?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
A number of strontium-based iron oxides are available for the industrial use. Among them, strontium hexaferrite cobalt iron oxide (Sr2Co2Fe12O22) is preferred over others due to its better magnetic and electrical properties than others. This material is also extensively used for the absorption of microwave radiations. This study was conducted to account for the synthesis and characterization of Sr2Co2Fe12O22 nanoparticles and to report their biocompatibility in albino mice. Nanoparticles were synthesised by normal microemulsion, XRD analysis confirmed the single and scanning electron microscopy (SEM) revealed average particle size ranged between 30 and 50 nm. Nine-week-old male mice were intraperitoneally administered with 50 mg/mL of solvent/kg body weight of strontium hexaferrite cobalt iron oxide nanoparticles for 22 days. Control group was maintained in parallel. A series of neurological tests (rota rod, light and dark box, open field and Morris water maze) were conducted in both groups. Blood samples were collected from direct cardiac puncture, and parameters of complete blood count, serum biochemical parameters and antioxidant were determined in liver and brain tissues of all subjects. Analysis of result revealed that all studied neurological test performances varied nonsignificantly (P > 0.05) between the two treatments except clockwise rotations during open field test that were significantly reduced (P = 0.05) in Strontium hexaferrite Cobalt Iron Oxide nanoparticle-treated male albino mice than in the control group. All studied complete blood count and serum parameters varied nonsignificantly (P > 0.05) between two treatments. It was observed that superoxide dismutase concentration was significantly higher (P = 0.05) in the liver of nanoparticle-treated male mice. In conclusion, we are reporting that applied dose of strontium hexaferrite cobalt iron oxide nanoparticles is affecting the exploratory behaviour and antioxidant metabolites of male albino mice.
1. Introduction
Compounds having iron oxide as their major component are called ferrites and are generally used as insulating ferromagnetic ceramic compounds due to their unique properties such as electrical sensitivity, corrosion resistance and chemical and mechanical stabilities [Citation1,Citation2]. Their magnetic properties are attributed to their hexagonal crystal structure [Citation3,Citation4]. Strontium hexaferrite cobalt iron oxide (Sr2Co2Fe12O22) nanoparticles belong to Y-type ferrites [Citation5]. The high electrical resistivity, decrease in coercivity and increase in saturation magnetization are the prosperities due to which these materials are used in manufacturing microwave, recording devices [Citation6] telecommunications, magneto-optical and permanent magnets [Citation7]. Hexaferrite nanoparticles have capability to bind with proteins, antibody, enzymes or drugs and targeted to tumour or tissues in the presence of external magnetic field and are used in cancer therapy [Citation8]. Despite their extensive utilization, limited information is available in literature regarding the biocompatibility of strontium hexaferrite cobalt iron oxide. This study was designed to account for the synthesis and characterization of strontium hexaferrite cobalt iron oxide nanoparticles and to report their effects on behaviour, blood chemistry and antioxidant metabolites of liver and brain of albino mice in a gender-specific manner.
2. Materials and methodology
2.1. Synthesis of strontium hexaferrite cobalt iron oxide nanoparticles
The regents used for the synthesis of Y-type hexaferrite were Fe(NO3)3·9H2O (97%; Riedel-de Haen, China), Co(NO3)2·6H2O (>99%; Merck, USA), Sr(NO3)2 (99%; Merck), cetyltrimethyl ammonium bromide (CTAB) (97%; Merck) as a surfactant, NH3 (35%; Fisher Scientific) as a precipitating agent and methanol (99%; Merck) as a washing agent. The Y-type hexaferrite sample with nominal composition Sr2Co2Fe12O22 was prepared by the normal microemulsion method. The required amounts of metal salts i.e. iron nitrate, cobalt nitrate and strontium nitrate were dissolved in the deionised water to form the solution of required molarities and mixed in a baker. CTAB was also added in metals solution in 1:15 ratio (metals:CTAB). The solution was stirred on the magnetic hot plate by maintaining the temperature at 333 K. The ammonia solution (2 M) was added drop wise to form the precipitates. After that, these precipitates were washed with deionised water until the pH of the mixture became neutral and, finally, the precipitates were washed with methanol to remove the water insoluble impurities. The precipitates were then dried in an oven at 373 K. Final annealing was carried out at 1323 K for 8 h, using box furnace (Heyaius, Hanau, Germany).
