ABSTRACT
Alternanthera philoxeroides (Mart.), a weed, mainly from tropical origin and easily available worldwide. People used to eat it as a food mainly in South Africa. In our previous report we have thoroughly characterise several important phenolics, monoterpene and phenylpropane from methanol soluble fraction of Alternenthera leaves (fraction X) and also reported their α-glucosidase inhibitory, antimicrobial and antioxidant activities. All these isolated natural compounds are well characterised and widely studied. In our present study we try to use this beneficial fraction (named fraction X) in green synthesis of gold nanoparticles (X-GNP). We also try to explore the beneficial aspects of green synthesis in comparison with commonly used chemical synthesis method (GNP) in context with their antimicrobial activity. UV/Vis spectroscopy, DLS, Zeta potential, FT-IR, EDAX and other microscopic techniques namely: SEM, AFM were used to characterise the synthesised nanoparticles. Different important microbial strains were used to evaluate the antimicrobial activity of prepared nanoparticles. Overall the studies suggest successful synthesis of green nanoparticles (X-GNP) and also showed the improvement in antimicrobial activity of X-GNP nanoparticles.
1. Introduction
Nanotechnology now a days become an exciting tool to design different types of particles which have a vast application in the field of medicine, diagnostic, imaging, drug discovery, tissue engineering and many more [Citation1]. It is quite easy to prepare nanoparticles with different size, shape and chemical compositions. Thus the use of these engineered nanomaterials is well accepted worldwide [Citation2]. But along with the increasing use of these nanoparticles, scientists have also noticed the negative sides of these particles. Generally chemically synthesised nanoparticles show an enormous range of side effects namely: hepatic problems, metabolic disorder, etc., which are quite annoying [Citation3]. Gold and silver nanoparticles are the two most widely used nanoparticles but they are a relevant example of this scenario [Citation3]. Gold nanoparticles mainly synthesised by using well established chemical method (citrate reduction) and due to its small size, inert character it has been widely used in different drug targeting and medicinal studies [Citation4]. But often use of a chemical reduction method may cause several side effects. Thus to reduce the side effects several biological methods are recently used to synthesise gold nanoparticles [Citation5]. It is now well accepted that biological methods for nanoparticle synthesis using different plant extracts are eco-friendly and possible alternative of the generalised chemical methods and it also causes less toxic effects [Citation5–7].
Most of the cases, lack of knowledge on the phytochemical constituents of the extract ruin the fate of entire prospects of the study. Thus use of a properly (phytochemical) characterised extracts will be preferable for green synthesis of gold nanoparticles [Citation8]. Leaf extract of Zingiber officinale [Citation9] and Cinnamom camphora [Citation10], floral extract of Mirabilis jalapa [Citation11], biomass of Stevia rebaudiana [Citation12], etc. has been successfully used to synthesise gold nanoparticles. Aqueous extracts of Withania coagulans [Citation13], Cichorium endivia [Citation14], Sesbania sesban (L) [Citation15], Scoparia dulcis [Citation16] and Trichosanthes dioica ([Citation17]), as well as the combination of Cassia auriculata and Aegle marmelos [Citation18] were also used in the biological synthesis of gold nanoparticles which exhibited profound medicinal properties.
In our present study, we have used methanol soluble fraction of Alternanthera philoxeroides (Mart.) Grisb. (Amaranthaceae) leaves to synthesise gold nanoparticles (X-GNP) and also evaluated the antimicrobial property. A. philoxeroides (Mart.) Grisb a tropical weed, mainly originates from South America [Citation19] and recently available almost all around the world [Citation20]. The weed is commonly known as alligator weed and found on the upper surface of water (lake or pond). In our previous study we have thoroughly characterised the methanol soluble fraction of A. philoxeroides leaves [Citation21]. We have reported the presence of five major phenolics viz. kaempferol, ferulic acid, salicylic acid, syringic acid, chlorogenic acid, one monoterpene (safrole) and one phenylpropane (myrcene) in the methanol soluble fraction called fraction-X [Citation21]. These purified methanolic fractions also possess antimicrobial, antioxidant and as well as α-glucosidase inhibitory activities [Citation21].
