ABSTRACT
Highly dispersed poly(amic acid) salt-stabilised palladium nanoparticles(PdNPs/PAAS) were synthesised via a facile reduction strategy in aqueous media at room temperature and characterised by transmission electron microscopy, powder X-ray diffraction, X-ray photoelectron spectroscopy, Fourier transform infrared spectroscopy and inductively coupled plasma optical emission spectrometer. The nanoparticles were used as catalyst for Suzuki–Miyaura coupling reaction to synthesise biphenyl compounds in short reaction time and high yields under mild conditions, benefiting from highly efficient quasi-homogeneous catalytic activity. The catalyst could be recycled 5 times without obvious loss attributing to the pH response of the PAAS.
1. Introduction
In organic chemistry, palladium catalyst has long been regarded as a ‘universal catalyst’, especially for the reactions of C-C bond formation, among which Suzuki–Miyaura coupling reaction has invariably been chosen to test palladium catalytic systems and synthesise biaryl compounds [Citation1–6]. In the early stage, the Suzuki–Miyaura coupling reaction with high efficiency and selectivity was often performed in homogeneous systems using palladium-based complexes as the catalysts [Citation7,Citation8]. Unfortunately, homogeneous catalytic systems suffered from various disadvantages such as high operational costs, short life time and the difficulty of separation from the reaction mixture, which was detrimental to recyclability of the catalyst and posed a serious limitation to their industrial-scale applications [Citation7–9]. Until 1996, Reetz et al. reported heterogeneous Pd and Pd-Ni nanoparticles stabilised by four ammonium salts or polyvinylpyrrolidone for Suzuki–Miyaura coupling reaction for the first time [Citation10]. Since then, various stabilisers or carriers, such as Al2O3, SiO2, magnetic nanoparticles, carbonaceous materials, silicon oxide mesoporous materials, natural porous materials, mesoporous polymer (extruded starch) and other synthetic polymer (polyaniline nanofibres, polysilane, polyamidoamine dendrimers, etc.) have been used in the preparation of nano-palladium owing to their ease of removal from the reaction media [Citation11–19]. However, the reaction condition is rather harsh because of the difficulty of mass transfer in multiphase system [Citation4,Citation12,Citation20–22].
Nowadays, quasi-homogeneous ‘smart’ nanocatalyst combining the efficiency of a homogeneous catalyst and the durability of a heterogeneous catalyst, has been receiving significant attention [Citation23]. The performance of a Nano catalyst is dependent on both the size (dimension) and shape (morphology) based on metal Nano catalysts were extensively studied in fundamental research and involve practical applications in the catalyst industry [Citation24–27]. Besides, stimuli-responsive polymers are chosen as stabilisers or carriers to endow the catalysts with facile reusability by simple environmental changes [Citation28]. Thanks to strong cation-complexing ability and pH responsiveness, water soluble poly (amic acid) salt can be used as a novel polymer stabiliser [Citation29,Citation30]. In recent years, our group has been working on the synthesis various poly (amic acid) salts with different structures and the development of catalysts for useful new synthetic methodologies. Previously, we have explored the preparation process of silver nanoparticles and used them to catalyse the proper reaction [Citation23]. In this paper, we present synthesis of highly dispersed poly (amic acid) salt-stabilised palladium nanoparticles (PdNPs/PAAS) via reduction of sodium borohydride in aqueous media at room temperature [Citation23,Citation31,Citation32]. To our delight, these nanoparticles with uniform shape and small size showed good catalytic activity in Suzuki–Miyaura coupling reactions under mild conditions and were easily recycled after the reaction by regulating the pH of system.
2. Experimental
2.1. Materials and methods
Palladium chloride (PdCl2, 99.9%), phenylboronic acid (97%), bromobenzene (99%) were all purchased from J&K Scientific Ltd. Sodium borohydride (NaBH4), triethanolamine (TEA), ethanol (EtOH), potassium hydrate (KOH), sodium chloride (NaCl), sodium tetraborate decahydrate (Na2B4O7·10H2O) and any other reagents were obtained from Sinopharm Chemical Reagent Co., Ltd. All reagents were of analytical grade or better without further purification. Deionised water was used throughout the experiments.
