsulating food antimicrobials
2.3.1.1. Encapsulation efficiency
The EE% of antimicrobials entrapped in liposomes has been shown in . In an effort for optimising the entrapment process of antimicrobial peptides by liposomes, EE ranging from 9.5% to 47% was reported for the liposomes entrapping nisin Z.[Citation76] The EE found to be optimal for hydrogenated PC liposomes (34%) compared to other unsaturated phospholipids (11%) or phospholipid mixtures (26%), and slightly decreased with the increasing cholesterol concentration in liposomes up to 20% (w/w).[Citation76] The decreased EE of liposomes with the increasing cholesterol concentration was in agreement with results reported by Were [Citation77] and Colas.[Citation49] It was suggested that cholesterol introduction reduced polypeptide affinity, thus reducing the concentration of antimicrobials that can be incorporated.[Citation76]
A relatively high EE of 54%–63% was reported by Were [Citation77] for nisin (co-encapsulated with calcein) incorporated into liposomes prepared from PC, PG, and cholesterol at different molar ratios, as well as a higher EE of 70%–90% by Taylor [Citation19] for nisin-entrapped liposomes consisting of DSPC and DSPG. While, Colas [Citation49] reported lower EE of nisin ranged from 11%–13% to 50%–54% for cationic vesicles (containing stearylamine (SA)) and anionic vesicles (containing dicetyl phosphate (DCP)), respectively. The former resulted from electrostatic repulsion between the positively charged nisin and the cationic vesicles, indicating the influence of variation in the liposomal constituents on the EE of the same antimicrobial entrapped in lipid vesicles.
2.3.1.2. Stability of liposomes encapsulating food antimicrobials
Some examples of assessing stability of liposomes encapsulating antimicrobials are given below. Antimicrobials may negatively interact with liposome membranes and disrupt the bilayer structure.[Citation77] Were,[Citation77] encapsulating nisin and lysozyme with calcein in liposomal formulations, observed the low antimicrobial-induced leakage of calcein (<40%) in all formulations with the highest leakage for PC liposomes at high concentration of nisin (375 μM), while it was the highest for PG-containing liposomes at 150 μM nisin, indicating a concentration-dependent effect of nisin-induced leakage of PC and PG liposomes. With respect to the size of liposomes, Were [Citation77] also observed slight fluctuations in effective diameter during the two-week storage of liposomes at 4 °C. Laridi [Citation76] reported stability of liposome-entrapped nisin Z (as the quantity of released nisin Z) for 27 days at 4 °C in different media, with the least stability of vesicles in whey (78% release after 18 days) compared to milk (39%–63% release) and phosphate buffer saline (PBS) (71% release), attributed to higher content of bivalent ions such as calcium and magnesium in whey, causing the formation of large aggregates. Liposome stability was also adversely affected by milk fat content and decreased with increasing milk fat content (0%–3.25%).[Citation76] It has been suggested that some interactions between liposome and fat globule membranes could be responsible for this destabilisation of the liposome membrane.[Citation51,Citation53,Citation76]
Long-term stability of nanoliposomes encapsulating nisin was also reported for at least 14 months at 4 °C (DPPC:DCP:Chol vesicles) and for 12 months at 25 °C (DPPC:SA:Chol vesicles) by Colas.[Citation49] Also, Taylor [Citation19] reported that DSPC/DSPG liposomes entrapping nisin were stable (in terms of EE) despite exposure to elevated temperature (25–60 °C) and high or low pH (5.5–11.0), suggesting that liposomes containing nisin may be suitable for use in low- or high-pH foods subjected to moderate heat treatments. However, decreased EE of hydrogenated PC vesicles by increasing pH from 3.6 to 6.6 was reported by Laridi,[Citation76] with no large fluctuation in EE of unsaturated PC vesicles, which indicates critical role of formulation on the stability of liposomes containing antimicrobials in different media.
2.4. Essential oil carrying liposomes
Essential oils are volatile, natural, complex compounds, characterised by a strong odour, formed by aromatic plants as secondary metabolites.[Citation78] They possess a wide spectrum of biological properties such as the antifungal, antioxidant, and bactericidal activities.[Citation79–84] Anti-proliferative effect of some essential oils on tumour cells has also been reported.[Citation85] However, it is well known that most essential oils are biologically instable, and are sensitive to oxygen, light, and temperature.[Citation86] They are also poorly soluble in water and distribute defectively to target sites in the final product.[Citation87] For these reasons, different approaches have been proposed to improve their solubility and bioavailability, to protect them, as well as to control their release, such as inclusion in polymeric nanoparticles,[Citation88–90] liposomes,[Citation91–94] cyclodextrins,[Citation95,Citation96] and solid lipid nanoparticles.[Citation97,Citation98]
Many studies have been carried out to evaluate the stability and antimicrobial activity of essential oils entrapped in MLVs and ULVs (). Valenti [Citation91] showed that Santolina insularis essential oil can be incorporated in high amounts in the liposomes and kept from degradation. Higher oxidative stability of liposome-incorporated Thymus species extracts [Citation92] and carvacrol, thymol (major constituents of essential oils from Origanum dictamnus L.), and/or their mixture,[Citation93] represented as oxidation onset temperature (To), was also observed than their free forms. Similarly, Detoni [Citation94] reported higher To of Zanthoxylum tinguassuiba essential oil by incorporating the oil into liposomes. Also, Wen [Citation99] demonstrated that liposomes could protect heat-sensitive molecules in rose essential oil from thermo-degradation. The protective effect of essential oils on oxidation of lipid bilayers has been known, as well.[Citation92–94]
Enhanced antimicrobial and biological activities of essential oils incorporated into liposomes have also been reported. Sinico [Citation100] demonstrated increased antiherpetic activity of Artemisia arborescens L. essential oil incorporated into MLVs compared with free oil. Also, much stronger antimicrobial activity of the essential oil from Citrus limon (Lemon Greek cultivar) [Citation101] and Thymus spp. extracts,[Citation92] as well as carvacrol and thymol, or their mixtures (6:1) [Citation93] entrapped in MLV liposomes, was reported than their pure forms. An improved antifungal activity of Eucalyptus camaldulensis leaf essential oil loaded into lipid vesicles was also observed by Moghimipour.[Citation102] Enhanced biological activity of liposomal Zanthoxylum tinguassuiba for glioma cells was also reported by Detoni,[Citation94] with superior response of SUVs than MLV system. This was in contrast to Sinico et al., [Citation100] who reported much stronger antiviral activity of MLV-incorporated A. arborescens L. essential oil than SUVs. This was attributed to the better settling of the largest MLV next to the cells in culture, and also higher leakage of the essential oil components from the smallest ULV in the presence of the culture medium containing several compounds capable of interacting with the liposomal bilayers.[Citation100]
2.4.1. Characteristics of liposomes encapsulating essential oils
2.4.1.1. Encapsulation efficiency
High EE% of essential oils incorporated into liposomes has been reported by several authors, ranging from 55% (Anethum graveolens essential oil) to 95% (E. camaldulensis leaf essential oil) (). While EE% as low as 4% has also been obtained for essential oil pure compounds, such as carvacrol.[Citation93] Also, Varona [Citation103] reported poor EE of lavandin essential oil by using particles from gas-saturated solution (PGSS) drying process (3%–14.5%), compared to 66% from the thin-film method, indicating the influence of the physico-chemical properties of each essential oil as well as the preparation method on the loading values of essential oils.