2.2. Characterization of strontium hexaferrite cobalt iron oxide
The crystalline phase of the synthesised material was investigated by using X-ray diffractometer (X’pert PRO PAN Analytical Diffractometer) which uses CuKα as a source of radiation (λ = 1.54056 Å), while the CuKβ was filtered using Ni filter. The elemental composition of the synthesize Y-type hexaferrite was carried out by the energy dispersive X-ray (EDX) analysis. The surface morphology was done by scanning electron microscopy (SEM). The crystallites’ sizes and lattice parameters (lattice constants and cell volume) were calculated from XRD data. The average crystallite size (D) was estimated by using the following Scherer’s formula:
(1)
(1)
where λ represents the wave length of incident X-rays, β stands for the full width half maxima, θ is the Bragg angle and the value of shape factor K is 0.89 for hexaferrites’ materials.
The lattice constants (a and c) and the cell volume (Vcell) were calculated by using the following relations:
(2)
(2)
(3)
(3)
where d is the spacing between the two consecutive planes and hkl are the Miller Indices.
2.3. Experimental animals and design
Adult albino mice (9-week old) were used as experimental animals. Mice were kept individually in cages and provided with standard mice diet and water ad libitum. Room temperature was maintained at 22 ± 1 °C. The room was illuminated with artificial light from 8 am to 6 pm. All the experimental protocol and animal handling procedures were approved by the Ethical Committee of Institute of Pure and Applied Biology at Bahauddin Zakariya University Multan. Mice of both genders were divided into two groups. Treated group (N = 8 for male and female mice) received 50 mg/kg body weight of strontium hexaferrite cobalt iron oxide nanoparticles for 22 days through intraperitoneal injection, while control mice (N = 8 for male and female mice) intraperitoneally received saline (0.9% NaCl) solution for 22 days. A series of neurological tests such as rota rod, light and dark, open field and Morris water maize were carried out after 14 days of dose supplementation in both experimental treatments. Dose was supplemented 30 min before the conduction of each test. Throughout the experiment, the changes in weight for each mouse were recorded to demonstrate the effect of experiment material on body weight of each mouse.
2.4. Rota rod test
Rota rod apparatus is used to test the balance and neuromuscular coordination of an animal. Rota rod test was performed by using a locally manufactured apparatus comprising of rotating drum with the acceleration of 40 rpm. During experimentation, each mouse received three training trials followed by three experimental trials. Mean time spent on rotating drum was compared between the control and strontium hexaferrite cobalt iron oxide treated groups following Allahyar et al. [Citation9]
2.5. Open field test
Open field test is used to assess locomotory and exploratory behaviours of an animal Iqbal et al. [Citation10] A computational tracking system, ANY-maze (Stoeling, Wood Dale, IL, USA) connected with video camera (XPod-058, Shanghai, China), was used to detect the behaviour of mice in the open field chamber (40 cm × 40 cm × 70 cm). Each mouse was released in the corner of the open field box for 10 min of test duration. Maximum speed (m), mean speed (m/s), time mobile and time immobile (s), mobile episodes, immobile episodes and rotations (clockwise and anticlockwise) were noted following Allahyar et al. [Citation9]
2.6. Light/dark box test
The light/dark box test equipment was having an area of (45 cm × 27 cm × 27 cm) made up of plywood and consisted of one-third dark safe chamber (18 cm × 27 cm) and two-third light aversive chamber (27 cm × 27 cm) with the light intensity of 200 W. The two chambers were connected by an opening (7.5 cm × 7.5 cm) located in the center of the dividing wall adjacent to floor. The floor was divided into 9 cm × 9 cm squares and was covered with Plexiglas. A mouse was placed in the centre of light chamber keeping its snout towards opening in the wall. Time spent in each chamber, transition frequency, rearing, stretch attended, defecation and urination were counted over a 5 min test following Zahra et al. [Citation11]
2.7. Morris water maze
Morris water maze (MWM) consists of a circular pool of a diameter of 122 cm and a depth of 76 cm. In the pool, mice were trained to swim to find a platform concealed (1.5 cm) under water surface. In order to identify the position of concealed platform, distal extra-maze cues of different colors and dimensions were attached to the room walls. During the whole experiment, both colors and dimension of visual cues were kept constant. Water temperature was maintained at 21 ± 1 °C. The circular pool was partitioned into four quadrants (compass locations: NE, NW, SW and SE) by a computerised tracking/image analyzer system (video camcorder Addlink Software; Scientific, Barcelona, Spain) coupled to computational tracking system, ANY-maze. The middle of the south-east quadrant was selected as a place for concealed platform where it remained during the whole experiment.