The morphology and size of the palladium nanoparticles were characterised by a transmission electron microscope (TEM, model G2 60–300). Placing drops of PdNPs/PAAS solution on a carbon-coated copper grid and drying them under the infrared light could obtain test sample. Element states of the catalyst were acquired by X-ray photoelectron spectroscope (XPS, model ESCALAB 250Xi) and powder X-ray diffraction (XRD) patterns were collected using a Japan Rigaku X-Ray diffractometer (D/max 2500/PC). The XPS sample was prepared by precipitating the PdNPs in an acidic solution (pH < 3), followed by washing the precipitate with water and acetone at least once, and finally drying it in a vacuum at room temperature for 2 h. Moreover, the XRD sample was prepared under the same conditions. Fourier transform infrared spectroscopy (FTIR) was used to characterise the structure of poly (amic acid) and analyse the interaction between functional groups and palladium. Catalytic yield was analysed by gas chromatography (GC-FID) carried out using a Shimadzu gas chromatograph (GC-2010) equipped with a 30-m capillary column using N2 as the carrier. Mass spectrometry was used to determine the products by a Shimadzu gas chromatograph mass spectrometer (GCMS-QP2010 Plus). The recovery of the catalyst was determined by evaluating the actual content of the metallic elements by inductively coupled plasma optical emission spectrometer (ICAP-6300).
2.2. Catalyst preparation
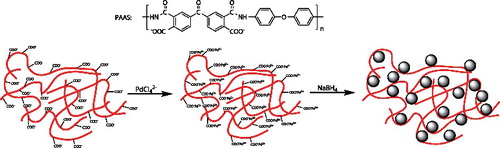
Poly(amic acid) (PAA) was synthesised by the polycondensation of 4,4′-oxydianiline (ODA) and 3,3′,4,4′-benzophenonetetracarboxylic dianhydride (BTDA) in DMF (15 wt% solid, 1.65 dL g−1 inherent viscosity at 25 °C). Mixing the same molar equivalents of TEA and PAA afforded water-soluble PAAS. Sodium tetrachloropalladate (Na2PdCl4) was obtained by mixing palladium chloride (PdCl2) and two molar equivalents of NaCl in water. The experiment procedure for the synthesis of PdNPs/PAAS is as follows: First, the aqueous solution of Na2PdCl4 (20 mM, 5 mL) and PAAS (10 mM, 10 mL) were added into Na2B4O7·10H2O solution (10 mM, pH = 9.18, 77.5 mL) under stirring of 800 rpm for 15 min at room temperature. Next, NaBH4 (100 mM, 7.5 mL) was rapidly injected, and the solution became dark. To ensure the complete reduction of palladium ions, the solution should be stirred for 2 h. The formation of palladium nanoparticles in solution contained two processes, as shown in . First, a complexing reaction between the carboxylate anions (-COO−) and palladium chloride ions (PdCl42−) to generate self-assembled Pd(II)/PAA complexes ensured Pd ions loaded into PAA chains in aqueous solution. Subsequently, palladium was converted into zero-valent Pd by fast reduction of NaBH4, then nucleated and grew into PdNPs in situ. The obtained PdNPs/PAAS solution was stored in a refrigerator at 4 °C for further use.