Entrapment efficiency of essential oils may also be affected by the composition of the lipid vesicle membrane. For example, Ortan [Citation78] reported a slight increase in the EE of the essential oil at higher lipid concentrations of liposomes prepared by thin-film hydration method. However, entrapping lavandin and linalool essential oils into liposomes prepared by PGSS-drying method, a decrease in the EE occurred with increasing the lipid concentration.[Citation103] Also, reduction in the oil encapsulation with increasing cholesterol content was observed by Ortan [Citation78] and Varona.[Citation103] Liposome size can also affect EE. Less EE of essential oil containing SUVs than MLVs has been proved, due to their smaller size and unilamellar structure. For example, the EE of A. graveolens essential oil was lesser in SUV (60.5%) liposomes than MLV (67.5%) liposomes.[Citation78] Encapsulation efficiencies, ca. 79% and 68%, were also obtained by MLVs and SUVs encapsulating Zanthoxylum tingoassuiba essential oil, respectively.[Citation94] Also, Sinico [Citation100] reported less EE of SUVs containing A. arborescens L. (∼66%) than that of MLVs (71%–75%).
2.4.1.2. Stability of liposomes encapsulating essential oils
Liposomal formulations could be promising alternatives for protecting essential oils during long-term storage. Valenti [Citation91] showed that vesicle dispersions entrapping S. insularis were stable for at least one year and neither oil leakage nor vesicle size alteration occurred during this period. Also, MLV and SUV liposomes encapsulating A. arborescens L. [Citation100] were shown to be very stable for six months in terms of size and oil retention. After one year of storage, the oil retention was still good, while the stability decreased slightly, and the size of the liposomes increased due to vesicle fusion. Similarly, Ortan [Citation78] showed that liposomes incorporating A. graveolens essential oil were stable during storage at 4 °C with a slight oil leakage and slight increase in the size for the first six months. After one year, the oil incorporation was at least 90% but the size of the liposomes was clearly modified to become at least 40% larger especially for SUV liposomes.
Moreover, Wen [Citation104] observed that the EE of essential oil from Atractylodes macrocephala Koidz incorporated into liposomes prepared by using modified rapid expansion of the supercritical solution method, was approximately stable during six-month storage at a constant temperature of 25 °C and a relative humidity of 60%. However, the size of liposomes varied from 173 nm at day 0 until 245 nm at the sixth month of storage. In another study, Varona [Citation103] showed a considerable increase in the size of liposomes containing lavandin essential oil prepared by thin-film method during the first 10 days of storage at 5 °C, while the size remained stable afterward during one month of storage. Also, higher stability of vesicles in terms of oil release was reported at lower ratio of essential oil/lipid (1.8) compared with the higher ratios (3.5).[Citation103]
2.5. Phenolic compounds carrying liposomes
Polyphenols constituting one of the most numerous and ubiquitous groups of plant metabolites are an integral part of both human and animal diets and possess a wide range of biological functions, including antioxidant, anti-inflammatory, antibacterial, and antiviral activities.[Citation105–107] By slowing the progression of certain cancers, reducing the risks of cardiovascular disease, neurodegenerative diseases, diabetes, or osteoporosis, they might also act as potential chemopreventive and anti-cancer agents in humans.[Citation108–110]
However, potential health benefits of polyphenols and their application in functional foods as additives have been limited due to their limited stability, conditioned solubility,[Citation111] and poor bioavailability.[Citation112] They are also known to cause unpleasant taste, such as astringency, when added into food products. They may associate with food components, such as proteins, causing significant aggregation and precipitation, and quantity and/or functional loss of the polyphenols, as well.[Citation113] Therefore, encapsulation of phenolic compounds can provide an approach to solve these drawbacks and make possible increasing the concentration of antioxidants within food products [Citation114] to improve the antioxidant properties and shelf-life of foods.
An increasing number of studies have aimed at designing liposomal formulations to stabilise and protect phenolic compounds, to improve their aqueous solubility and bioavailability, and to achieve targeted and/or sustained release (). Caddeo [Citation40] reported that liposomes prevented the cytotoxicity of resveratrol at high concentrations, avoiding its immediate and massive intracellular distribution, and increased the ability of resveratrol to stimulate the proliferation of the cells and their ability to survive under stress conditions caused by UV-B light. Similarly, Isailović [Citation115] reported reduced cytotoxicity of liposome-entrapped resveratol compared to deteriorative effects of resveratrol solution of the same concentration. Anti-oxidative activity of resveratrol was also retained upon liposomal encapsulation.[Citation115] Takahashi [Citation116] reported a faster rate and better absorption of encapsulated curcumin, a natural polyphenolic phytochemical extracted from the powdered rhizomes of turmeric (Curcuma longa) spice, than its free form or a mixture of curcumin and lecithin. Plasma antioxidant activity following oral liposome-entrapped curcumin was also significantly higher than that of two other treatments.[Citation116] Better antimicrobial and antiviral activities of quercetin-enriched lecithin formulations have also been observed compared to individual lecithin or quercetin.[Citation117]
2.5.1. Characteristics of liposomes encapsulating phenolic compounds
2.5.1.1. Encapsulation efficiency
Encapsulation efficiencies ranging from 20% to above 90% have been reported for liposomes entrapping phenolic compounds, depending on the physico-chemical properties of the phenolic compound and variations in the preparing method (). Relatively low EE% of about 20%–57% for salidroside (concentration of 5%–10%) entrapping liposomes prepared from different methods was reported by Fan,[Citation118] which was the highest (28%–57%) through the freeze–thawing method, followed by thin-film evaporation, reverse-phase evaporation, melting, and sonication. Also, Isailović [Citation115] reported lower EE of resveratrol-loaded sonicated vesicles (44%–55%) when compared with EE > 90% of liposomes prepared by thin-film hydration, proliposome, and extrusion methods. The reported EE for extruded liposomes (92%–96%) in this study was also higher than that reported by Caddeo [Citation40] for resveratrol-loading liposomes prepared by the same method (∼80%). Also, Fang [Citation119] reported high loading capacity (>90%) for a modified liposome system encapsulating resveratrol with the encapsulated particles called ‘acoustically active lipospheres’ (AALs) or ‘microbubbles’, possessing high sensitivity to ultrasound treatment with potential to be ‘magic bullet’ agents for the delivery of active compounds to precise locations in the body, with the locations being determined by focusing the ultrasound energy.[Citation119]
High loading efficiencies were also reported for vesicles entrapping curcumin (68%),[Citation116] polyphenolic grape seed extract (83.5%),[Citation120] and catechin or epigallocatechin gallate (EGCG) (∼70%).[Citation113] In another study, Fang [Citation121] indicated much higher EE of 84%–99% for EGCG compared to only 39%–57% for (+)-catechin and 31%–64% for (−)-epicatechin, which was attributed to the presence of a galloyl group in EGCG, causing greater lipophilicity of EGCG.