In the present study, gold nanoparticles were synthesised using fraction-X of A. philoxeroides leaves (X-GNP) in comparison to chemically synthesised nanoparticles (GNP). Synthesised nanoparticles were characterised by using spectroscopic (UV/Vis, DLS, Zeta potential, FT-IR, EDAX), microscopic (SEM, AFM) methods and antimicrobial property was evaluated against Pseudomonas aeruginosa (MTCC 10462), Escherichia coli (MTCC 1680), Micrococcus luteus (MTCC 8101), Acinetobacter lwoffii (MTCC 8288) and Bacillus subtilis (MTCC 10073).
2. Materials and methods
2.1. Material
Chloroauric acid and trisodium citrate was purchased from Sigma-Aldrich Company, USA. All other chemicals were of analytical grade and purchased from Sisco Research Laboratory Pvt. Ltd., India.
2.2. Preparation of leaf extract
A. philoxeroides (Mart.) Grisb were collected from the local ponds. Collected plants were washed under running tap water and rinsed with double distilled water. Plants were verified with their taxonomic classification and their herbarium specimens were also prepared. Specimen data is available in our previous article [Citation21]. Leaves were detached and dried in oven at 60 °C for 30 min. Dry leaves (100 g) were crushed in a mortar-pestle with methanol. Step wise separation of the crude extract was done using a silica column (10 × 1 cm). Entire separation process was done according to our previous published method [Citation22]. After separation, resulting methanolic fraction was sonicated for 1 h under cold condition. Samples were then filtered through a Whatmann-1 filter paper. Fine powder (46 mg) was obtained after lyophilisation of the filtrate. Powder thus obtained was used to prepare a methanolic stock solution (1 mg/ml) named as Fraction-X. Active components present in fraction-X (1 mg/ml) was previously characterised and reported by Bhattacherjee et al. [Citation21] and Bhattacherjee and Chakraborti [Citation22]. Fraction-X was used for synthesis of gold nanoparticle.
2.3. Synthesis of gold nanoparticles
Chemical synthesis of gold nanoparticles (GNP) was done by citrate reduction method [Citation23]. According to the method chloroauric acid is reduced by trisodium citrate. To synthesise GNPs, 0.5 ml of tri-sodium citrate (1 mg/ml) was added to 10 ml of 0.01% chloroauric acid under boiling condition. After a few minutes the colourless solution became wine red.
In the green synthesis method, 10 ml of 0.01% gold chloride solution (freshly prepared) and 0.5 ml of fraction-X (1 mg/ml stock solution) was mixed at boiling condition. Wine red color of gold chloride solution indicates formation of gold nanoparticles. Gold nanoparticles prepared by fraction-X are represented as X-GNP in the manuscript. Fraction-X was coated onto the nanoparticles during synthesis of gold nanoparticles. For preparation of dry nanoparticles, the nanoparticle solutions (GNP and X-GNP) were centrifuged at 16000 g for 20 min and the precipitates were lyophilised. Resulting solid precipitates again can be dissolved in aqua's solution to prepare nano suspension and used in spectroscopic studies. After synthesis of GNP and X-GNP particles, solution was dried and 1 mg/mL stock solution was prepared. Equal amount (1 mg/mL) of GNP, X-GNP and fraction X was compared during bioactivity assays.
2.4. Characterisation of X-GNP and GNP
2.4.1. UV-Vis spectroscopic study
The UV-Vis spectra of both GNP and X-GNP were recorded from 250 to 650 nm in Hitachi spectrophotometer using 1 cm optical path quartz cuvette [Citation24,Citation25].
2.4.2. Size and surface charge measurement
Particular size of X-GNP in suspension was measured by dynamic light scattering (DLS) method using Zeta nano sizer (nano-ZS). Surface charge of the particles was estimated by measuring zeta potential by the same instrument [Citation9].
2.5. Morphological study using microscopic methods
Morphological topography of both nanoparticles (GNP and X-GNP) was visualised by atomic force microscopy (Nanoscope IIIA system) and scanning electron microscopy (Quanta-200). Briefly, nanoparticle suspensions were dried on a glass side and visualised under AFM. A part of the dehydrated sample was washed with isoamyl acetate, dried, stained with gold solution, and examined under a Quanta-200 scanning electron microscope [Citation26–28].