2.3. General procedure for Suzuki–Miyaura coupling reaction
For Suzuki–Miyaura reaction, 0.6 mmol of arylboronic acid, KOH aqueous solution (1 M, 1 mL), appropriate amount of the catalyst (1 mM, 5 mL) and pure ethanol (3 mL) were mixed (EtOH/H2O: v/v = 1/2) and stirred at desired temperature. After 3 min of stirring, 0.5 mmol of aryl halide was added. The process of the reaction was monitored by regularly aliquoting 0.5 mL of samples. After extraction with ether (3 × 2 mL), the samples were analysed by GC. In most cases, the reaction mixture was further analysed by GC-MS when reaction completed and extracted. To investigate the cycling performance, the catalyst acidified by hydrochloric acid (HCl, 5 mol/L) was first centrifuged, and then washed with ether and deionised water to remove organic or inorganic impurities. After that, the solid catalyst was mixed with Na2B4O7·10H2O solution (10 mM, pH = 9.18, 5 mL), resulting in complete redispersion of the PdNPs/PAAS precipitate under stirring of 800 rpm for 30 min. This ensured that the pH of catalyst was consistent during the 5 times recovery process.
3. Results and discussion
3.1. Morphology and characterisation of catalysts
The TEM images in (a,b) clearly show that PdNPs are highly dispersed. The particles are usually elliptical or spherical in nature with a diameter of 4.6 ± 2.4 nm. This was due to the alkaline environment of the Na2B4O7·10H2O solution (10 mM, pH = 9.18), which made the carboxyl group of PAAS completely dissociate to the carboxylate ions. Besides, the nucleation and growth of Pd nanoparticles occurred in the polymer chains. Repulsion of the same charge and steric hindrance of polymer chains avoided agglomeration of nanoparticles, which guarantied the small size of nanoparticles, in addition to the rapid reduction by NaBH4.
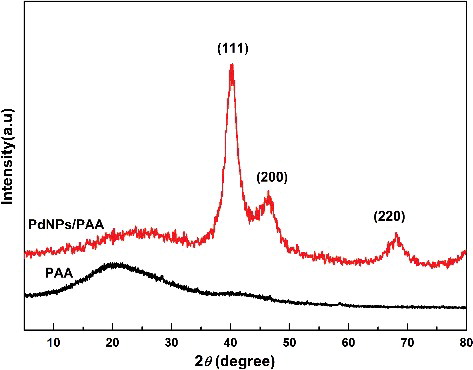
The XRD patterns in further prove the existence of palladium nanocrystals. Broadening of diffraction peaks showed the small size of the particles. In addition, the size of grain determined by Scherrer equation was 3.7 nm, which matched with the TEM results. The wide and intense peak at around 20.1° is due to polymer of PAA. When the preparation was complete, three additional peaks appeared at around 40.1°, 46.4° and 68.0° attributing to the (111), (200) and (220) crystal planes of face centred cubic (fcc) crystalline palladium appeared, indicating the reduction of Pd2+ to Pd0.
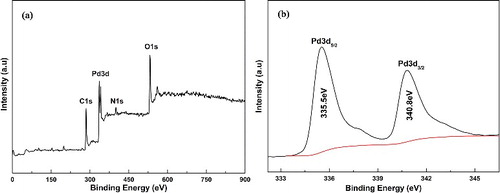
To confirm the status of palladium in PAA, catalyst was characterised by XPS, one of powerful surface analysis technologies. The XPS survey spectrum of the catalyst in (a) confirmed the presence of Pd, C, N and O. The peaks centred at the binding energies of 284.8, 287.6 and 289.2 eV correspond to the sp2 carbon, C–O, C=O species. In addition, O1s at 532.1 and 533.0 eV might also suggest the presence of C–O, C=O species, the N1s spectra of amides for the peaks centred at 400.1 eV. As shown in (b), the Pd 3d spectrum could be divided into two groups. The characteristic peaks at 335.5 and 340.8 eV represented the electron binding energy of metallic Pd, whereas the oxidation state of Pd(II) corresponds to the peaks at 337.8 and 343.2 eV, proving that almost all palladium existed in zero-valent state.
Figure 3. (a) Global XPS spectrum of the PdNPs/PAAS catalyst; (b) high-resolution XPS spectra of Pd 3d.