In the spatial acquisition phase, mice were subjected to 16 training trials: four training trials per day and four training days with an intertrial interval of 30 min. Mice were released (randomly) in the circular pool from the four compass locations for swimming. For 120 s, mice were allowed to search the platform by swimming. Then, the mice were allowed to stay on the platform for another 30 s after failure of subject to find the concealed platform within 120 s.
In acclimatization training session of first training day in the MWM, each mouse was manually placed on the platform and was subsequently guided back to the platform after the swimming of 30 s. Parameters such as average time, distance travelled to reach the hidden platform, time immobile during the trial, time mobile and mean speed were recorded. After the abovementioned phase of acquisition, each mouse was subjected to probe trial. Probe trials were administered by the removal of platform. Animals were released from the north start point to swim freely for 60 s. The mouse swimming path was tracked and analysed for the proportion of swimming time and/or path length spent to reach platform, time mobile, rest time during the trail, total latency, mean visit to platform and average speed as followed by Kipnis et al. [Citation12].
2.8. Blood and serum collection
Following the intraperitoneal injections for 22 days, mice were anaesthetised and blood was collected either from retro-orbital sinus or through direct cardiac puncture and divided into two parts. One part was used for the study of complete blood count parameters by using haematology analyzer SYSMEX, 21 (Japan), while the second part was centrifuged at 13000 rpm for 10 min, and the extracted serum was used for the estimation of cholesterol, creatinine, low-density lipoprotein, high-density lipoprotein and triglycerides by using diagnostic kits.
2.9. Determination of antioxidant metabolites in liver and brain
Following sacrifice, liver and brain were surgically removed, rinsed in isotonic saline solution and stored immediately at –20 °C. In both organs, concentrations of superoxide dismutase were calculated following Chidambara et al. [Citation13], estimation of lipid peroxidation was carried out as described by Haider et al. [Citation14] and catalase activity was determined following Lateef and Qureshi [Citation15].
2.10. Statistical analysis
Statistical package Minitab (version 16; PA, USA) was used for data analysis. All data were expressed as mean ± standard error of mean. Significance level was set at P < 0.05. Two sample Student’s t-test was applied to compare all studied parameters of behaviour, complete blood count, serum and antioxidant profile between strontium hexaferrite cobalt iron oxide nanoparticle-treated and untreated albino mice of both genders.
