ABSTRACT
The synthesis of metal nanoparticles is an emerging area of advanced research and technology with potential application in plant protection. In the current study, with an eco-friendly approach, a convenient method was adopted, where copper nanoparticles are biosynthesised extracellularly by using Streptomyces griseus. Further, the existence of nanoparticles was confirmed by UV–visible spectroscopy, transmission electron microscopy, X-ray diffraction analysis and Fourier transform infrared spectroscopy characterisation. We assessed the field effectiveness of copper nanoparticles through soil application in P. hypolateritia infested tea plants. In response to seven different treatments, carbendazim exhibited superior control followed by nanocopper at 2.5 ppm dosage. However, maximum leaf yield was observed in plants treated with nanocopper. In addition, nanocopper-treated plants showed improved soil macronutrients considerably when compared to bulk copper and carbendazim treated plants. In addition, there was trivial variation in population dynamics of microbes noted in plants treated with nanocopper. These encouraging results confirmed that nanocopper could act as an efficient novel fungicide which may be used for the management of red root-rot disease in tea plantations.
1. Introduction
Nanoscale materials exhibit unique properties, when compared at their bulk level.[Citation1] In general, synthesis of copper nanoparticles involves different chemical and physical methods such as sol gel methods, chemical vapour deposition, precipitation-pyrolysis method, sonochemistry, electrochemistry, one-step solid-state reactions, cathodic vacuum arc and solvothermal reactions.[Citation2] However, these methods involve utilisation of toxic chemicals which are environmental hazards. Therefore, it is necessary to develop an eco-friendly approach for the synthesis of nanomaterials. It has been well established that when microbes are kept in harsh toxic metal environment, they evolve mechanism of bioremediation to survive in stringent conditions by transforming toxic metal ions into their corresponding non-toxic forms.[Citation3]
It has been reported that several kinds of metallic nanoparticles such as silver, copper and gold exhibit strong activity against some microbes and microorganisms. According to Dang et al.,[Citation4] the nanoparticles have strong antifungal activity against various plant pathogens such as Phytophthora and C. salmonicolor. However, antimicrobial studies of copper nanoparticles received more attention recently because the nanoparticles are much cheaper than that of silver or gold nanoparticles. This advantage could offer the antifungal applications of the copper nanoparticles in agriculture.[Citation5]
In 1761, copper was used in agriculture for the first time, when it was discovered that seed grains soaked in a weak solution of copper sulphate inhibit the seed-borne fungi. The copper compounds have wide application in agricultural practices as metallic fungicide.[Citation6] Bordeaux and burgundy were used to control downy-mildew in garden, nursery, vineyard and in farm. At present, the search for new fungicide is important due to the gradual increase of new resistant fungal strains. Nanotechnology enhances antimicrobial activity of copper metal when it is transformed into corresponding nanoparticle.[Citation7]
Among the different diseases reported in tea plants, red root-rot disease is considered as a primary root disease caused by Poria hypolateritia Berk. ex Cooke, which is of more important because it affects the entire root system of tea plants, resulting in the sudden death of bushes leading to capital loss. It is a fast-growing and slow-killing pathogen which spread to other tea bushes by means of root, water or through soil at elevations above 600 MSL.[Citation8] Here, we report the synthesis of copper nanoparticles using Actinomycetes spp., namely, Streptomyces griseus. This organism was isolated from the rhizosphere soil of tea plants in Valparai, which lies in the Western Ghats of Tamil Nadu. In this study, we reported that when S. griseus biomass is treated with aqueous copper sulphate salt solutions, copper nanoparticles are formed extracellularly, under aerobic conditions. The formation of copper nanoparticles could be mediated by the induction of secreted proteins by bacteria that convert bulk metal to nanoparticles. Finally, the antifungal activity of copper nanoparticles was tested for fungicidal efficacy by field evaluation against red root-rot infested tea plants.
2. Materials and methods
2.1. Culture and growth conditions
Streptomyces griseus belonging to Actinomycetes spp. was initially grown in starch casein nitrate agar medium.[Citation9] For mass multiplication, the cells were grown to the stationary phase in starch casein nitrate broth at 37 °C under shaking conditions (120 rpm) for 72 h. After incubation, the cells were harvested by centrifugation (5000 rpm for 10 min at room temperature). The cell pellet was resuspended and centrifuged three times in deionised water.