2.6. Elemental analysis by EDAX study
The synthesised nanoparticles were subjected for elemental analysis through Energy Dispersive X-ray (EDAX) spectrum by using FEI Quanta-200 MK2 (Philips Co.) according to the method of Vankar and Bajpai [Citation11].
2.7. FT-IR study
Both GNP and X-GNP suspensions were dried in a lyophiliser and solid nano powders were used for FT-IR study (FT-IR 8400S, Shimadzu) according to the method of Daisy and Saipriya [Citation27] and Bhattacherjee et al. [Citation29]. The scanning range was 400–4000 cm−1 with resolution of 4 cm−1.
2.8. Antimicrobial activities
2.8.1. Broth assay
Antimicrobial potential of GNP and X-GNP was assayed against five bacterial strains Pseudomonas aeruginosa (MTCC 10 462), Escherichia coli (MTCC 1680), Micrococcus luteus (MTCC 8101), Acinetobacter lwoffii (MTCC 8288) and Bacillus subtilis (MTCC 10 073). For quantitative studies, 100 ml of nutrient broth was mixed with 5%, 10%, 20%, and 25% of GNP and X-GNP [Citation30,Citation31]. Broth without GNP was treated as negative control and broth with fraction-X was taken as positive control. Pre-sterilised broths were inoculated with 1% of overnight grown seed cultures and allowed to grow at 37 °C. Bacterial growth was then monitored at every hour for 24 h by measuring the increase in absorbance at 600 nm in a spectrophotometer (Cecil Aquarious, CE7200, Double Beam UV/VIS Spectrophotometer, 7000 series, Cambridge, UK). Percent inhibit of bacterial growth was calculated based on the difference in growth between the control and media containing fraction X after 24 h. Cell suspension was again monitored based on the colony forming units (CFU) per ml by plating on nutrient agar medium for viable cell count at every 6 h.
2.8.2. Immobilisation of GNP and X-GNP on cotton cloth diffusion studies
Antibacterial activity is commonly tested using a disk (containing desired amount of drug) diffusion test [Citation32]. A similar test with GNP and X-GNP loaded cotton cloth was used in this study. Desired amount of GNP and X-GNP (20, 40 and 60 µl) were loaded on the pre-sterilised white cotton cloth (25 mm2) in aseptic condition and their antimicrobial activity was visualised on nutrient agar spread plates [Citation33]. The negative control plates were prepared without GNP and X-GNP. Plates were incubated for 24 h at 37 °C and the average diameter of the inhibition zone surrounding the cloth was measured with a ruler with up to 0.1 mm resolution. The mean and standard deviation (±SD) reported for each type of GNP and X-GNP and with each microbial strain were based on three replicates.
3. Results and discussion
Objective of the present work is to synthesise phyto-chemically gold nanoparticles with help of A. philoxeroides leaves and try exploring the beneficial effect of these biologically synthesised nanoparticles against certain microbial strains. Improvement in the antimicrobial activity of biologically synthesised nanoparticles (X-GNP) with comparison to gold nanoparticles (GNP) was also demonstrated in this work.
Nanotechnology is one of most widely studied topic mainly deals with synthesis and manipulation of small particles (less than 100 nm). Several metallic nanoparticles (namely: gold, silver, etc.) has been widely studied now a days. In spite of the high benefits of these metallic nanoparticles they often failed to deliver in humal trails due to severe side effects [Citation3]. Biological synthesis or green syntheses of these nanoparticles are thus needed. Biosynthesis method is more eco-friendly in comparison to conventional chemical synthesis [Citation34]. Mainly the secondary metabolites present in the plant extracts play the major role in the green synthesis [Citation35]. In our previous report [Citation21], we have thoroughly characterised different important secondary metabolites present in the methanol soluble fraction of A. philoxeroides leaves (fraction X). In the present work fraction X was used for bio-reduction of gold ions into nanoparticles. The reduction of chloroaurate ions to nanoparticles were facilities by addition of fraction X [Citation36].