3. Results
3.1. Structural analysis of strontium hexaferrite cobalt iron oxide nanoparticles
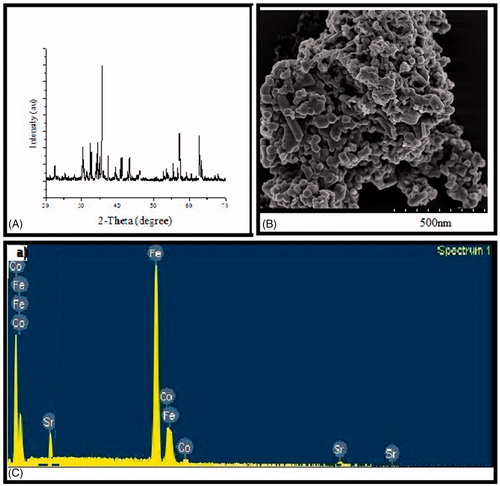
The XRD pattern for the synthesised Y-type strontium hexaferrite cobalt iron oxide nanoparticles is shown in . All the peaks in the pattern were well matched with the standard pattern (ICSD-01-072-0750), which indicates that the material was crystallised into rhombohedral single phase. The peaks were appeared at 2θ = 22.6, 24.7, 28.5, 30.8, 31.9, 32.7, 33.7, 35.1, 35.6, 37.4, 39.3, 40.9, 42.1, 43.2, 45.9, 50.08, 52.7, 54.8, 56.02, 57.3, 59.02, 60.8, 62.4, 64.1, 64.4, 66.7 and 68.6°, which were related to the Miller indices 107, 0012, 0111, 110, 1013, 116, 0114, 021, 119, 1016, 1112, 0210, 2011, 1019, 2014, 128, 1211, 2113, 1214, 039, 0222, 2023, 1028, 0030, 226, 2026 and 2212, respectively. The calculated lattice parameters such as lattice constants (a and c) and cell volume were found to be 5.92 and 43.48 Å and 1319.6 Å3, respectively, which was also in agreement with the standard pattern indicating that the synthesised material was in pure single phase. The average crystallite size was also calculated by using EquationEquation (1)(1)
(1) , and it was found to be around 40 nm ().
3.2. Morphological and compositional analysis of strontium hexaferrite cobalt iron oxide nanoparticles
The SEM image of Sr2Co2Fe12O22 is shown in . The particles were in hexagonal plate-like shape with clear boundaries. The particle size calculated from SEM image was found to be 30–50 nm range, which is in good agreement with that of calculated from XRD data ().
The composition of the synthesize sample was determined by EDX, and the pattern is shown in . All the peaks in the pattern were related to the Fe, Co and Sr, which confirm that there was no other element in the materials. The experimental composition for Sr, Co and Fe was found to be 1.97, 1.93 and 11.91, respectively. This indicates that the theoretical and experimental compositions are in good agreement with each other.
3.3. Rota rod test
Rota rod test results indicated that mice injected with 50 mg/mL of solvent/kg body weight strontium hexaferrite cobalt iron oxide nanoparticles spent more time on rota rod apparatus than mice injected with saline water but difference in rota rod test performance did not reached statistical significance for both male (P = 0.7) and female (P = 0.4) albino mice.
3.4. Light/dark box test
Analysis of the data revealed that all the studied parameters of light/dark box test varied nonsignificantly (P > 0.05) when compared between strontium hexaferrite cobalt iron oxide nanoparticle-treated and saline water-treated albino mice ().
Table 1. Comparison of various studied parameters of light/dark box between 50 mg/mL of solvent/kg body weight strontium hexaferrite cobalt iron oxide (50 mg/mL of solvent/kg body weight) and saline treated adult albino mice.
3.5. Open field test
Results of open field test indicated that all studied parameters of open field test varied nonsignificantly when compared between strontium hexaferrite cobalt iron oxide nanoparticle- and saline-treated albino mice as compared to their respective control groups except clockwise rotations that were significantly reduced (P = 0.05) in strontium hexaferrite cobalt iron oxide nanoparticle-treated male albino mice than the control group ().
Table 2. Comparison of studied parameters of open field test between saline and 50 mg/mL of solvent/kg of body weight strontium hexaferrite cobalt iron oxide nanoparticles treated albino mice.
3.6. Morris water maze test
3.6.1. Acquisition phase test
Analysis of the results revealed that strontium hexaferrite cobalt iron oxide nanoparticle-treated male albino mice covered more distance on training days 2 (P = 0.03) and 4 (P = 0.04) to reach the platform area than saline-treated control group, while this parameter during training days 1 and 3 and all other studied parameters during the acquisition phase varied nonsignificantly (P > 0.05) when compared between two experimental treatments (data not shown here).
3.6.2. Probe trial
Analysis of results revealed that all studied parameters during probe trials varied nonsignificantly (P > 0.05) when compared between 50 mg/mL of solvent/kg body weight strontium hexaferrite cobalt iron oxide-treated and untreated albino mice (data not shown here).