2.2. Biosynthesis of copper nanoparticles
Copper sulphate was purchased from Hi-Media, and was used for the synthesis of copper nanoparticles. In a classical way of nanoparticles synthesis, carefully weighed 0.1 g biomass of S. griseus was added to 100 ml of 1 mM aqueous copper sulphate solution in 250-ml conical flasks. The flasks were then incubated in incubator shaker (150 rpm) at room temperature.[Citation10] During the process of biosynthesis, the flasks were noted for colour change to confirm the formation of nanoparticles visually.
2.3. Characterisation of copper nanoparticles by UV–visible spectroscopy, transmission electron microscopy and particle size analyser
The bioreduction of the copper sulphate using cells of S. griseus was monitored and the appearance of reddish brown colour indicates the formation of copper nanoparticles. The reduction of copper sulphate to copper was monitored by measuring the UV–visible spectrum over a wavelength range of 200–800 nm. It was done after diluting the reaction medium in a small aliquot of the sample with deionised water. The measurements were carried out on ELICO UV spectrophotometer at a resolution of 1 nm. For transmission electron microscopy (TEM) analysis the copper nanoparticles were suspended in pure acetone by ultra-sonication and a few drops of particles were placed onto copper grids and dried in a desiccator. TEM measurements were performed on a Technai G2 ZOU Twin instrument operated at an accelerating voltage of 200 kV. This microscopy was used for the estimation of crystalline structure, morphology and mean size of nanoparticles. In addition, the average size of nanoparticle was determined using particle size analyser.
2.4. Analysis of copper nanoparticles by X-ray diffraction studies and Fourier transform infrared spectroscopy
The microbial reduced copper nanoparticle formation and quality measurements were carried out using powder X-ray diffractometer 6000 X-ray diffraction instrument (Shimadzu). The pattern was checked in drop-coated films of synthesised nanoparticles on glass substrate recorded at a scan speed of 4° per minute with Cu Kα radiation (λ = 1.54060 Å) in a θ–2θ configuration operated at a voltage of 40 KV and current of 30 mA. The crystallite domain size was calculated by using Debye-Scherrer formula. The Fourier transform infrared (FTIR) measurements were carried out for copper nanoparticles to identify the possible bioactive molecules responsible for the reduction of the copper ions and the capping ability of the bioreduced copper nanoparticles by S. griseus and the spectra was recorded in the wavelength interval of 4000–450 cm−1 using a resolution of 4 cm−1. This molecular analysis of the sample was performed using FTIR spectroscopy Perkin-Elmer-1600 (USA).
2.5. Field location and treatments
Field experiments were conducted in naturally infected red root-rot disease tea fields at two different estates of Parry Agro Tea Industries, Valparai, Tamil Nadu, India, during 2011–2014 in the Western Ghats of southern India (10°30′ N, 77°O′E, altitude 1050 m above MSL). Susceptible clone UPASI-9 with a population of 15,000 plants/ha was studied. The field experiments were devised as completely randomised block designs. Each experiment consisted of seven treatments and each plot consisted of 45 bushes. Similarly, systemic fungicides such as carbendazim (Bavistin- 50 WP) were applied at the rate of 0.05% with 1 l/bush. Copper nanoparticles were sprayed to P. hypolateritia infected plants applied at the different rate of 1–2.5 ppm concentration with 1.5 l/bush.
2.6. Determination of edaphic analysis of soil samples
All the collected soil samples were subjected to analysis of various soil edaphic parameters such as soil pH, electrical conductivity (EC), water holding capacity (WHC), organic carbon,[Citation11] nitrogen, available phosphorous, exchangeable potassium, exchangeable calcium, sodium and magnesium contents.[Citation12]
2.7. Disease assessment
The disease incidence was recorded on 25 bushes (25 root samples) selected randomly per treatment. Disease assessment was recorded two times per year during pre-monsoon (April--May) and post-monsoon (November–December) seasons. Percentage of disease incidence was calculated as PDI = (A +B + C + D + E)/25 × 100 in which the method of Ziedan [Citation13] was adopted for disease assessment with slight modification.