2.5.1.2. Stability of liposomes encapsulating phenolic compounds
Comparing the stability of liposomes prepared by different methods including thin-film evaporation, sonication, reverse-phase evaporation, melting, and freezing–thawing, Fan [Citation118] reported better physico-chemical stability of liposomes containing salidroside obtained from melting method (10% and 15% leakage after one month of storage at 4 and 30 °C, respectively) than other techniques, which was explained by different morphologies of prepared liposomes. More stability of salidroside containing liposomes prepared by melting method than empty liposomes was also observed in terms of particle size, suggesting the role of salidroside in preventing the aggregation and fusion of liposomes.[Citation118] Also, Rashidinejad [Citation113] reported more stability of liposomes containing catechin or EGCG than empty liposomes in terms of autoxidation of polar lipids in liposomes, which was in agreement with the results found by Ramadan,[Citation122] who reported greater oxidative stabilities of quercetin-enriched lecithin models than models containing either quercetin or lecithin, due to the anti-oxidative synergism between quercetin and polar phospholipids.
Long-term physical and oxidative stability (for up to 150 days) of polymer-coated liposomes entrapping polyphenolic grape seed extract was also reported by Gibis [Citation120] with significantly less hexanal formation (<15 µmol/L compared to >717 µmol/L for liposomes without extract) and no aggregation during storage for up to 150 days. Liposomes entrapping resveratol were also reported to be physically stable for three weeks, and provided prolonged release of resveratrol.[Citation115] Also, Caddeo [Citation40] showed no significant changes in the stability of liposomes loading resveratrol during storage time over 60 days at 4 °C, in terms of the zeta potential and the average size, as well as the amount of resveratrol loaded. Assessing the stability of liposomal phenolic compounds in food matrices, Rashidinejad [Citation113] observed effective retaining of liposomes containing catechin or EGCG within a low-fat hard cheese and very little of the polyphenols partitioned into the whey.
3. Challenges and future prospective of liposomes in food industry
Liposomes are flexible carriers for encapsulation of both hydrophilic and lipophilic compounds simultaneously. They have been widely used as delivery systems in pharmaceutical and cosmetic industries. However, the widespread utilisation of liposomes has been currently restricted in food sector due to a number of challenges, such as poor stability under the complex environments, poor loading capacity for hydrophilic bioactive components,[Citation123] high cost, bioactive compound leakage, and fast release.[Citation124] Nevertheless, many attempts have been carried out to elevate the usefulness of liposomes.
After initial attempts for improving the stability of liposomes through the using of cholesterol and neutral long-chain saturated phospholipids,[Citation125] currently substitution of cholesterol with plant sterols (phytosterols) in liposome structure has been considered,[Citation126–128] since people suffering from hypercholesterolaemia should severely restrict the intake of cholesterol even in low concentrations.[Citation126] Phytosterols not only could be suitable substitutes for cholesterol in liposome structure, but also add several bioactive properties with possible benefits for human health such as lowering blood cholesterol levels and reduction in the risk of heart disease,[Citation129] anti-inflammatory, anti-bacterial, anti-artherosclerotic, anti-oxidative, anti-ulcerative, anti-tumour, and anti-carcinogenic functions.[Citation130,Citation131] On the other hand, incorporation of phytosterols in liposome structure may solve the problems related to the direct addition of phytosterols to food products due to their high melting point and tendency to form insoluble crystals.[Citation132]
Successful results in improving the stability of liposomes have also been obtained by the modification of liposome with several substances, such as poly(ethylene glycol) (PEG),[Citation133–135] poloxamer,[Citation136] polysorbate 80,[Citation137] carboxymethyl chitin,[Citation138] carboxymethyl chitosan,[Citation139] chitosan,[Citation140–142] and dextran derivatives.[Citation143,Citation144] A variety of stimulus-sensitive liposomes, which release their contents in response to various physical or chemical stimuli, have also been designed for targeted release of core materials. For example, temperature-sensitive liposomes have been obtained by incorporating thermo-sensitive polymers such as poly(N-isopropylacrylamide) [Citation145–147] into liposome structures. These types of liposomes have been mostly applied for specific delivery of drugs to a target site to increase their efficacy and decrease their side effects. In food industry, these kinds of carriers could be attempted in baking industry, for example, for flavour release by increasing the cooking temperature of the ready meals.[Citation7]
The pH-sensitive liposomes, other types of trigger-release liposomes designed to release the loaded compounds in response to the change of pH in the surrounding medium, have been prepared by the combination of unsaturated species of phosphatidylethanolamine (such as diacetylenic-phosphatidyl-ethanolamine (DAPE), palmitoyl-oleoyl-phosphatidyl-ethanolamine (POPE) and dioleoyl-phosphatidyl-ethanolamine (DOPE)) and an amphiphilic stabiliser such as oleic acid, palmitoylhomocystein, or cholesteryl hemisuccinate, inserting the pH-sensitive peptide/proteins, such as GALA, the N-terminus of hemaglutinin (INF peptides from influenza) or the listeriolysin O into the phospholipid double-fusion peptide or protein and/or addition of pH-sensitive polymers based on poly (alkyl acrylic acid)s, succinylated PEG, and N-isopropylacrylamide (NIPAM) copolymers to liposome structure.[Citation148] These types of liposomes have been appropriately designed to encapsulate biological macromolecules, such as drugs (especially for cancer, pulmonary and infectious diseases),[Citation149] enzymes,[Citation150], antibodies [Citation151] and antisense oligonucleotide (ODN),[Citation152] plasmids,[Citation153] proteins and peptides.[Citation154] pH-sensitive liposomes have not been employed in the encapsulation of food ingredients to date. Nevertheless, they seem to have significant potential in food industry, for example, for the release of antimicrobials upon pH changes as a result of increased microbial activity.[Citation7]
Besides, it should be considered that liposomes must be prepared from GRAS or food-grade ingredients for food applications. Since many substances that are permitted for application in pharmaceutical or cosmetic industries are not allowed in food in large quantity, further studies are required for the selection of appropriate materials for food applications.[Citation155] The ingredients should not also unpleasantly influence the physico-chemical or sensory properties of food or beverage product that is incorporated into, for example, appearance, texture, or flavour.[Citation156]
Finally, suitability of liposomal entrapment cannot be determined without considering the viability of the economics of using this process. Since food products compared to the medical and cosmetic products are required to be prepared by using less expensive techniques to be marketable, and if the economics are viable, this technology could dramatically contribute to develop more novel food products.