3.7. Body weight analysis
Analysis of the data indicated that body weight varied nonsignificantly (P > 0.05) at all the studied time points (22 consecutive days) when compared between 50 mg/mL of solvent/kg body weight of strontium hexaferrite cobalt iron oxide nanoparticle-treated and untreated albino mice (data not shown here).
3.8. Effect of strontium hexaferrite cobalt iron oxide nanoparticles on the complete blood count of albino mice
Upon comparison of complete blood count parameters of albino mice treated with hexaferrite strontium cobalt iron oxide with saline-treated control, it was observed that all studied parameters varied nonsignificantly (P > 0.05) between the two experimental treatments for both genders ().
Table 3 Comparison of studied complete blood count parameters between Strontium Hexaferrite Cobalt Iron Oxide nanoparticles (50 mg/ml of solvent/Kg body weight) (N = 16) and saline treated albino mice (N = 16) of both gender. All values are expressed as mean ± standard error of mean. P value indicates the results of 2-sample t-tests calculated for each parameter.
3.9. Effect of strontium hexaferrite cobalt iron oxide nanoparticles on serum biochemical profile of albino mice
When the studied serum parameters were compared between strontium hexaferrite cobalt iron oxide nanoparticle-treated and untreated mice, it was observed that all the parameters varied nonsignificantly (P > 0.05) between the two treatments for both genders ().
Table 4 Comparison of various studied serum parameters between Strontium Hexaferrite Cobalt Iron Oxide nanoparticles (50 mg/ml of solvent/Kg body weight) (N = 16) and saline treated albino mice (N = 16) of both gender. All values are expressed as mean ± standard error of mean. P value indicates the results of 2-sample t-tests calculated for each parameter.
3.10. Effect of strontium hexaferrite cobalt iron oxide nanoparticles on antioxidants profile of albino mice
Data analysis revealed that superoxide dismutase concentration in liver was significantly higher (P = 0.005) in strontium hexaferrite cobalt iron oxide nanoparticle-treated mice than their control group. While all other studied parameters from liver and brain varied nonsignificantly (P > 0.05) when compared between the two treatment groups in both genders ().
Table 5. Comparison of studied antioxidant metabolites of liver and brain in albino mice treated with strontium hexaferrite cobalt iron oxide nanoparticles (50 mg/mL of solvent/kg body weight) (N = 16) with their saline-treated control mice (N = 16) of both gender
4. Discussion
Nanotechnology is the area of active research in these days due to its wide range of applications in medicine, environmental protection, industry and consumer products [Citation16]. Nanoparticles (NPs) can enter in human body by various routes, through ingestion, inhalation, injection and dermal penetration and can induce toxicity on environment and human either deliberately or accidently [Citation17]. NPs are small in size and have large surface area due to which they can easily cross barriers and enter in circulatory system, transported to organs and tissues lead to oxidative damage [Citation18]. Pathways by which nanoparticles’ uptake may occur include hemolysis and thrombogenicity. These pathways include numerous activities such as reduced number of blood cells, antimitotic properties and increasing the number of cells involved in the immune processes [Citation19]. They also lead to the formation of free radicals due to oxidative stress. These free radicals interact with lipids, proteins and nucleic acids changing the cell signalling and transcription [Citation20]. Despite the large scale industrial use of strontium hexaferrite cobalt iron oxide nanoparticle, limited information is available in literature regarding their biocompatibility in living systems.
It has been reported that the particles of smaller size have higher dissolution rate as compared to agglomerated (larger particles) in the gastric fluid and the animals are more sensitive to smaller particles as compared larger one [Citation21]. Internalization of magnetic nanoparticles strongly depends upon the size of the particles. After administration, larger particles with a diameter higher than 200 nm are easily sequestered by the spleen and eventually removed by the cells of the phagocyte system, resulting in decreased nanoparticles concentrations in blood. Small particles with diameters less than 10 nm are rapidly removed through extravasations and renal clearance. Particles with a diameter ranging from 10 to 100 nm are optimal for intravenous injection and have the most prolonged blood circulation times. These particles are small enough to evade the RES of the body as well as to penetrate small capillaries of the tissues and offer the most effective distribution in targeted tissues [Citation22]. In the present investigation, some of the particles agglomerate into larger particles as indicated by the SEM image (). The nonagglomerated (smaller) particles play a major toxic role due to their higher dissolution. During the present study, we applied the nonagglomerated, fine nanoparticles to demonstrate their biocompatibility in albino mice and our results indicated that the crystalline structure of nanoparticles was around 40 nm in size and nanoparticles of this size can be effectively distributed to blood capillaries [Citation21].