2.8. Green leaf yield assessment
Yield data per plot were recorded regularly during the course of the experimental period. Yield and yield attributes in terms of productivity index were monitored at every plucking round. The green leaf yield was converted to made tea in kg ha−1 using the formula: yield = green leaf yield (kg) × 15,000 × 0.225/number of bushes in the experimental plot, where 15,000 is the total bush number per hectare and 0.225 is the conversion factor for green leaf (22.5% out turn) to make tea.[Citation14]
2.9. Enumeration of microbial population
Periodically, soil samples were collected for the enumeration of microbes including bacterial, fungal and actinomycetes population level with specific medium following the dilution plate technique to estimate the survival rate of microbes in the soil samples.[Citation15]
2.10. Statistical analysis
All mean data obtained were subjected to analysis of variance (ANOVA) and when the significant differences were obtained Duncan's multiple range test (DMRT) was carried out (at P = 0.05) using SPSS 17.0 statistical package (SPSS, Inc. Chicago, IL).[Citation16]
3. Results
3.1. UV–vis spectroscopy analysis of copper nanoparticles
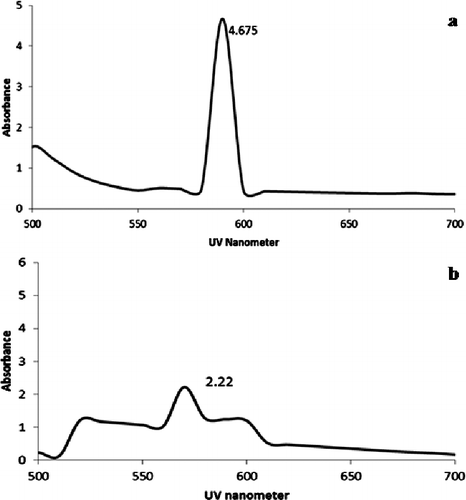
The presence of biosynthesised copper nanoparticles was primarily identified by visual inspection, which produced yellow colour which later turned to reddish brown when heated over water bath at 80 °C. The existence was further confirmed followed by UV–visible spectroscopy technique in which the absorption spectra of copper nanoparticles ranged between 580 and 600 nm (). The spectral analysis result indicated that maximum peak absorption was found at 592 nm (4.675 abs) when recorded by continuous wavelength scan. Beyond 600 nm the absorption band of nanoparticles abruptly changed and gradually decreased to zero with linear band. In control sample, the UV absorption for copper solution without biomass read against distilled water exhibited a peak at 570 nm ().
3.2. TEM characterisation of copper nanoparticles
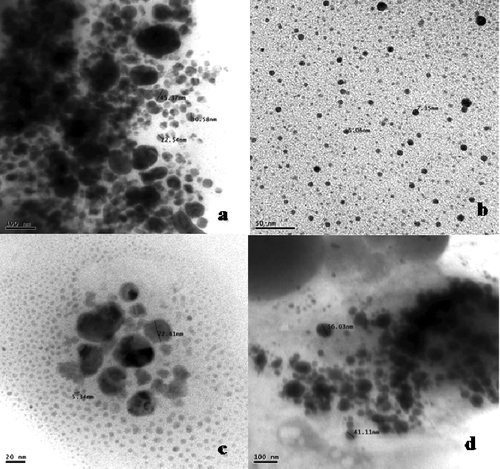
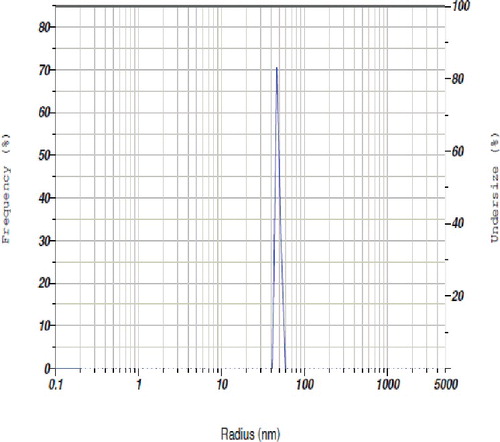
The spectral analysis was followed by TEM analysis to determine the structure and morphology of copper nanoparticles. The size and shape obtained from the image of nanoparticles were recorded on the film of extracellular product of the S. griseus--copper sulphate reaction. A representative picture recorded from the copper nanoparticle film deposited on a grid is shown in . It showed that the particles were polydispersed and varied from 5 to 50 nm in diameter. The particles appeared to be reasonably monodispersed as well as in aggregated form. The majority of morphology was spherical in shape. Occasional micro-particles were observed at 2-μm magnifications. TEM images confirmed that the copper nanoparticles are in nano range and further they are approximately spherical in shape. The particle size of biosynthesised nanoparticles were determined by particle size analyser and the results are presented in . The results revealed that copper nanoparticles were heterogeneously distributed in the culture filtrate in which the size varied between 30 and 50 nm and particles were strongly existed at nanoscale level.