4. Conclusions
Due to possessing a number of benefits including possibility of large-scale production using natural ingredients, entrapment, and release of water-soluble, lipid-soluble, and amphiphilic materials, as well as targetability, liposomal entrapment is an emerging system to be used widely in food sector. There are many potential applications for liposomes in the food industry, ranging from the protection of sensitive ingredients to increasing the efficacy of food additives. The rate and extent of degradation of vitamins in food systems can be limited by their entrapping into the liposomes. Liposomal encapsulation of enzymes may protect them to be inactivated by the conditions of a food system. The incorporation of antimicrobials into liposomes might also represent an alternative to overcome some problems related to the direct application of antimicrobials in food. Liposome entrapment of essential oils can also be used to improve their stability, solubility, and bioavailability. Finally, utilisation of encapsulated polyphenols instead of free compounds can overcome the drawbacks of their instability, alleviate unpleasant tastes or flavours, as well as improve the bioavailability and half-life of the compound in vivo and in vitro.
Disclosure statement
The authors declare that there are no conflicts of interest.
References
- Augustin MA, Sanguansri L. Challenges in developing delivery systems for food additives, nutraceuticals and dietary supplements. In: Garti N, McClements DJ, editors. Encapsulation technologies and delivery systems for food ingredients and nutraceuticals. Philadelphia (PA): Woodhead Publishing; 2012. p. 19–48.
- Nedovic V, Kalusevic A, Manojlovic V, et al. An overview of encapsulation technologies for food applications. Procedia Food Sci. 2011;1:1806–1815.
- Taylor TM, Weiss J, Davidson PM, et al. Liposomal nanocapsules in food science and agriculture. Crit Rev Food Sci Nutr. 2005;45:587–605.
- Mozafari MR, Johnson C, Hatziantoniou S, et al. Nanoliposomes and their applications in food nanotechnology. J Liposome Res. 2008;18:309–327.
- Thompson AK. Structure and properties of liposomes prepared from milk phospholipids [PhD thesis]. Palmerston North: Massey University; 2005.
- Laouini A, Jaafar-Maalej C, Limayem-Blouza I, et al. Preparation, characterization and applications of liposomes: state of the art. J Colloid Sci Biotechnol. 2012;1:147–168.
- Fathi M, Mozafari M, Mohebbi M. Nanoencapsulation of food ingredients using lipid based delivery systems. Trends Food Sci Technol. 2012;23:13–27.
- Gregoriadis G. Liposome technology: liposome preparation and related techniques. London: Informa Health Care; 2007.
- New RRC. Preparation of liposomes. In: New RRC, editor. Liposomes: A practical approach. Oxford: IRL press; 1990. p. 33–104.
- Lasic DD. Handbook of biological physics: Structure and dynamics of membranes, from cells to vesicles. Amsterdam: Elsevier; 1995. Chapter 10, Applications of liposomes; p. 491–519.
- Gregoriadis G. Liposome technology. Boca Raton (FL): CRC Press; 1984.
- Thompson AK, Mozafari MR, Singh H. The properties of liposomes produced from milk fat globule membrane material using different techniques. Le Lait. 2007;87:349–360.
- De Leeuw J, De Vijlder H, Bjerring P, et al. Liposomes in dermatology today. J Eur Acad Dermatol Venereol. 2009;23:505–516.
- Koopman JS, Turkish VJ, Monto AS. Infant formulas and gastrointestinal illness. Am J Public Health. 1985;75:477–480.
- Peel M. Liposomes produced by combined homogenization/extrusion. GIT Lab J. 1999;3:37–38.
- Huwiler A, Kolter T, Pfeilschifter J, et al. Physiology and pathophysiology of sphingolipid metabolism and signaling. Biochim Biophys Acta. 2000;1485:63–99.
- Crook T, Petrie W, Wells C, et al. Effects of phosphatidylserine in Alzheimer's disease. Psychopharmacol Bull. 1992;28:61–66. Epub 1992/01/01. PubMed PMID: 1609044.
- Crook T, Tinklenberg J, Yesavage J, et al. Effects of phosphatidylserine in age‐associated memory impairment. Neurology. 1991;41:644–649.
- Taylor TM, Gaysinsky S, Davidson PM, et al. Characterization of antimicrobial-bearing liposomes by ζ-potential, vesicle size, and encapsulation efficiency. Food Biophys. 2007;2:1–9.
- Ball GF. Bioavailability and analysis of vitamins in foods. London: Chapman and Hall; 1998.
- Ordoñez JA, Campero M, Fernández L, et al. Food technology: Food components and processes. Madrid: Síntesis; 1998. p. 60–74.
- Yang S, Liu W, Liu C, et al. Characterization and bioavailability of vitamin C nanoliposomes prepared by film evaporation-dynamic high pressure microfluidization. J Dispers Sci Technol. 2012;33:1608–1614.
- Kirby C, Whittle C, Rigby N, et al. Stabilization of ascorbic acid by microencapsulation in liposomes. Int J Food Sci Technol. 1991;26:437–449.
- Lee S-C, Yuk H-G, Lee D-H, et al. Stabilization of retinol through incorporation into liposomes. J Biochem Mol Biol. 2002;35:358–363.
- Wechtersbach L, Poklar Ulrih N, Cigić B. Liposomal stabilization of ascorbic acid in model systems and in food matrices. LWT Food Sci Technol. 2012;45:43–49.
- Helgason T, Awad TS, Kristbergsson K, et al. Impact of surfactant properties on oxidative stability of β-carotene encapsulated within solid lipid nanoparticles. J Agric Food Chem. 2009;57:8033–8040.