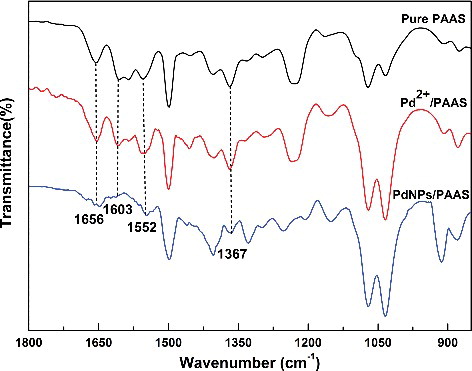
Palladium nanoparticles could be dispersed uniformly and stably in solution because of sustaining of PAAS, a polymer chain with a considerable steric hindrance and functional groups possessing strong complexing ability. Then, pure PAAS, Pd2+/PAAS and PdNPs/PAAS were analysed by Fourier transform infrared spectroscopy, as shown in , as many functional groups of PAAS have obvious characteristic infrared absorption. The FTIR spectrum of the PAAS has two anti-symmetric and symmetric absorption peaks for carboxylate anion (−COO−) at 1603 and 1367 cm−1, respectively. In addition, two characteristic bands at 1656 and 1552 cm−1 attributed to the C=O and N–H, respectively, and are regarded as an evidence for the existence of secondary amide (−CONH−). When the PdNPs were obtained, the intensity of many peaks, especially at 1603 cm−1, decreased, relating to the complexing between the carboxylate anions (−COO−) and PdNPs. This indicates that carboxylate anions played an important role in the formation of nanoparticles.
3.2. PdNPs/PAAS catalysed Suzuki–Miyaura coupling reactions
The applications of PdNPs/PAAS were explored for the synthesis of biphenyl compounds by Suzuki–Miyaura coupling reactions. Bromobenzene and phenylboric acid were chosen as model substrates to investigate the catalytic performance of the composites for Suzuki reaction. Subsequently, a series of experiments were carried out. The reaction did not proceed at 40 °C without catalyst used even after 3 h, the same situation to the reaction in blank PAAS and Na2B4O7·10H2O solution, ensuring that the reaction was not catalysed by PAAS or Na2B4O7·10H2O. However, when the supported PdNPs/PAAS were used as the catalyst, the desired products were detected within 0.5 h, indicating that Pd participated in the reaction.
To determine optimum reaction conditions, the effects of temperature, base, and solvent parameters on the coupling reaction yield were investigated, and results are listed in . It was obvious that the yield increased inconspicuously (>98%) within 0.5 h with increasing temperature (entries 1–4) in range of 40–80 °C. Besides, the reaction could still be carried out even at 25 °C. However, high yield might be achieved for longer time. Hence, 40 °C was chosen as optimum temperature to obtain a decent yield of products in a short time under mild condition. The choice of the base and solvent was based on the reports available in the literature. Common inorganic bases such as NaOH, KOH, Na2CO3 and K2CO3 were employed in Suzuki–Miyaura coupling reactions under the same conditions (entries 2, 5–7). Among these bases, Na2CO3, K2CO3 and KOH were found to be efficient, affording >90% yield of biphenyl, but NaOH only gave 23% yield. In contrast, KOH, affording the highest yield, was the best choice. Appropriate solvents could be used to optimise the mass transfer efficiency. Even if time was extended, the reaction still exhibited low yield when water was chosen as a solvent (entry 8). Fortunately, protic solvents ethanol and methanol afforded significantly higher yield, and the addition of aprotic solvents such as N, N-dimethylformamide (DMF) also increased the yield (entries 2, 12, 13). In addition, the proportion of water and organic solvents had a great impact on the reaction outcome. The experimental results show that the yield increased tremendously with the increase of organic solvent ratio (entries 2, 9–11), especially the ethanol/water system (v/v = 1/2 and 1/1, entries 2 and 11) afforded yield >98%. In view of the cost and availability in this study, the ethanol/water (v/v = 1/2) was selected as the most suitable solvents for the catalytic system.