3.3. X-ray diffraction pattern of copper nanoparticles
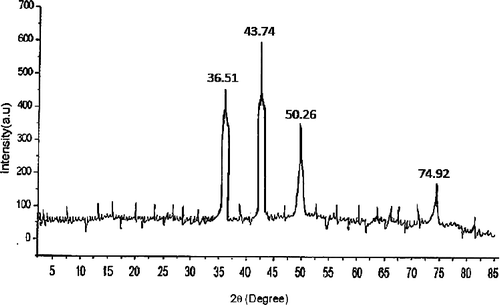
Analysis of copper nanopowder through X-ray diffraction (XRD) revealed the diffraction pattern related to size and shape produced from the peak intensities. A consistent result obtained from XRD patterns of nanoparticles is presented in . The peak positions arose at around 2θ degree with values 36.51°, 43.74°, 50.26° and 74.92° of the spectrum corresponding to (111), (111), (200) and (220) respectively. This clearly indicated the interaction between nanoparticles and stabilising medium. There were no other sharp peaks observed in the spectra, which confirmed that the synthesised particles were of high purity.
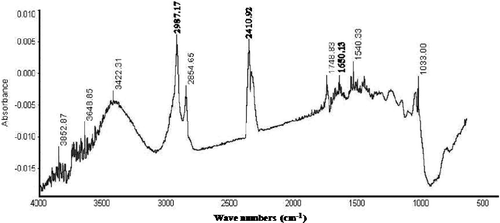
3.4. FTIR spectrum of copper nanoparticles
The dried copper nanoparticles were subjected to FTIR measurements primarily to identify the possible biomolecules and allow to determine the functional group responsible for the reduction, capping and efficient stabilisation. According to , the absorption peaks from 3852 cm−1 to 3413 cm−1 corresponding to OH stretching H-bonded alcohols and phenols groups which confirmed the presence of aromatic ring in proteins required for synthesis and stabilisation of copper nanoparticles. The bands at 2987 and 2854 cm−1 were assigned to –CH2 and C–H stretching modes in alkanes presented as aliphatic organic molecules present in biosynthesised nanoparticles. Further, two bands at 1650 and 1550 cm−1 obtained, respectively, represent the presence of amide I and amide II bands of protein due to capping of nanoparticles. In continuous, the band obtained at 1748 cm−1 may be related to ester derived from a carboxylic acid and an alcohol. The band detected near 1033 cm−1 was described to be C–N stretching vibrations of aromatic and aliphatic amines. These analyses strongly confirmed that the formation of copper nanoparticles in the culture filtrate which was mainly due to the protein or enzymatic induced/reduction reactions.
3.5. Impact of copper nanoparticles on soil and its nutrients
The influence of copper nanoparticles on nutrient status of P. hypolateritia infested tea soil samples collected randomly from various locations of tea plants is presented in . The pH of the tea soils showed that they were acidic, with pH ranging from 4.5 to 5.3. The electrical conductivity of tea soils was determined in terms of soluble salt index, which ranged from 0.15 to 0.23 dS/m. The greatest amount of total organic carbon (3.5%) content was recorded in treatment applied with nanocopper followed by control soil sample (3.3%). Similarly, the highest amount of total nitrogen (3.6%) content was noted in soil applied with both nano- and bulk copper. Likewise, the maximum amount of phosphorus (17.3%) and potassium (71.3%) was observed in soil treated with copper nanoparticles. In contrast, there were no significant changes detectable in the rest of the soil nutrients including calcium, magnesium and sodium contents.
Table. 1. Edaphic parameters of P. hypolateritia infested tea soil in response to bulk and nano copper during 2011–2014.