Analysis of neurological test data indicated that the studied parameters of rota rod, light/dark box and MWM test performance varied non significantly (P > 0.05) when compared between saline-treated and strontium hexaferrite cobalt iron oxide nanoparticle-treated albino mice of both genders indicating that either these nanoparticles are unable to cross the blood–brain barrier effectively or the receptors may not be present everywhere in the central nervous system. Our open field test results showed that all studied parameters varied nonsignificantly when compared between NP-treated and untreated albino mice except clockwise rotations that were significantly reduced in strontium hexaferrite cobalt iron oxide nanoparticle-treated male mice than saline-treated control group (). These results indicate that cerebellum part of male brain has potentially the receptor for strontium hexaferrite cobalt iron oxide nanoparticles as this part is reported to regulate the locomotory and exploratory behaviours resulting in improved performance than in the control group.
Blood has special importance in the living system and involves in the transport of carbon dioxide, oxygen and various metabolites [Citation23]. Hematological parameters are affected by stress, environmental factors and nutritional deficiencies and hence can be used as good health indicator and are used on regular basis for the diagnosis of various diseases [Citation24]. Gaharwar and Paulraj [Citation25] had reported a significant decrease in red blood cells in rat that were exposed to either 15 or 30 mg/kg iron oxide. They had suggested that these changes in red blood cells are due to abnormalities induced by iron oxide in hemopoietic system that is responsible for erythrocyte production. Our results revealed that total red blood cell count was lower in strontium hexaferrite cobalt iron oxide-treated mice as compared to saline-treated male albino mice, although the difference did not reached statistical significance (P = 0.7) (). The difference in the two studies is probably due to the nature of chemicals (as we used NPs containing iron oxide as a component while the mentioned study used iron oxide only) and due to the different concentrations of the applied chemicals.
Our results revealed decrease in white blood cells (P = 0.8) and lymphocytes (P = 0.8) in NP-treated male mice (). Tabish et al. [Citation26] had reported decreased white blood cells, lymphocytes and platelets’ concentration in rabbits following a 10 mg/kg body weight implantation of cobalt iron oxide and suggested that nanoparticles at high doses can disturb the immune system in animals through affecting white blood cell production.
Nanoparticles induce oxidative stress that leads to the formation of reactive oxygen species that can disturb the antioxidant system that is consists of enzymes superoxide dismutase (SOD), catalase and nonenzymes such as vitamins E and C and glutathione [Citation27]. Oxidative stress also causes lipid peroxidation of membranes. Superoxide dismutase generates H2O2 from super oxide free radicals that are more toxic than oxygen-derived free radicals and are detoxify by catalase and reduced glutathione [28]. Gahrawar and Pulraj [Citation25] had reported a reduction in SOD and increased lipid peroxidation in iron oxide nanoparticle-treated rats as compared to control. In contrast, our results revealed increased SOD activity (P = 0.005) and reduced lipid peroxidation (P = 0.8) in liver and brain of strontium hexaferrite cobalt iron oxide-treated male mice as compared to saline-treated control group (). Increased level of SOD in liver during present study indicates increased H2O2 production in liver of the strontium hexaferrite cobalt iron oxide nanoparticle-treated male mice. These differences in results are probably due to different experimental designs, animals model used, applied dosage and exposure time.
Our results revealed lower value of catalase enzyme (P = 0.7) in strontium hexaferrite cobalt iron oxide-treated male mice as compared to control treatment. Our results are in agreement with Gahrawar and Pulraj [Citation25] who had reported reduced catalase activity in liver and kidney of iron oxide nanoparticle-treated rat.