Table 1. Optimisation of reaction conditions for the PAAS-stabilised PdNPs catalysed Suzuki–Miyaura coupling.
Subsequently, the catalytic scope of PdNPs/PAAS catalyst was examined by using various substrates under optimised conditions listed in . The biphenyl yields of different halobenzenes were diverse owing to the difference in electronegativity of halogen elements (entries 1–3, 16), the order of which was Cl (3.16) > Br (2.96) > I (2.66). Reaction proceeded smoothly even in pure water when iodobenzene was chosen. On the contrary, inert chlorobenzene whose strength of carbon-halogen bond was stronger required higher temperature and longer time to achieve the coequal yield. As for the substituted bromobenzene or phenylboronic acid, electron-donating 4-OCH3, 4-CH3, 3-CH3, and 2-CH3 gave decent yields during 1.5 h; whereas electron-withdrawing 4-F, 4-Cl, and 4-NO2 groups especially F and Cl decreased the reaction time (entries 4–15). Generally speaking, the reaction is not only relative to the electronic effect, but also sensitive to the steric effect. Fortunately, the results indicated that reaction rate and yield were almost the same by choosing 4-CH3, 3-CH3, and 2-CH3 substituted phenylboronic acids as substrates (entries 7–9), indicating the steric hindrance was not obvious due to the small size of the methyl group. To sum up, both electron-rich substituted and electron-deficient substituted substrates were efficiently coupled using PdNPs/PAAS catalyst, affording the desired products in excellent yields. To the best of our knowledge, this is of great value for Pd-mediated Suzuki–Miyaura coupling reactions at such a low temperature. Comparing the results of the coupling of bromobenzene with phenylboronic acid with the previous reported procedure in the literature, our catalyst showed shorter reaction time and high reaction yield than the others as listed in .
Table 2. Generality of PdNPs/PAAS catalyst in Suzuki–Miyaura coupling involving different substrates under stated reaction conditions.
Table 3. Suzuki reaction catalysed by various palladium catalysts.
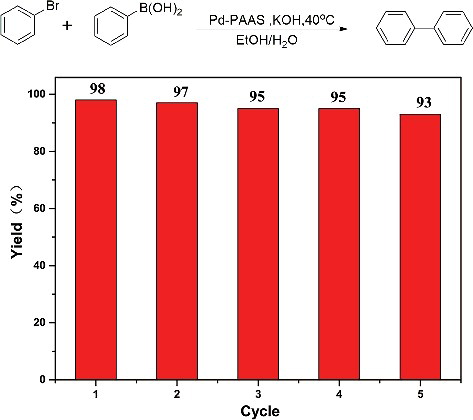
In addition to the activity, stability is also an important property for the catalyst. Because the pH-induced precipitation–redispersion process of PdNPs/PAAS is highly reversible, catalyst could be easily separated by adjusting the pH of solution below 3. Then dark precipitate of PdNPs/PAAS was collected and redissolved in Na2B4O7·10H2O solution (10 mM, pH = 9.18, 5 mL), resulting in complete redispersion under stirring of 800 rpm for 30 min. To evaluate the recovery efficiency of catalyst, ICP-OES analysis was used to determine the content of Pd. The concentration of Pd in the fresh catalyst was 106.5 mg/L (1 mM), whereas 104.4 mg/L (0.98 mM) in the first recycled catalyst. Such an extremely low loss of the catalyst during recycling indicates that the pH regulation was a simple and effective method. Subsequently, the synthesis of biphenyl from bromobenzene and phenylboric acid under the optimised conditions was used to assess the activity of the recycled catalysts. As shown in , the PdNPs/PAAS was repeatedly reused five times with no significant loss in the activity.