3.6. Impact of copper nanoparticles application on disease incidence and leaf yield of tea plants
There was a significant reduction in red root-rot disease incidence, in response to soil application of copper nanoparticles (at different dosage), fungicide and bulk copper (). Systemic fungicide carbendazim showed the greatest disease control which accounted for 57.2% disease reduction. Among varying concentration of copper nanoparticles applied, the maximum disease control was observed at 2.5 ppm dosage with 52.7% disease reduction when compared to bulk copper (45.3%). In contrast, among seven different treatments, the highest leaf yield of 3565 kg ha−1 made tea was recorded in the treatment containing nanocopper at 2.5 ppm concentration. This was followed by treatment with carbendazim with 3256 kg ha−1 made tea. An appreciable increase in yield with 3177 kg ha−1 made tea was detected in the plants treated with bulk copper. In general, the infected control was noticed with defoliation and heavy flowering where the highest disease incidence (51.7%), lowest disease protection (−27.6%) and least leaf yield (2734 kg ha−1 made tea) was observed.
Table 2. Effect of fungicide, bulk and nanocopper on red root-rot disease incidence and leaf yield during 2011–2014.
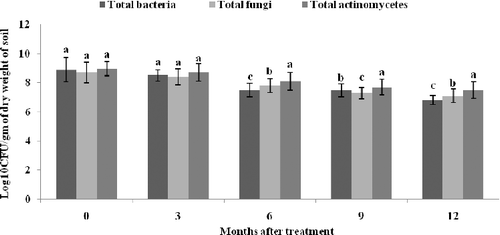
3.7. Population dynamics of microbes
The population density of microbes including total bacteria, fungi and actinomycetes was monitored periodically after delivery of copper nanoparticles to tea soil, in order to conclude the persistent existence in rhizosphere (). The total population level of all the microbes were nearly 8.7 log10 cfu g−1 soil at the time of delivery. The same trend existed approximately after three months. At the end of nine months, the population densities of microbes vary differentially in which maximum population was identified in actinomycetes (7.8 log10 cfu g−1 soil) followed by bacteria (7.4 log10 cfu g−1 soil) and fungi (7.1 log10 cfu g−1 soil) populations. Finally, after 12 months, the least population density was noted in bacteria with 6.3 log10 cfu g−1 soil. The enumeration graph strongly showed that there was a trivial decline at 10% level in population densities of microbes due to nanoparticles in tea plantations.
4. Discussion
Effective management of fungal diseases for economically important cash crops is of vital need because an efficient control strategy is required that are less harmful to animals and human beings. This study focused on antifungal activity of copper nanoparticles on suppression of red root-rot disease in tea plants.
The present study was initiated with primary detection of copper nanoparticles by visual observation from the culture filtrate of S. griseus. The reddish brown colour appearance indicated the reduction of copper sulphate and the formation of nanoparticles. Previously, Krithiga et al.[Citation17] described that the presence of copper nanoparticles was confirmed by visual appearance and UV–visible spectroscopy technique, which has been frequently used to characterise metal nanoparticles to give a convenient signature for indication.
In UV–vis spectroscopy analysis, an absorption peak of copper nanoparticles was sharply observed λmax at 592 nm. In parallel, Varshney et al. [Citation10,Citation18] reported the spectral analysis of copper nanoparticles synthesised by P. stutzuri showed an inter-band transition which produced yellow hydrosol at 570 and 780 nm due to plasmon resonance (). This showed that UV spectroscopy measurements provide strong evidence to detect the presence of copper nanoparticles and also it revealed that synthesised particles have broad range of absorption spectrum.
In earlier work, TEM analysis determined the shape, size and protein coating of copper oxide nanoparticles synthesised by the cyanobacterium Phormidium. It described the topography of average particle size ranges between 10-40 nm which are quasi-spherical shaped nanoparticles.[Citation2] In our study, TEM analysis was carried out and spherical shaped copper nanoparticles were identified both in monodispersed and polydispersed forms. In addition a well defined spherical shaped particles in a dense assembly and at higher magnifications a better idea of morphology and size was viewed. In general, TEM is a useful tool to delineate size, shape, morphology, symmetry and nature of nanoparticles.
According to [Citation19], a consistent result was obtained for the XRD patterns of the copper nanoparticles. The peaks at 36.79°, 43.49°, 50.65° and 74.15° of the spectrum correspond to (111), (111), (200) and (220) respectively, which represent crystal face-centred cubic (fcc) of copper (copper XRD reference number 01-089-2838). Similarly, our result for diffraction patterns of copper nanoparticles was noted with similar raised peaks. The particles sizes obtained from the expanded XRD line correlates well with that obtained from TEM. Thus, this record proved strong evidence in favour of UV–visible spectra and TEM images for the presence and size of copper nanoparticles.