5. Conclusion
In conclusion, our results indicated that 50 mg/mL of solvent/kg body weight of strontium hexaferrite cobalt iron oxide nanoparticles did not affected the studied neurological, hematological and serological parameters of albino mice of both genders, when compared with saline-treated control mice. Analysis of antioxidant metabolites in liver has indicated that the applied dose of strontium hexaferrite cobalt iron oxide can disturb the H2O2-associated metabolic pathways that resulted in increased SOD concentrations in male mice. As these nanomaterials are part of our everyday life, so it is recommended that their effects in living systems should be explored under variable dose and exposure time conditions to get a broader vision regarding their biocompatibility.
Disclosure statement
Authors declare that they do not have conflict of interest of any sort with anyone.
Additional information
Notes on contributors
Sana Akhtar
Sana Akhtar and Sehrish Saba has performed behavioral experiments.
Sauma Rehman
Sauma Rehman, Ahmad Hassan and Noreen Samad performed hematological tests.
Ahmad Hassan
Sauma Rehman, Ahmad Hassan and Noreen Samad performed hematological tests.
Muhammad Amir
Muhammad Amir, Mudassar Akram, Maryam Ijaz and Kiran Asif did the antioxidant analysis and MNA synthesize and characterize the nano material.
Mudassar Akram
Muhammad Amir, Mudassar Akram, Maryam Ijaz and Kiran Asif did the antioxidant analysis and MNA synthesize and characterize the nano material.
Maryam Ijaz
Muhammad Amir, Mudassar Akram, Maryam Ijaz and Kiran Asif did the antioxidant analysis and MNA synthesize and characterize the nano material.
Furhan Iqbal
Furhan Iqbal has designed and supervised the project.
References
- Ozgur U, Alivov Y, Morkoc, H. Microwave ferrites, part 1: fundamental properties. J Mater Sci Mater Electron. 2009;20:786–834.
- Dhage VN, Mane ML, Keche AP, et al. Structural and magnetic behaviour of aluminium doped barium hexaferrite nanoparticles synthesized by solution combustion technique. Phys B: Condens Matter. 2011;406:789–793. doi: 10.1016/j.physb.2010.11.094
- Pullar RC. Hexagonal ferrites: a review of the synthesis, properties and applications of hexaferrites ceramics. Prog Mater Sci. 2012;57:1191–1334. doi: 10.1016/j.pmatsci.2012.04.001
- Bayrakdar H. Fabrication, magnetic and microwave absorbing properties of Ba2Co2 Cr2Fe12O22 hexagonal ferrites. J Alloys Comp. 2016;674:185–188. doi: 10.1016/j.jallcom.2016.03.055
- Auwal IA, Unal B, Gungunes H, et al. Dielectric properties, cationic distribution calculation and hyperfine interactions of La3+ and Bi3+ doped strontium hexaferrites. Ceram Int. 2016;42:9100–9115. doi: 10.1016/j.ceramint.2016.02.175
- Ashiq MN, Iqbal MJ, Gul IH. Structural, magnetic and dielectric properties of Zr–Cd substituted strontium hexaferrite (SrFe12O19) nanoparticles. J Alloys Comp. 2009;487:341–345. doi: 10.1016/j.jallcom.2009.07.140
- Sousa MH, Tourinho FA, Depeyrot J, et al. New electric double-layered magnetic fluids based on copper, nickel, and zinc ferrite nanostructures. J Phy Chem B. 2001;105:1168–1175. doi: 10.1021/jp0039161
- Gupta AK, Gupta M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 2005;26:3995–4021. doi: 10.1016/j.biomaterials.2004.10.012
- Allahyar R, Akbar A, Iqbal F. Efect of creatine monohydrate supplementation on learning, memory and neuromuscular coordination in female albino mice. Acta Neuropsychiatr. 2016. doi: 10.1017/neu.2016.28.