4. Conclusions
In summary, a novel quasi-homogeneous poly (amic acid) salt-stabilised palladium nanocatalyst has been developed via a facile reduction approach. TEM observation clearly reveals that palladium nanoparticles are highly dispersed and usually elliptical or spherical in nature with a diameter of 4.6 ± 2.4 nm. The PdNPs/PAAS catalyst here combines the advantages of the high efficiency, easy recovery and reuse, attributing Suzuki–Miyaura coupling reaction to proceed smoothly under such mild conditions. Moreover, such an extremely low loss of the catalyst during recycling and shirtsleeve operation will provide a prospect for large-scale industrial application.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Shah D, Kaur H. Supported palladium nanoparticles: a general sustainable catalyst for microwave enhanced carbon-carbon coupling reactions. J Mol Catal A: Chem. 2016;424:171–180.
- Sharma V, Kumar S, Bahuguna A, et al. Plant leaves as natural green scaffolds for palladium catalyzed Suzuki-Miyaura coupling reactions. Bioinspir Biomim. 2017;12(1):016010.
- Chawla M, Kumar R, Siril PF. High catalytic activities of palladium nanowires synthesized using liquid crystal templating approach. J Mol Catal A: Chem. 2016;423:126–134.
- Sahu D, Silva AR, Das P. Facile synthesis of palladium nanoparticles supported on silica: an efficient phosphirie-free heterogeneous catalyst for Suzuki coupling in aqueous media. Catal Commun. 2016;86:32–35.
- Kumar BS, Amali AJ, Pitchumani K. Cubical palladium nanoparticles on C@Fe3O4 for nitro reduction, Suzuki-Miyaura coupling and sequential reactions. J Mol Catal A: Chem. 2016;423:511–519.
- Xia HW, Fu YS, He GY, et al. Core-shell-like Ni-Pd nanoparticles supported on carbon black as a magnetically separable catalyst for green Suzuki-Miyaura coupling reactions. Appl Catal B. 2017;200:39–46.
- Fihri A, Bouhrara M, Nekoueishahraki B, et al. Nanocatalysts for Suzuki cross-coupling reactions. Chem Soc Rev. 2011;40(10):5181–5203.
- Arpad M. Efficient, selective, and recyclable palladium catalysts in carbon-carbon coupling reactions. Chem Rev. 2011;111(3):2251–2320.
- Puthiaraj P, Ahn WS. Highly active palladium nanoparticles immobilized on NH2-MIL-125 as efficient and recyclable catalysts for Suzuki-Miyaura cross coupling reaction. Catal Commun. 2015;65:91–95.
- Reetz MT, Breinbauer R, Wanninger K. Suzuki and Heck reactions catalyzed by preformed palladium clusters and palladium/nickel bimetallic clusters [Article]. Tetrahedron Lett. 1996;37(26):4499–4502.
- Gniewek A, Ziolkowski JJ, Trzeciak AM, et al. Palladium nanoparticles supported on alumina-based oxides as heterogeneous catalysts of the Suzuki-Miyaura reaction. J Catal. 2008;254(1):121–130.
- Yu DD, Bai J, Wang JZ, et al. Assembling formation of highly dispersed Pd nanoparticles supported 1D carbon fiber electrospun with excellent catalytic active and recyclable performance for Suzuki reaction. Appl Surf Sci. 2017;399:185–191.
- Hossain AMS, Balbin A, Erami RS, et al. Synthesis and study of the catalytic applications in C–C coupling reactions of hybrid nanosystems based on alumina and palladium nanoparticles. Inorg Chim Acta. 2017;455:645–652.
- Das DD, Sayari A. Applications of pore-expanded mesoporous silica 6. Novel synthesis of monodispersed supported palladium nanoparticles and their catalytic activity for Suzuki reaction. J Catal. 2007;246(1):60–65.
- Budarin VL, Clark JH, Luque R, et al. Palladium nanoparticles on polysaccharide-derived mesoporous materials and their catalytic performance in C–C coupling reactions. Green Chem. 2008;10(4):382–387.
- Zhang ZH, Wang ZY. Diatomite-supported Pd nanoparticles: an efficient catalyst for Heck and Suzuki reactions. J Org Chem. 2006;71(19):7485–7487.