Further FTIR spectrum revealed that biosynthesised copper nanoparticles bounded by various functional groups. Absorption peaks obtained at different wavenumbers confirmed the presence of protein in the sample and assisted to identify the possible biomolecules responsible for the reduction, capping and efficient stabilisation of the bioreduced copper nanoparticles. Earlier, Vasudev and Pramod [Citation20] confirmed that the FTIR analysis was used to identify the capping, reducing and stabilising capacity of the leaf extract from copper nanoparticles. Hence, FTIR spectrum suggested that copper nanoparticles were surrounded by different organic molecules such as terpenoids, alcohols, ketones, aldehydes and carboxylic acid.
Application of copper nanoparticles in P. hypolateritia infested tea soil has noteworthy impact on edaphic parameters. There was slight fluctuation in pH and EC of tea soil. On the other hand, there was considerable variation in the amount of organic carbon, nitrogen, phosphorus and potassium in response to nanoparticles application. On contrary, previously Karunakaran et al. [Citation21] reported that delivery of nano and bulk zirconia and titania particles to the agricultural soil decrease the important macronutrients. However, there was no trivial change observed other than in rest of the micronutrients. Hence, these findings suggested that delivery of nanoparticles in soil produce considerable effect of soil nutrients.
In the current work copper nanoparticles were evaluated as fungicides against P. hypolateritia causing red root-rot disease in tea plants. Based on previous in vitro antagonistic studies, copper nanoparticles were selected as efficient metal fungicides and applied to P. hypolateritia infected tea plants with different dosage of treatments (data not shown). Among seven treatments, systemic fungicide carbendazim gave a better protection than the other treatments tested. This may be due to the mechanism of instantaneous action on fungal mycelium. It exhibited a maximum control over P. hypolateritia infection where the disease incidence was 17.5%. This was followed by treatment with nanocopper at 2.5 ppm dosage where the disease incidence was 18.0%. Earlier, it has been reported that ultrafine synthesised colloidal solution at 7 ppm of copper nanoparticles exhibited a powerful antifungal activity against Corticium salmonicolor when sprayed on pink disease-infected rubber trees.[Citation5]
Recently, Tanubay et al. [Citation22] reported that chemical reduction of carboxymethylated chitosan-stabilised copper nanoparticles was found have significant antifungal and antibacterial activities when evaluated against Candida tropicalis and Escherichia coli under in vitro condition. In parallel, copper nanoparticles were successfully prepared from copper salt via chemical reduction method with sodium citrate dispersant and polyvinyl alcohol capping polymer which was used as an efficient fungicide.[Citation5] In contrary, we have reported a convenient way of biological method for the synthesis of copper nanoparticles using S. griseus for suppression of red root-rot disease.
In addition, amendment of copper nanoparticles in tea soil was reflected with trivial change in beneficial microbes. This may be due to slight fluctuations in soil nutrient status of NPK contents. Therefore, population dynamics of important beneficial microbes was noted with minor changes at 10% level difference in response to nanoparticle application. Conversely, the change in nutrient content due to the incorporation of bulk and nanoparticles may be due to their impact on microbial population of plant growth promoting rhizhobacteria (PGPR). They improved soil properties and nutrient content by recovering the soil N, P and K values. Thereby exploitation of nanoparticles automatically decreases the soil nutrient value according to the report of Karunakaran et al.[Citation21]
The outcome of the current investigation disclosed the significance of green way of copper nanoparticles synthesis which provided a simple, fast and efficient method. In this work, the copper nanoparticles were prepared via a biological reduction method at a high concentration with good stability and encouraging results were obtained when sprayed on red root-rot infected tea plants. It promises to be an effective nanometallic fungicide application as a cheap eco-fungicide for agriculture. Therefore, from our study, it may be concluded that biologically synthesised copper nanofungicide could be a possible alternative control measure for tea root disease, which in turn have a very high surface area with a tremendous driving force for diffusion in rhizosphere that ultimately reduces the dependency on commercially available bulk copper fungicide.