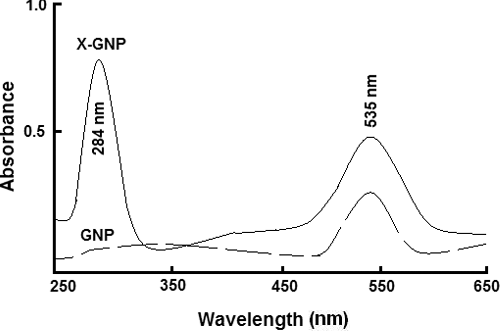
Spectroscopic methods were used to evaluate successful synthesis of X-GNP and GNP. UV-Vis spectra of X-GNP and GNP are shown in . GNP was characterised by its specific absorbance maxima at 535 nm. While in case of X-GNP we observed two absorption maxima. In addition to peak at 535 nm, we found another peak at 284 nm region. This additional peak at 284 nm region is mainly due to addition of phenolic compounds present within fraction X. Generally it is reported that phenolics, flavonoids, secondary metabolites has a characteristic absorbance around 280 nm region. Thus presence of the additional peak at 284 nm region in X-GNP indicates successful formulation of phenolics-tagged nanoparticles in case of X-GNP. Vankar and Bajpai [Citation11], also reported similar kind of observation during biosynthesis of gold nanoparticles by using Mirabilis jalapa flowers. Similar observation was also reported by Daisy and Saipriya [Citation27]. Our result in agreement with other reports, suggest successful tagging of phenolics with gold nanoparticles in X-GNP.
Figure 1. Spectroscopic studies: Ultraviolet-visible spectroscopic analysis of biologically synthesised gold nanoparticles (X-GNP) and chemically prepared gold nanoparticles (GNP).
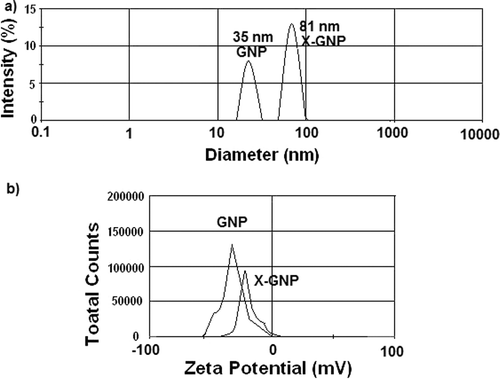
Diameters of GNP and X-GNP were respectively 35 and 81 nm as obtained from DLS studies ((a)) and a surface charges were −26.54 and −14.21 mV ((b)), respectively. It has been reported in different studies (Sastry et al., [Citation34]; [Citation9,Citation37]), due to addition of phenolics or secondary metabolites, surface charge of GNP is reduced significantly, as observed in our findings. Similarly increased size of X-GNP also indicates addition of the phenolics on the outer surface of gold nanoparticles [Citation38]. Both these results together indicate successful formation of phenolics-tagged nanoparticles in X-GNP formulation.
Figure 2. Size and charge distribution: Determination of size and surface charge of synthesised nanoparticles (GNP and X-GNP): (a) Dynamic light scattering (DLS) analysis of GNP and X-GNP suspension. (b) Estimation of surface charge of GNP and X-GNP by zeta potential measurement.