- Qian C, Decker EA, Xiao H, et al. Impact of lipid nanoparticle physical state on particle aggregation and β-carotene degradation: potential limitations of solid lipid nanoparticles. Food Res Int. 2013;52:342–349.
- Khayata N, Abdelwahed W, Chehna M, et al. Preparation of vitamin E loaded nanocapsules by the nanoprecipitation method: from laboratory scale to large scale using a membrane contactor. Int J Pharm. 2012;423:419–427.
- Noronha CM, Granada AF, de Carvalho SM, et al. Optimization of α-tocopherol loaded nanocapsules by the nanoprecipitation method. Ind Crops Prod. 2013;50:896–903.
- Deeth HC. Lipoprotein lipase and lipolysis in milk. Int Dairy J. 2006;16:555–562.
- Tesoriere L, Bongiorno A, Pintaudi AM, et al. Synergistic interactions between vitamin A and vitamin E against lipid peroxidation in phosphatidylcholine liposomes. Arch Biochem Biophys. 1996;326:57–63.
- Banville C, Vuillemard J, Lacroix C. Comparison of different methods for fortifying Cheddar cheese with vitamin D. Int Dairy J. 2000;10:375–382.
- Ko S, Lee S-C. Effect of nanoliposomes on the stabilization of incorporated retinol. Afr J Biotechnol. 2010;9:6158–6161.
- Kulkarni S, Vargha-Butler E. Study of liposomal drug delivery systems 2. Encapsulation efficiencies of some steroids in MLV liposomes. Colloids Surf B. 1995;4:77–85.
- Marsanasco M, Márquez AL, Wagner JR, et al. Liposomes as vehicles for vitamins E and C: an alternative to fortify orange juice and offer vitamin C protection after heat treatment. Food Res Int. 2011;44:3039–3046.
- Lee S-C, Lee K-E, Kim J-J, et al. The effect of cholesterol in the liposome bilayer on the stabilization of incorporated retinol. J Liposome Res. 2005;15:157–166.
- Lee S-C, Kim J-J, Lee K-E. Effect of β-sitosterol in liposome bilayer on the stabilization of incorporated retinol. Food Sci Biotechnol. 2005;14:604–607.
- Liu N, Park H-J. Chitosan-coated nanoliposome as vitamin E carrier. J Microencapsulation. 2009;26:235–242.
- Sharma A, Sharma US. Liposomes in drug delivery: progress and limitations. Int J Pharm. 1997;154:123–140.
- Caddeo C, Teskač K, Sinico C, et al. Effect of resveratrol incorporated in liposomes on proliferation and UV-B protection of cells. Int J Pharm. 2008;363:183–191.
- Coderch L, Fonollosa J, De Pera M, et al. Influence of cholesterol on liposome fluidity by EPR: relationship with percutaneous absorption. J Control Release. 2000;68:85–95.
- Lariviere B, El Soda M, Soucy Y, et al. Microfluidized liposomes for the acceleration of cheese ripening. Int Dairy J. 1991;1:111–124.
- Picon A, Gaya P, Medina M, et al. Proteinases encapsulated in stimulated release liposomes for cheese ripening. Biotechnol Lett. 1997;19:345–348.
- Picon A, Gaya P, Medina M, et al. The effect of liposome-encapsulated bacillus subtilis neutral proteinase on Manchego cheese ripening. J Dairy Sci. 1995;78:1238–1247.
- Picon A, Serrano C, Gaya P, et al. The effect of liposome-encapsulated cyprosins on Manchego cheese ripening. J Dairy Sci. 1996;79:1699–1705.
- Kheadr EE, Vuillemard JC, El Deeb SA. Accelerated Cheddar cheese ripening with encapsulated proteinases. Int J Food Sci Technol. 2000;35:483–495.
- Thompson AK. Liposomes: from concepts to applications. Food New Zealand. 2003;13:S23–S32.
- Wilkinson M, Kilcawley K. Mechanisms of incorporation and release of enzymes into cheese during ripening. Int Dairy J. 2005;15:817–830.
- Colas J-C, Shi W, Rao V, et al. Microscopical investigations of nisin-loaded nanoliposomes prepared by Mozafari method and their bacterial targeting. Micron. 2007;38:841–847.
- Law BA, King JS. Use of liposomes for proteinase addition to Cheddar cheese. J Dairy Res. 1985;52:183–188.
- Kirby C, Brooker B, Law B. Accelerated ripening of cheese using liposome‐encapsulated enzyme. Int J Food Sci Technol. 1987;22:355–375.
- Walde P, Ichikawa S. Enzymes inside lipid vesicles: preparation, reactivity and applications. Biomol Eng. 2001;18:143–177.
- Laloy E, Vuillemard J-C, Dufour P, et al. Release of enzymes from liposomes during cheese ripening. J Control Release. 1998;54:213–222.
- Kheadr EE, Vuillemard JC, El‐Deeb S. Acceleration of Cheddar Cheese Lipolysis by Using Liposome‐entrapped Lipases. J Food Sci. 2002;67:485–492.
- Matsuzaki M, McCafferty F, Karel M. The effect of cholesterol content of phospholipid vesicles on the encapsulation and acid resistance of β‐galactosidase from E. coli. Int J Food Sci Technol. 1989;24:451–460.
- Chawan CB, Penmetsa PK, Veeramachaneni R, et al. Liposomal encapsulation of β-galactosidase: effect of buffer molarity, lipid composition and stability in milk. J Food Biochem. 1992;16:349–357.
- Rao D, Chawan C, Veeramachaneni R. Liposomal encapsulation of β-galactosidase: comparison of two methods of encapsulation and in vitro lactose digestibility. J Food Biochem. 1995;18:239–251.
- Kirby C, Gregoriadis G. Dehydration–rehydration vesicles: a simple method for high yield drug entrapment in liposomes. Nat Biotech. 1984;2:979–984.
- Alkhalaf W, Piard J-C, El Soda M, et al. Liposomes as proteinase carriers for the accelerated ripening of Saint-Paulin type cheese. J Food Sci. 1988;53:1674–1679.
- Alkhalaf W, El Soda M, Gripon JC, et al. Acceleration of cheese ripening with liposomes-entrapped proteinase: influence of liposomes net charge. J Dairy Sci. 1989;72:2233–2238.
- Scolari G, Vescovo M, Sarra P, et al. Proteolysis in cheese made with liposome-entrapped proteolytic enzymes. Le Lait. 1993;73:281–292.
- Jay JM, Loessner MJ, Golden DA. Modern food microbiology. New York: Springer; 2005.
- Jung D-S, Bodyfelt FW, Daeschel MA. Influence of fat and emulsifiers on the efficacy of nisin in inhibiting listeria monocytogenes in fluid milk. J Dairy Sci. 1992;75:387–393.