Acknowledgments
The authors are thankful to the Chairman and Principal of K.S. Rangasamy College of Technology, Tiruchengode, Tamil Nadu, India, for providing necessary facilities and constant encouragement to carry out this study. Dr. P. Mohan kumar, Director, UPASI Tea Research Institute, Valparai, is gratefully acknowledged for his excellent support in conducting field trails.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Klabunde KJ. Nanoscale materials in chemistry. New York: Wiley; 2002. p. 1.
- Rahman A, Ismail A, Jumbianti D, et al. Synthesis of copper oxide nanoparticles by using Phormidium cyanobacterium. Indo J Chem. 2009;9:355–360.
- Neis DH. Microbial heavy-metal resistance. Appl Microbiol Biotechnol. 1999;51:730–750.
- Dang VP, Vo TKL, Nguyen TKL, et al. Synthesis and antimicrobial effects of colloidal silver nanoparticles in chitosan by c-irradiation. J Exp Nanosci. 2010;5:169–179.
- Van Du C, Nguyen PP, Khuong VQ, et al. Ultrafine copper nanoparticles exhibiting a powerful antifungal/killing activity against Corticium salmonicolor. Bull Korean Chem Soc. 2014;35(9):2645–2648.
- García PC, Rivero RM, Ruiz JM, et al. The role of fungicides in the physiology of higher plants: implications for defense responses. Bot Rev. 2003;69:162–172.
- Kanhed P, Birla S, Gaikwad S, et al. In vitro antifungal efficacy of copper nanoparticles against selected crop pathogenic fungi. Mat Lett. 2014;115:13–17.
- Shanmuganathan N. Poria root disease of tea. Ceylon, Sri Lanka: Tea Research Institute, Advisory pamphlet 1/66; 1997. p. 13.
- Kuster E, Williams ST. Selection of media for isolation of Streptomyces. Nature. 1964;202:928–929.
- Varshney R, Bhadauria S, Gaur MS, et al. Characterization of copper nanoparticles synthesized by a novel microbiological method. JOM: J Miner Met Mater Soc. 2010;62(12):102–104.
- Walkley A, Black CA. An examination of the Degtjareff method for determining soil organic matter and proposed modification of chromic valid titration method. Soil Sci. 1934;37:29–38.
- AOAC. Estimation of various biochemical parameters. In: Helrich K, editor. Official methods of analysis of the association of official analytical chemist. 15th ed. Gaithersburg; 1990; p. 152–155.
- Ziedan EH. Soil treatment with biofertilizers for controlling peanut root and pod rot diseases in Nobaria province. Egypt J Phytopathol. 2000;28(1–2):17–26.
- Ponmurugan P, Baby UI. Evaluation of fungicides and biocontrol agents against phomopsis canker of tea under field condition. Aust J Plant Pathol. 2007;36:68–72.
- Elango V, Manjukarunambika K, Ponmurugan P, et al. Evaluation of Streptomyces spp. for effective management of Poria hypolateritia causing red root-rot disease in tea plants. Biol Control. 2015;89:75–83.
- Gomez KA, Gomez AA. Statistical procedure for agricultural research, 2nd ed. Los Banos, The Phillippines: International Rice Research Institute; 1984; p. 183.
- Krithiga N, Jayachitra A, Rajalakshmi A. Synthesis, characterization and analysis of the effect of copper oxide nanoparticles in biological systems. Ind J Ns. 2013;1:6–15.
- Varshney R, Bhadauria S, Gaur MS, et al. Copper nanoparticles synthesis from electroplating industry effluent. Nano Biomed Eng. 2011;3(2):115–119.
- Usman MS, Zowalaty ME El, Shameli K, et al. Synthesis, characterization, and antimicrobial properties of copper nanoparticles. Int J Nanomed. 2013;13(8):4467–4479.
- Vasudev DK, Pramod SK. Green synthesis of copper nanoparticles using Ocimum sanctum leaf extract. Int J Chem Studies. 2013;1(3):1–4.
- Karunakaran G, Suriyaprabha R, Manivasakan P, et al. Impact of nano and bulk ZrO2, TiO2 particles on soil nutrient contents and PGPR. J Nanosci Nanotechnol. 2013;13:678–685.
- Tantubay S, Mukhopadhyay S, , Himani Kalita SK, et al. Carboxymethylated chitosan-stabilized copper nanoparticles: a promise to contribute a potent antifungal and antibacterial agent. J Nanoparticle Res. 2015;17:243.