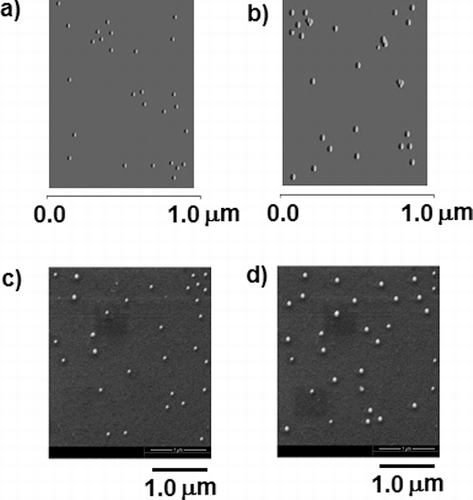
The surface topography and three dimensional images of GNP and X-GNP were visualised by AFM (). The particles from different fields were considered to calculate the mean lateral size, which was found to be 21.25 ± 3.21 ((a)) and 72.11 ± 2.87 nm ((b)) (n = 50) for GNP and X-GNP respectively. The outer surface of the nanoparticles appeared to be smooth. (c) and d represents the SEM image of the GNP and X-GNP respectively. The mean particle size was 23.67 ± 2.01 and 69.81 ± 2.34 nm (n = 30) respectively for GNP and X-GNP, calculated by image processing software attached with the electron microscope. The particles appeared to be round shaped. The diameters measured by microscopic method are little less than that calculated by DLS method, probably due to the contribution of hydrodynamic radii in DLS [Citation10]. Fierascu et al. [Citation35] reported increased diameter in biologically synthesised gold nanoparticles in comparison with chemically prepared gold nanoparticles due to successful addition of phenolics on the outer surface of gold nanoparticles. XRD analysis of nano-composites was also conducted to establish the crystalline nature of the nano-particles. Detail methodology and result (Figure S1) are documented in supplementary materials. Our results in corresponds with Parida et al. [Citation39] demonstrated the signature gold nanoparticle peaks in X-GNP solution.
Figure 3. Microscopic study to determine the size and shape of the nanoparticles: Atomic force microscopic (AFM) images of GNP (a) and X-GNP (b). Scanning electron microscopy analysis was done for gold nanoparticles (c) and phytochemical-tagged gold nanoparticles (X-GNP).
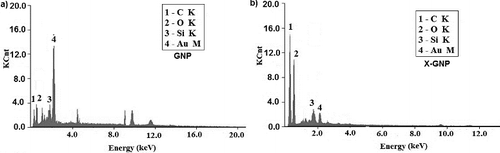
Elemental analysis of synthesised nanoparticles was done by EDAX. The EDAX spectra of both GNP and X-GNP are shown in . In GNP ((a)), 56% gold was present but in case of X-GNP ((b)) it reduced to 6.9%. Carbon and oxygen content in X-GNP has increased in significantly, i.e. 48.25%–35.80% and 12.61%–15.80%, respectively. Significant increment in carbon and oxygen content directly suggest the attachment of polyphenolics on the surface of gold nanoparticles [Citation6,Citation40]. Si (10%) was present in both cases due to the glass slide base for the sample preparation.
Figure 4. Elemental analysis: EDX analysis was done for both GNP (a) and X-GNP (b) to determine elemental components of synthesised nanoparticles.
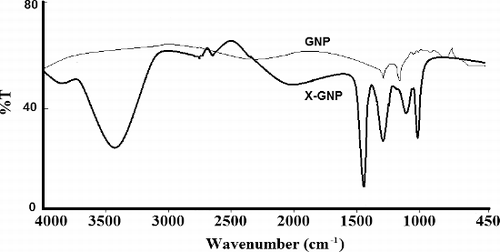
FT-IR analysis was done to characterise specific functional groups of formulated nanoparticles. We observed specific peaks at 3435.17 cm−1 (specific for OH groups of phenolics), 1465.32 cm−1 for C-H bending, 1368.23 cm−1 for C-H bend, 675.09 cm−1 for C-Br stretching in case of X-GNP (). These peaks are totally absent in case of GNP. Similar results were obtained by Daisy and Saipriya [Citation27]; Agnieszka et al. [Citation37] during biological synthesis of gold nanoparticles. Presence of these characteristic peaks in X-GNP mainly suggests the successful incorporation of polyphenolics on the outer surface of gold nanoparticles.
Figure 5. Characteristic Fourier-transform infrared absorption (FT-IR) spectra for GNP and biologically synthesised X-GNP.
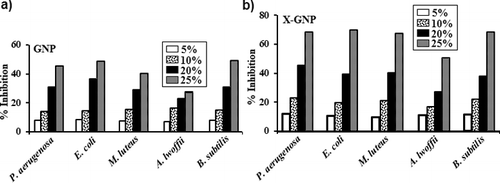
GNP and X-GNP were showed different inhibitory effect against five bacterial strains P. aeruginosa (MTCC 10462), E. coli (MTCC 1680), M. luteus (MTCC 8101), A. lwoffii (MTCC 8288) and B. subtilis (MTCC 10073). shows different inhibition percentages of the above strains with GNP and X-GNP at 5%, 10%, 20% and 25% additional stages.