- Makhubela BCE, Jardine A, Smith GS. Pd nanosized particles supported on chitosan and 6-deoxy-6-amino chitosan as recyclable catalysts for Suzuki-Miyaura and Heck cross-coupling reactions. Appl Catal A. 2011;393(1–2):231–241.
- Nasrollahzadeh M, Sajadi SM, Rostami-Vartooni A, et al. Green synthesis of Pd/Fe3O4 nanoparticles using Euphorbia condylocarpa M. bieb root extract and their catalytic applications as magnetically recoverable and stable recyclable catalysts for the phosphine-free Sonogashira and Suzuki coupling reactions. J Mol Catal A: Chem. 2015;396:31–39.
- Erathodiyil N, Ooi S, Seayad AM, et al. Palladium nanoclusters supported on propylurea-modified siliceous mesocellular foam for coupling and hydrogenation reactions. Chem Eur J. 2008;14(10):3118–3125.
- Luque R, Budarin V, Clark JH, et al. Glycerol transformations on polysaccharide derived mesoporous materials. Appl Catal B. 2008;82(3–4):157–162.
- Cuenca T, Filice M, Palomo JM. Palladium nanoparticles enzyme aggregate (PANEA) as efficient catalyst for Suzuki-Miyaura reaction in aqueous media. Enzyme Microb Technol. 2016;95:242–247.
- Ye YX, Liu WL, Ye BH. A highly efficient and recyclable Pd(II) metallogel catalyst: a new scaffold for Suzuki-Miyaura coupling. Catal Commun. 2017;89:100–105.
- Li J, Tang GN, Wang YC, et al. Poly(amic acid) salt-stabilized silver nanoparticles as efficient and recyclable quasi-homogeneous catalysts for the aqueous hydration of nitriles to amides. New J Chem. 2016;40(1):358–364.
- Zhang QH, Deng WP, Wang Y. Effect of size of catalytically active phases in the dehydrogenation of alcohols and the challenging selective oxidation of hydrocarbons. Chem Commun. 2011;47(33):9275–9292.
- Zhang JF, Feng C, Deng YD, et al. Shape-controlled synthesis of palladium single-crystalline nanoparticles: the effect of HCl oxidative etching and facet-dependent catalytic properties. Chem Mater. 2014;26(2):1213–1218.
- Kundu S, Lau S, Liang H. Shape-controlled catalysis by cetyltrimethylammonium bromide terminated gold nanospheres, nanorods, and nanoprisms. J Phys Chem C. 2009;113(13):5150–5156.
- Laskar M, Skrabalak SE. Decoupling the geometric parameters of shape-controlled Pd nanocatalysts. ACS Catal. 2014;4(4):1120–1128.
- Baran NY, Baran T, Mentes A. Fabrication and application of cellulose Schiff base supported Pd(II) catalyst for fast and simple synthesis of biaryls via Suzuki coupling reaction. Appl Catal A. 2017;531:36–44.
- Qi SL, Shen XY, Lin ZW, et al. Synthesis of silver nanocubes with controlled size using water-soluble poly(amic acid) salt as the intermediate via a novel ion-exchange self-assembly technique. Nanoscale. 2013;5(24):12132–12135.
- Zhang L, Wu JT, Sun N, et al. A novel self-healing poly(amic acid) ammonium salt hydrogel with temperature-responsivity and robust mechanical properties. J Mater Chem A. 2014;2(21):7666–7668.
- Le XD, Dong ZP, Liu YS, et al. Palladium nanoparticles immobilized on core-shell magnetic fibers as a highly efficient and recyclable heterogeneous catalyst for the reduction of 4-nitrophenol and Suzuki coupling reactions. J Mater Chem A. 2014;2(46):19696–19706.
- Nabid MR, Bide Y. H40-PCL-PEG unimolecular micelles both as anchoring sites for palladium nanoparticles and micellar catalyst for Heck reaction in water. Appl Catal A. 2014;469:183–190.