- Kirby C. Microencapsulation and controlled delivery of food ingredients. Food Sci Technol Today. 1991;5:74–78.
- Roberts RFJ. Development of a nisin-producing starter-culture for use during cheddar cheese manufacture to inhibit spoilage in high moisture pasteurized process cheese spreads. Minneapolis: University of Minnesota; 1991.
- Thomas LV, Delves-Broughton J. Nisin In: Davidson PM, Sofos JN, Branen AL, editors. Antimicrobials in Food. New York: CRC Press; 2005. p. 237–274.
- Bouksaim M, Lacroix C, Audet P, et al. Effects of mixed starter composition on nisin Z production by Lactococcus lactis subsp. lactis biovar. diacetylactis UL 719 during production and ripening of Gouda cheese. Int J Food Microbiol. 2000;59:141–156.
- Johnson EA, Larson AE. Lysozyme. In: Davidson PM, Sofos JN, Branen AL, editors. Antimicrobials in foods. New York: CRC Press; 2005. p. 361–387.
- Delves-Brougthon J. Nisin and its uses as a food preservative. Food Technol. 1990;44:100–117.
- Thapon J, Brule G. Effets du pH et de la forme ionize sur raffinit lysozymes-caseines [Effect of pH and ionic strength on lysozyme-caseins affinity]. Lait (France). 1986;66:19–30.
- Degnan A, Luchansky J. Influence of beef tallow and muscle on the antilisterial activity of pediocin AcH and liposome-encapsulated pediocin AcH. J Food Prot. 1992;55:552–554.
- Degnan A, Buyong N, Luchansky JB. Antilisterial activity of pediocin AcH in model food systems in the presence of an emulsifier or encapsulated within liposomes. Int J Food Microbiol. 1993;18:127–138.
- Benech R-O, Kheadr E, Laridi R, et al. Inhibition of Listeria innocua in cheddar cheese by addition of nisin Z in liposomes or by in situ production in mixed culture. Appl Environ Microbiol. 2002;68:3683–3690.
- Were LM, Bruce B, Davidson PM, et al. Encapsulation of nisin and lysozyme in liposomes enhances efficacy against Listeria monocytogenes. J Food Prot. 2004;67:922–927.
- Weiss J, Gaysinsky S, Davidson M, et al. Nanostructured encapsulation systems: food antimicrobials. In: Barbosa-Canovas G, Mortimer A, Lineback D, et al., editors. Global issues in food science and technology. San Diego (CA): Academic Press; 2009. p. 425–479.
- Laridi R, Kheadr E, Benech R-O, et al. Liposome encapsulated nisin Z: optimization, stability and release during milk fermentation. Int Dairy J. 2003;13:325–336.
- Were LM, Bruce BD, Davidson PM, et al. Size, stability, and entrapment efficiency of phospholipid nanocapsules containing polypeptide antimicrobials. J Agric Food Chem. 2003;51:8073–8079.
- Ortan A, Campeanu G, Dinu–Pirvu C, et al. Studies concerning the entrapment of Anethum graveolens essential oil in liposomes. Roum Biotechnol Lett. 2009;14:4411–4417.
- Chami N, Chami F, Bennis S, et al. Antifungal treatment with carvacrol and eugenol of oral candidiasis in immunosuppressed rats. Braz J Infect Dis. 2004;8:217–226.
- Tian J, Ban X, Zeng H, et al. Chemical composition and antifungal activity of essential oil from Cicuta virosa L. var. latisecta Celak. Int J Food Microbiol. 2011;145:464–470.
- Esmaeili A. Biological activities of Eremostachys laevigata Bunge grown in Iran. Pak J Pharm Sci. 2012;25:803–808.
- Boukhris M, Bouaziz M, Feki I, et al. Hypoglycemic and antioxidant effects of leaf essential oil of Pelargonium graveolens L'Hér in alloxan induced diabetic rats. Lipids Health Dis. 2012;11:81–90.
- Gill A, Holley R. Disruption of Escherichia coli, Listeria monocytogenes and Lactobacillus sakei cellular membranes by plant oil aromatics. Int J Food Microbiology. 2006;108:1–9.
- Mahapatra SK, Chakraborty SP, Das S, et al. Methanol extract of Ocimum gratissimum protects murine peritoneal macrophages from nicotine toxicity by decreasing free radical generation, lipid and protein damage and enhances antioxidant protection. Oxidative MedCell Longev. 2009;2:222–230.
- Jaganathan SK, Mazumdar A, Mondhe D, et al. Apoptotic effect of eugenol in human colon cancer cell lines. Cell Biol Int. 2011;35:607–615.
- Martín Á, Varona S, Navarrete A, et al. Encapsulation and co-precipitation processes with supercritical fluids: applications with essential oils. Open Chem Eng J. 2010;4:31–41.
- Shoji Y, Nakashima H. Nutraceutics and delivery systems. J Drug Target. 2004;12:385–391.
- Woranuch S, Yoksan R. Eugenol-loaded chitosan nanoparticles. I. Thermal stability improvement of eugenol through encapsulation. Carbohydr Polym. 2013;96:578–585.
- Hosseini SF, Zandi M, Rezaei M, et al. Two-step method for encapsulation of oregano essential oil in chitosan nanoparticles: preparation, characterization and in vitro release study. Carbohydr Polym. 2013;95:50–56.
- de Oliveira EF, Paula HC, Paula R. Alginate/cashew gum nanoparticles for essential oil encapsulation. Colloids Surf B. 2014;113:146–151.
- Valenti D, De Logu A, Loy G, et al. Liposome-incorporated Santolina insularis essential oil: Preparation, characterization and in vitro antiviral activity. J Liposome Res. 2001;11:73–90.
- Gortzi O, Lalas S, Chinou I, et al. Reevaluation of antimicrobial and antioxidant activity of Thymus spp. extracts before and after encapsulation in liposomes. J Food Prot. 2006;69:2998–3005.
- Liolios C, Gortzi O, Lalas S, et al. Liposomal incorporation of carvacrol and thymol isolated from the essential oil of Origanum dictamnus L. and in vitro antimicrobial activity. Food Chem. 2009;112:77–83.
- Detoni CB, de Oliveira DM, Santo IE, et al. Evaluation of thermal-oxidative stability and antiglioma activity of Zanthoxylum tingoassuiba essential oil entrapped into multi-and unilamellar liposomes. J Liposome Res. 2012;22:1–7.
- Samperio C, Boyer R, Eigel III WN, et al. Enhancement of plant essential oils' aqueous solubility and stability using alpha and beta cyclodextrin. J Agric Food Chem. 2010;58:12950–12956.