Figure 6. Antimicrobial activity: Growth inhibition percentages of five MTCC strains in the presence of (a) GNP and (b) X-GNP at different concentrations in broth assay.
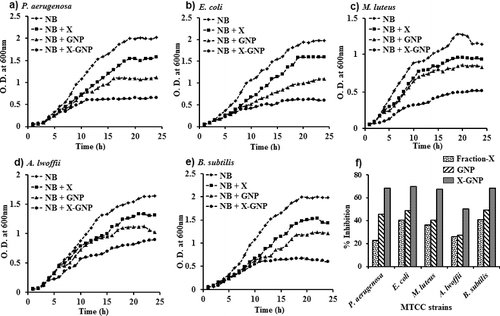
shows the optical density (at 600 nm) of the five strains in presence of GNP and X-GNP for 24 h for 25% of test supplement. In all the cases, the normal growth (nutrient broth) were inhibited up to 68.2% for P. aeruginosa, 69.6% for E. coli, 67.5% for M. luteus, 50.3% for A. lwoffii and 68.2% for B. subtilis when X-GNP was supplemented. Whereas only GNP supplement shows growth inhibition up to 45.4% for P. aeruginosa, 48.7% for E. coli, 40.2% for M. luteus, 27.0% for A. lwoffii and 49.0% for B. subtilis. These results are in agreement with previous findings proving GNP a good antimicrobial agent. The fraction-X also exhibit antimicrobial activity and it was provided in the previous study [Citation21]. In our study, X-GNP was exhibit highest antimicrobial activity in comparison to GNP and Fraction-X due to combinatorial effect. X-GNP actually made up of major polyphenols like kaempferol, ferulic acid, salicylic acid, syringic acid and chlorogenic acid [Citation21] tagged GNP. Greater inhibitory effect on microbial growth by X-GNP was very much satisfactory and may useful as antibiotics.
Figure 7. Batch growth profiles of P. aeruginosa (a), E. coli (b), M. luteus (c), A. lwoffii (d) and B. subtilis (e) with 25% supplements of GNP and X-GNP. NB (Nutrient broth, pH 7.0) with fraction-X was taken as (+) ve control and NB without any supplement was taken as (-) ve control. The maximum percentage of growth inhibition (f) with 25% supplements of fraction-X, GNP and X-GNP was plotted after 24 h of incubation at 37 °C.
The antimicrobial property of GNP and X-GNP was assessed against the same five strains (P. aeruginosa, E. coli, M. luteus, A. lwoffii and B. subtilis). shows the efficacy of GNP and X-GNP expressed as the zone of inhibition in spread plate cotton cloth diffusion studies. E. coli showed highest zone of inhibition with 60 µl load of X-GNP (62.13 mm), whereas other strains (except A. lwoffii) showed more or less similar zones. A. lwoffii showed lowest zone with X-GNP (35.25 mm) because of its special morphological characteristic. A. lwoffii often remained in folded form, thus lowering the available surface area remain in contact with GNP and X-GNP.
Table 1. Efficacy of GNP and X-GNP expressed as the zone of inhibition in spread plate cotton cloth diffusion studies.
Hence all these results suggest successful synthesis of X-GNP by using A. philoxeroides (Mart.) leaf extract and also showed improved antimicrobial activity of X-GNP in comparison with chemically synthesised GNP. Successful tagging of several important phenolics of fraction X, on the surface of biologically synthesised nanoparticles is the major cause of the improvement in antimicrobial activity of X-GNP. Synthesised antioxidant-tagged nanoparticles have a tremendous therapeutic potential.
Supplementary_Data.docx
Download MS Word (33.1 KB)Disclosure statement
The authors declare that they have no competing interests. There is no financial interest in this work or any financial support for publication.
Reference
- Davis ME, Chen ZG, Shin DM. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat Rev Cancer. 2008;7:771–782.
- Emerich DF, Thanos CG. Nanotechnology and medicine. Expert Opin Biol Ther. 2003;3:655–663.