- Iqbal S, Ali M, Iqbal F. Long term creatine monohydrate supplementation, following neonatal hypoxic ischemic insult, improves neuromuscular coordination and spatial learning in male albino mouse. Brain Res. 2015;1603:76–83. doi: 10.1016/j.brainres.2014.10.006
- Zahra K, Khan M, Iqbal F. Oral supplementation of Ocimum basilicum has the potential to improves the locomotory, exploratory, anxiolytic behavior and learning in adult male albino mice. Neurol Sci. 2015;36:73–78. doi: 10.1007/s10072-014-1913-3
- Kipnis J, Cohen H, Cardon M, et al. T cell deficiency leads to cognitive dysfunction: implications for therapeutic vaccination for schizophrenia and otherpsychiatric conditions. Proc Natl Acad Sci USA. 2004;101:8180–8185. doi: 10.1073/pnas.0402268101
- Chidambara MKN, Jayaprakasha GK, Singh RP. Studies on antioxidant activity of Pomegranate (Punica granatum) peel extract using in vivo models. J Agric Food Chem. 2002;50:4791–4795. doi: 10.1021/jf0255735
- Haider S, Naqvi F, Batool Z, et al. Pretreatment with curcumin attenuates anxiety while strengthens memory performance after one short stress experience in male rats. Brain Res Bull. 2015;115:1–8. doi: 10.1016/j.brainresbull.2015.04.001
- Lateef T, Qureshi SA. Centratherum anthelminticum ameliorates antiatherogenic index in hyperlipidemic rabbit. Int J Pharm. 2013;3:698–704.
- Bradely EL, Castle L, Chaudhry Q. Application of nanomaterials in food packaging with a consideration of opportunities for developing countries. Trends Food Sci Technol. 2011;22:604–610. doi: 10.1016/j.tifs.2011.01.002
- Oberdorster G, Oberdorster E, Oberdorster J. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect. 2005;113:823–839. doi: 10.1289/ehp.7339
- Li N, Sioutas C, Cho A, et al. Ultrafine particulate pollutants induce oxidative stress and mitochondrial damage. Environ Health Perspect. 2003;111:455–459.
- Lian S, Wang E, Kang Z, et al. Synthesis of magnetite nanorods and porous hematite nanorods. Solid State Commun. 2004;129:485–490. doi: 10.1016/j.ssc.2003.11.043
- Monteiller C, Tran L, Macnee W, et al. The pro-inflammatory effects of low-toxicity low-solubilssity particles, nanoparticles and fine particles, on epithelial cells in vitro: the role of surface area. Occup Med. 2007;64:609–615. doi: 10.1136/oem.2005.024802
- Wang B, Feng W, Wang M, et al. Acute toxicological impact of nano- and submicro-scaled zinc oxide powder on healthy adult mice. J Nanopart Res. 2008;10:263–273. doi: 10.1007/s11051-007-9245-3
- Laurent S, Forge D, Port M, et al. Magnetic iron oxide nanoparticles: synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem Rev. 2008;108:2064–2110. doi: 10.1021/cr068445e
- Aaron SD, Vandemheen KL, Naftel SA, et al. Tropical tetracaine prior to arterial puture: a randomized, placebo- controlled clinical trial. J Res Med Sci. 2003;97:1195–1199.
- Solomon SG, Okomoda VT. Effects of photoperiod on the haematological parameters of Clarias gariepinus fingerlings reared in water recirculatory system. J Stress Physiol Biochem. 2012; 8:25–29.
- Gaharwar US, Paulraj R. Iron oxide nanoparticles induced oxidative damage in peripheral blood cells of rat. J Biomed Sci Eng. 2015;8:274–286. doi: 10.4236/jbise.2015.84026
- Tabish TA, Ashiq MN, Ullah MA, et al. Biocompatibility of cobalt iron oxide magnetic nanoparticles in male rabbits. Korean J Chem Eng. 2016;33:2222–2227. doi: 10.1007/s11814-016-0043-4
- Shohami E, Beit-Yannai E, Horowitz M, et al. Oxidative stress in closed-head injury: brain antioxidant capacity as an indicator of functional outcome. J Cereb Blood Flow Metab. 1997;17:1007–1019. doi: 10.1097/00004647-199710000-00002
- Blake DR, Allen RE, Lunec J. Free radicals in biological systems – a review orientated to inflammatory processes. Br Med Bull. 1987;43:371–385. doi: 10.1093/oxfordjournals.bmb.a072188