- Hill LE, Gomes C, Taylor TM. Characterization of beta-cyclodextrin inclusion complexes containing essential oils (trans-cinnamaldehyde, eugenol, cinnamon bark, and clove bud extracts) for antimicrobial delivery applications. LWT Food Sci Technol. 2013;51:86–93.
- ALHaj NA, Shamsudin MN, Alipiah NM, et al. Characterization of Nigella sativa L. essential oil-loaded solid lipid nanoparticles. Am J Pharmacol Toxicol. 2010;5:52–57.
- Lai F, Sinico C, De Logu A, et al. SLN as a topical delivery system for Artemisia arborescens essential oil: in vitro antiviral activity and skin permeation study. Int J Nanomed. 2007;2:419–425.
- Wen Z, You X, Jiang L, et al. Liposomal incorporation of rose essential oil by a supercritical process. Flavour Fragr J. 2011;26:27–33.
- Sinico C, De Logu A, Lai F, et al. Liposomal incorporation of Artemisia arborescens L. essential oil and in vitro antiviral activity. Eur J Pharm Biopharm. 2005;59:161–168. Epub 2004/11/30. doi: 10.1016/j.ejpb.2004.06.005.
- Gortzi O, Lalas S, Tsaknis J, et al. Enhanced bioactivity of Citrus limon (Lemon Greek cultivar) extracts, essential oil and isolated compounds before and after encapsulation in liposomes. Planta Med. 2007;73:184.
- Moghimipour E, Aghel N, Mahmoudabadi AZ, et al. Preparation and characterization of liposomes containing essential oil of Eucalyptus camaldulensis leaf. Jundishapur J Nat Pharm Prod. 2012;7:117–122.
- Varona S, Martín Á, Cocero MJ. Liposomal incorporation of lavandin essential oil by a thin-film hydration method and by particles from gas-saturated solutions. Ind Eng Chem Res. 2011;50:2088–2097.
- Wen Z, Liu B, Zheng Z, et al. Preparation of liposomes entrapping essential oil from Atractylodes macrocephala Koidz by modified RESS technique. Chem Eng Res Des. 2010;88:1102–1107.
- Bennick A. Interaction of plant polyphenols with salivary proteins. Crit Rev Oral Biol Med. 2002;13:184–196.
- Haslam E. Natural polyphenols (vegetable tannins) as drugs: possible modes of action. J Nat Prod. 1996;59:205–215.
- Quideau S, Feldman KS. Ellagitannin chemistry. Chem Rev. 1996;96:475–504.
- Arts IC, Hollman PC. Polyphenols and disease risk in epidemiologic studies. Am J Clin Nutr. 2005;81:317S–325S.
- Scalbert A, Johnson IT, Saltmarsh M. Polyphenols: antioxidants and beyond. Am J Clin Nutr. 2005;81:215S–217S.
- Surh Y-J. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3:768–780.
- Fang Z, Bhandari B. Encapsulation of polyphenols – a review. Trends Food Sci Technol. 2010;21:510–523.
- Mukhtar H, Ahmad N. Tea polyphenols: prevention of cancer and optimizing health. Am J Clin Nutr. 2000;71:1698s–1702s.
- Rashidinejad A, Birch EJ, Sun-Waterhouse D, et al. Delivery of green tea catechin and epigallocatechin gallate in liposomes incorporated into low-fat hard cheese. Food Chem. 2014;156:176–183.
- Sun-Waterhouse D, Wadhwa SS, Waterhouse GI. Spray-drying microencapsulation of polyphenol bioactives: a comparative study using different natural fibre polymers as encapsulants. Food Bioprocess Technol. 2013;6:2376–2388.
- Isailović BD, Kostić IT, Zvonar A, et al. Resveratrol loaded liposomes produced by different techniques. Innov Food Sci Emerging Technol. 2013;19:181–189.
- Takahashi M, Uechi S, Takara K, et al. Evaluation of an oral carrier system in rats: bioavailability and antioxidant properties of liposome-encapsulated curcumin. J Agric Food Chem. 2009;57:9141–9146.
- Ramadan MF, Asker S, Mohamed M. Antimicrobial and antiviral impact of novel quercetin-enriched lecithin. J Food Biochem. 2009;33:557–571.
- Fan M, Xu S, Xia S, et al. Effect of different preparation methods on physicochemical properties of salidroside liposomes. J Agric Food Chem. 2007;55:3089–3095.
- Fang J-Y, Hung C-F, Liao M-H, et al. A study of the formulation design of acoustically active lipospheres as carriers for drug delivery. Eur J Pharm Biopharm. 2007;67:67–75.
- Gibis M, Vogt E, Weiss J. Encapsulation of polyphenolic grape seed extract in polymer-coated liposomes. Food Funct. 2012;3:246–254.
- Fang J-Y, Lee W-R, Shen S-C, et al. Effect of liposome encapsulation of tea catechins on their accumulation in basal cell carcinomas. J Dermatol Sci. 2006;42:101–109.
- Ramadan MF. Antioxidant characteristics of phenolipids (quercetin-enriched lecithin) in lipid matrices. Ind Crops Prod. 2012;36:363–369.
- McClements DJ. Nanoparticle- and microparticle-based delivery systems: encapsulation, protection and release of active compounds. Boca Raton (FL): CRC Press; 2014.
- Mu X, Zhong Z. Preparation and properties of poly (vinyl alcohol)-stabilized liposomes. Int J Pharm. 2006;318:55–61.
- Kirby C, Clarke J, Gregoriadis G. Effect of the cholesterol content of small unilamellar liposomes on their stability in vivo and in vitro. Biochem J. 1980;186:591–598.
- Chan YH, Chen BH, Chiu CP, et al. The influence of phytosterols on the encapsulation efficiency of cholesterol liposomes. Int J Food Sci Technol. 2004;39:985–995.
- Hwang J-S, Tsai Y-L, Hsu K-C. The feasibility of antihypertensive oligopeptides encapsulated in liposomes prepared with phytosterols-β-sitosterol or stigmasterol. Food Res Int. 2010;43:133–139.
- Alexander M, Lopez AA, Fang Y, et al. Incorporation of phytosterols in soy phospholipids nanoliposomes: encapsulation efficiency and stability. LWT Food Sci Technol. 2012;47:427–436.
- Cheikh-Rouhou S, Besbes S, Lognay G, et al. Sterol composition of black cumin (Nigella sativa L.) and Aleppo pine (Pinus halepensis Mill.) seed oils. J Food Compos Anal. 2008;21:162–168.