- Gaiser BK, Hirn S, Kermanizadeh A, et al. Effects of silver nanoparticles on the liver and hepatocytes in vitro. Toxicol Sci. 2013;131:537–547.
- BarathManiKanth S, Kalishwaralal K, Sriram M, et al. Anti-oxidant effect of gold nanoparticles restrains hyperglycemic conditions in diabetic mice. J Nanobiotechnol. 2010;8:16–21.
- Mohamed M, Veeranarayanan S, Poulose AC, et al. Type 1 ribotoxin-curcin conjugated biogenic gold nanoparticles for a multimodal therapeutic approach towards brain cancer. Biochim Biophys Acta. 2013;13:548–555.
- Levchenko LA, Golovanova SA, Lariontseva NV, et al. Synthesis and study of gold nanoparticles stabilized by bioflavonoids. Russ Chem Bull. 2011;60:426–433.
- Spivak MY, Bubnov RV, Yemets IM, et al. Gold nanoparticles – the theranostic challenge for PPPM: nanocardiology application. EPMA Journal. 2013;4:18–24.
- Sona PS. Nanoparticulate drug delivery system for the treatment of diabetes. Dig J Nanomater Biostruct. 2010;5:411–418.
- Singh C, Sharma V, Naik PKR, et al. A green biogenic approach for the synthesis of gold and silver nanoparticles using Zingiber officinale. Dig J Nanomater Biostruct. 2011;6:535–542.
- Huang J, Li Q, Sun D. Biosynthesis of silver and gold nanoparticles by novel sundried Cinnanonum camphora leaf. Nanotechnology. 2007;18:105104.
- Vankar PS, Bajpai D. Preparation of gold nanoparticles from Mirabilis jalapa flowers. Indian J Biochem Biophys. 2010;47:157–160.
- Mishra AN, Bhadauria S, Gaur MS, et al. Synthesis of gold nanoparticles by leaves of zero-calorie sweetener herb (Stevia rebaudiana) and their nanoscopic characterization by spectroscopy and microscopy. Int J Green Nanotechnol Phys Chem. 2010;1:P118–P124.
- Jaiswal D, Rai PK, Watal G. Antidiabetic effect of Withania coagulans in experimental plants. Indian J Clin Biochem. 2009;24:88–93.
- Kamel ZH, Daw I, Marzouk M. Effect of Cichorium endivia leaves on some biochemical parameters in streptozotocin-induced diabetic rats. Aus J Basic App Sci. 2011;5:387–396.
- Pandhare RB, Sangameswaran B, Mohite PB, et al. Antidiabetic activity of aqueous leaves extract of Sesbania sesban (L) Merr. in streptozotocin-induced diabetic rats. Avicenna J Med Biotechnol. 2011;3:37–43.
- Das H, Chakraborty U. Anti-hyperglycemic effect of Scoparia dulcis in streptozotocin-induced diabetes. Res J Pharm Biol Chem Sci. 2011;2:334–342.
- Adiga S, Bairy KL, Meharban A, et al. Hypoglycemic effect of aqueous extract of Trichosanthes dioica in normal and diabetic rats. Int J Diab Dev Ctries. 2010;30:38–42.
- Sivaraj A, Devi K, Palani S, et al. Anti-hyperglycemic and Anti-hyperlipidemic effect of combined plant extract of Cassia auriculata and Aegle marmelos in streptozotocin (STZ) induced diabetic albino rats. Int J Pharmatech Research. 2009;1:1010–1016.
- Buckingham GR. Biological control of alligator weed, Alternanthera philoxeroides, the world's first aquatic weed success story. Castanea. 1996;61:232–243.
- Julien MH, Skarratt B, Maywald GF. Potential geographical distribution of alligator weed and its biological control by Agasicles hygrophila. J Aquat Plant Manage. 1995;33:55–60.
- Bhattacherjee A, Ghosh T, Sil R, et al. Isolation and characterization of methanol soluble fraction of Alternanthera philoxeroides (Mart.) – evaluation of their antioxidant, α-glucosidase inhibitory and antimicrobial activity in in vitro systems. Nat Prod Res. 2014;28:2199–2202.