- Cherif AO. Phytochemicals components as bioactive foods. In: Rasooli I, editor. Bioactive compounds in phytomedicine. Rijeka: InTech; 2012. p. 113–124.
- Normén L, Andersson S, Dutta P. Does phytosterol intake affect the development of cancer? In: Dutta PC, editor. Phytosterols as functional food components and nutraceuticals. New York: Marcel Dekker. 2004. p. 191–242.
- McClements DJ, Decker EA, Weiss J. Emulsion‐based delivery systems for lipophilic bioactive components. J Food Sci. 2007;72:R109–R124.
- Meyer O, Kirpotin D, Hong K, et al. Cationic liposomes coated with polyethylene glycol as carriers for oligonucleotides. J Biol Chem. 1998;273:15621–15627.
- Arifin DR, Palmer AF. Physical properties and stability mechanisms of poly (ethylene glycol) conjugated liposome encapsulated hemoglobin dispersions. Artif Cells Blood Substit Immobil Biotechnol. 2005;33:137–162.
- Allen TM, Mehra T, Hansen C, et al. Stealth liposomes: an improved sustained release system for 1-β-D-arabinofuranosylcytosine. Cancer Res. 1992;52:2431–2439.
- Jamshaid M, Farr SJ, Kearney P, et al. Poloxamer sorption on liposomes: comparison with polystyrene latex and influence on solute efflux. Int J Pharm. 1988;48:125–131. doi: http://dx.doi.org/10.1016/0378-5173(88)90255-4.
- Kronberg B, Dahlman A, Carlfors J, et al. Preparation and evaluation of sterically stabilized liposomes: colloidal stability, serum stability, macrophage uptake, and toxicity. J Pharm Sci. 1990;79:667–671.
- Dong C, Rogers J. Polymer-coated liposomes; stability and release of ASA from carboxymethyl chitin-coated liposomes. J Control Release. 1991;17:217–224.
- Alamelu S, Rao KP. Studies on the carboxymethyl chitosan-containing liposomes for their stability and controlled release of dapsone. J Microencapsulation. 1991;8:505–519.
- Gibis M, Rahn N, Weiss J. Physical and oxidative stability of uncoated and chitosan-coated liposomes containing grape seed extract. Pharmaceutics. 2013;5:421–433.
- Mady MM, Darwish MM. Effect of chitosan coating on the characteristics of DPPC liposomes. J Adv Res. 2010;1:187–191.
- Filipovic-Grcic J, Škalko-Basnet N, Jalšienjak I. Mucoadhesive chitosan-coated liposomes: characteristics and stability. J Microencapsulation. 2001;18:3–12.
- Elferink MG, de Wit JG, In't Veld G, et al. The stability and functional properties of proteoliposomes mixed with dextran derivatives bearing hydrophobic anchor groups. Biochim Biophys Acta. 1992;1106:23–30.
- Mumper RJ, Hoffman AS. The stabilization and release of hirudin from liposomes or lipid-assemblies coated with hydrophobically modified dextran. AAPS PharmSciTech. 2000;1:20–29.
- Kono K, Hayashi H, Takagishi T. Temperature-sensitive liposomes: liposomes bearing poly (N-isopropylacrylamide). J Control Release. 1994;30:69–75.
- Kono K, Nakai R, Morimoto K, et al. Thermosensitive polymer-modified liposomes that release contents around physiological temperature. Biochim Biophys Acta. 1999;1416:239–250.
- Kim J-C, Kim J-D. Release property of temperature-sensitive liposome containing poly (N-isopropylacrylamide). Colloids Surf B. 2002;24:45–52.
- Liu X, Huang G. Formation strategies, mechanism of intracellular delivery and potential clinical applications of pH-sensitive liposomes. Asian J Pharm Sci. 2013;8:319–328.
- Leite EA, dos Santos Giuberti C, Wainstein AJ, et al. Acute toxicity of long-circulating and pH-sensitive liposomes containing cisplatin in mice after intraperitoneal administration. Life Sci. 2009;84:641–649.
- Briscoe P, Caniggia I, Graves A, et al. Delivery of superoxide dismutase to pulmonary epithelium via pH-sensitive liposomes. Am J Physiol Lung Cell Mol Physiol. 1995;268:L374–L380.
- Mizoue T, Horibe T, Maruyama K, et al. Targetability and intracellular delivery of anti-BCG antibody-modified, pH-sensitive fusogenic immunoliposomes to tumor cells. Int J Pharm. 2002;237:129–137.
- Selvam MP, Buck SM, Blay RA, et al. Inhibition of HIV replication by immunoliposomal antisense oligonucleotide. Antivir Res. 1996;33:11–20.
- Kale AA, Torchilin VP. Enhanced transfection of tumor cells in vivo using ‘Smart’ pH-sensitive TAT-modified pegylated liposomes. J Drug Target. 2007;15:538–545.
- Nair S, Zhou F, Reddy R, et al. Soluble proteins delivered to dendritic cells via pH-sensitive liposomes induce primary cytotoxic T lymphocyte responses in vitro. J Exp Med. 1992;175:609–612.
- Tamjidi F, Shahedi M, Varshosaz J, et al. Nanostructured lipid carriers (NLC): a potential delivery system for bioactive food molecules. Innov Food Sci Emerging Technol. 2013;19:29–43.
- Ziani K, Fang Y, McClements DJ. Encapsulation of functional lipophilic components in surfactant-based colloidal delivery systems: vitamin E, vitamin D, and lemon oil. Food Chem. 2012;134:1106–1112.
- Gibbs BF, Kermasha S, Alli I, et al. Encapsulation in the food industry: a review. Int J Food Sci Nutr. 1999;50:213–224.
- Augustin M, Sanguansri L, Margetts C, et al. Microencapsulation of food ingredients. Food Aust. 2001;53:220–223.
- Barenholz Y, Lasic DD. An overview of liposome scaled-up production and quality control. In: Barenholz Y, Lasic DD, editors. Vol. 3, Handbook of nonmedical applications of liposomes. Boca Raton (FL): CRC Press; 1996. p. 23–30.
- Ye Q, Asherman J, Stevenson M, et al. DepoFoam™ technology: a vehicle for controlled delivery of protein and peptide drugs. J Control Release. 2000;64:155–166.
- Reineccius GA. Liposomes for controlled release in the food industry. In: Risch S, Reineccius GA, editors. Encapsulation and controlled release of food ingredients. Washington (DC): American Chemical Society; 1995. p. 113–131.
- Benech R-O, Kheadr E, Lacroix C, et al. Impact of Nisin producing culture and liposome-encapsulated nisin on ripening of Lactobacillus added-Cheddar cheese. J Dairy Sci. 2003;86:1895–1909.