- Bhattacherjee A., Chakraborti AS. Inhibitory effect of Piper betle Linn. leaf extract on protein glycation – quantification and characterization of the antiglycation components. Ind J Biochem Biophys. 2013;50:529–536.
- Turkevich J, Stevenson PL, Hillier J. Nucle-ation and growth process in the synthesis of colloidal gold. Discuss Faraday Soc. 1951;11:55.
- Bhattacherjee A, Chakraborti AS. Fructose-induced modifications of myoglobin: change of structure from met (Fe 3+) to oxy (Fe 2+) form. Int J Biol Macromol. 2011;48:202–209.
- Bhattacherjee A, Dhara K, Chakraborti AS. Argpyrimidine-tagged rutin-encapsulated biocompatible (ethylene glycol dimers) nanoparticles: synthesis, characterization and evaluation for targeted drug delivery. Int J Pharm. 2016;509:507–517.
- Bhattacherjee A, Chakraborti AS. Argpyrimidine-tagged rutin-encapsulated biocompatible (ethylene glycol dimers) nanoparticles: application for targeted drug delivery in experimental diabetes (Part 2). Int J Pharm. 2017;528:8–17.
- Daisy P, Saipriya K. Biochemical analysis of Cassia fistula aqueous extract and phytochemically synthesized gold nanoparticles as hypoglycemic treatment for diabetes mellitus. Int J Nanomed. 2012;7:1189–1202.
- Ghosh T, Sarkar, P, Turner AP. A novel third generation uric acid biosensor using uricase electro-activated with ferrocene on a Nafion coated glassy carbon electrode. Bioelectrochemistry. 2015;102:1–9.
- Bhattacherjee A, Dhara K, Chakraborti AS. Bimolecular interaction of argpyrimidine (a Maillard reaction product) in in vitro non-enzymatic protein glycation model and its potential role as an antiglycating agent. Int J Biol Macromol. 2017;102:1274–1285.
- Ghosh T, Sarkar P. Isolation of a novel uric-acid-degrading microbe Comamonas sp. BT UA and rapid biosensing of uric acid from extracted uricase enzyme. J Biosci. 2014;39:805–819.
- Mishra A, Kumari M, Pandey S, et al. Biocatalytic and antimicrobial activities of gold nanoparticles synthesized by Trichoderma sp. Bioresour Technol. 2014;166:235–242.
- Case CL, Jhonson TR. Laboratory experiments in microbiology. Menlo Park (CA): Benjamin Cummings Pub. Inc; 1984. p. 126–129.
- Sathishkumar M, Sneha K, Yun YS. Immobilization of silver nanoparticles synthesized using Curcuma longa tuber powder and extract on cotton cloth for bactericidal activity. Bioresour Technol. 2010;101:7958–7965.
- Sastry M, Ahmed A, Khan MI, et al. Biosynthesis of metal nanoparticles using fungi and actinomycete. Curr Sci. 2003;85:162–170.
- Fierascu RC, Ion RM, Dumitriu I. Noble metal nanoparticle synthesis in plant extracts. Optoelectron Adv Mater Rapid Commun. 2010;4:1297–1300.
- Shankar SS, Ahmad A, Pasricha R, et al. Bioreduction of chloroaurate ions by geranium leaves and its endophytic fungus yields gold nanoparticles of different shapes. J Mater Chem. 2003;13:1822–1826.
- Agnieszka SK, Dagmara M, Mulgorzata Z, et al. Characterization of gold nanoparticles for various medical application. Digest J Nanostruct Biomater. 2011;6:803–808.
- He YQ, Liu SP, Kong L, et al. A study on the sizes and concentrations of gold nanoparticles by spectra of absorption, resonance Rayleigh scattering and resonance non-linear scattering. Spectrochim Acta A Mol Biomol Spectrosc. 2005;61:2861–2866.
- Parida UK, Bindhani BK, Nayak P. Green synthesis and characterization of gold nanoparticles using onion (Allium cepa) extract. World J Nano Sci Eng. 2011;1(4):93.
- Elavazhagan T, Arunachalam KD. Memecylon edule leaf extract-mediated green synthesis of silver and gold nanoparticles. Int J Nanomed. 2011;6:1265–1278.
