Abstract
Nano- and micro-scale engineered surface structures are often used to control cell morphology and mimic the extracellular matrix in tissue engineering. However, there is little understanding of how toxins produced by common bacteria might affect cell adhesion to these structures. In this study, human dermal fibroblast (GM5565) cells were incubated on patterned tungsten/silicon oxide nanocomposite in media in the presence or absence of Antimycin A. This composite consists of parallel tungsten and silicon oxide lines with identical widths in the range of 0.18 and 50 μm. The morphology of the cells and of their mitochondria was characterized by using high-resolution scanning electron microscopy and fluorescence confocal microscopy. Results show that cells preferentially align along the line axes in a pattern-dependent manner, with a maximum population of cells oriented within 10° of the line axes on the structures containing 10 μm wide lines. Cells treated with Antimycin A, however, show a smaller proportion of cells oriented in this direction as compared to cells cultured in Antimycin A-free media (34.4% vs 53.0%). The majority of mitochondria in cells growing in Antimycin A-free media are tubular in shape and are preferentially positioned on the tungsten lines, whereas these organelles exhibit a circular geometry and are less attracted to the metal lines in the presence of Antimycin A.
1. Introduction
The ability to control the morphology of cells on engineered surfaces is important for the success of medical implants and other devices [Citation1]–[Citation3]. Cell adhesion mechanisms and morphologies on engineered surfaces are complex and can be influenced by local micro-environments, including surfaces differing with respect to pre-adsorbed protein species [Citation4, Citation5], surface structure geometries [Citation5]–[Citation7], roughness [Citation8]–[Citation13], and surface energy [Citation8, Citation14] of the substratum, and by cell type-specific characteristics [Citation15, Citation16]. Focal adhesions, the sites of cellular attachment to the substratum, are controlled by complex protein-protein interactions [Citation17], which often occur at the leading edge of a migrating cell where pseudopodia are constantly probing the surrounding environment. These cytoplasmic projections are regulated by different molecular signal transduction pathways: Nobes and Hall [Citation18] showed that Cdc42 activation promotes the formation of filopodia, while activation of Rac leads to lamellipodia in spreading Swiss 3T3 cells. Both processes assist the formation of focal adhesion complexes and actin polymerization, with Ridley and Hall [Citation19] showing that activated Rho is responsible for the formation of actin stress fibers.
While a significant amount of progress has been made in our understanding of cell adhesion to engineered structures, there is little understanding of how natural (or synthetic) toxins, such as antimycin A, affect normal cell adhesion to biomaterial surfaces such as surgical implants. Antimycin A is a compound naturally produced by Streptomyces bacteria, which are commonly found in soil. Antimycin A, which serves as a defensive mechanism against microorganisms in the environment, is a potent inhibitor of Complex III of the mitochondrial respiratory chain, which is housed in almost all eukaryotic cells. Exposure to Antimycin A interferes with the production of adenosine triphosphate (ATP), modifies the electrical potential across the mitochondrial membrane and results in elevated production of the superoxide molecule, one of the family of reactive oxygen species (ROS) [Citation20]. Antimycin A is also used as a piscicide in the aquaculture industry to control invasive fish populations. In large quantities, this chemical is lethal to fish, mammals, and plants. While high doses of Antimycin A are known to lead to cell apoptosis [Citation21], sub-lethal doses of Antimycin A have been used as a means of depleting ATP in models of ischemia in renal dysfunction; transient exposure to Antimycin A was shown to cause actin rearrangements and lead to poor cell adhesion [Citation22–24]. Interestingly, similar effects on the cytoskeleton and cell adhesion were observed when cells were treated with cyanide, a potent inhibitor of cytochrome c oxidase, which is the terminal electron acceptor of the mitochondrial respiratory chain [Citation25, Citation26], suggesting that depletion of ATP leads to defects in cell structure and morphology. However, the effect of Antimycin A on mitochondrial morphology remains undocumented; likewise, the mitochondrial morphology in cells adhering to engineered surfaces remains unknown.
The primary objective of this study was to characterize the adhesion behaviours of human fibroblast cells (GM5565) to tungsten-silicon oxide nanocomposite surfaces and determine how these engineered surface features would affect the morphology of the mitochondria, the organelles that produce the bulk of cellular ATP in most cells. In addition to studying the mitochondrial morphology and distribution in fibroblasts adhering to these engineered substrates, a secondary objective was to investigate the influences of a known mitochondrial poison that would affect the oxidative capacity of these organelles and decrease in ATP production.
Herein, human fibroblast cells were deposited on tungsten-silicon oxide parallel line comb structures fabricated by using advanced integrated circuit-based chemical-mechanical polish techniques. Parallel line comb structures were chosen for this work as cell alignment results on these test patterns are commonly reported in the literature [Citation4, Citation5, Citation27–32] to evaluate and compare cell manipulation efficiency produced by different surface modification techniques. Both tungsten- [Citation33]–[Citation36] and silicon oxide- [Citation37]–[Citation39] based materials are commonly found in medical implant devices. The morphology of adherent cells incubated in media containing Antimycin A was studied by using fluorescence confocal microscopy and scanning electron microscopy and the orientation of cell nuclei and mitochondria relative to the comb structure line axes was characterized. Our results show that the propensity for human fibroblasts, and their mitochondria, to align on comb structures is influenced by the pattern of the comb structure and can be inhibited in the presence of a mitochondrial toxin on substrates with 10 μm tungsten wires. To our knowledge, this is the first report to demonstrate that cell alignment on engineered wire-containing structures can be influenced by the inhibition of oxidative phosphorylation, a cellular function that is essential for survival in the aerobic environment.
2. Methods
2.1. Tungsten-silicon oxide nanocomposite
The nanocomposite tested in this work was fabricated by using advanced integrated circuit (IC) manufacturing techniques and damascene integration methods supplied by Versum Materials, LLC (Tempe, Arizona, USA). Detailed fabrication procedures have been described elsewhere [Citation5]. Briefly, plasma etching and photolithography techniques [Citation40]–[Citation42] were used to transfer the parallel line patterns to the silicon oxide thin films deposited on silicon substrates. The specimens were then deposited with a thin layer of titanium (∼ 20 nm) and tungsten. Excess tungsten was polished and removed with chemical-mechanical planarization methods [Citation43]–[Citation46]. Representative scanning electron micrographs of the final comb structure devices are displayed in Figure S1. The polished surface is smooth, flat, and free of residue.
2.2. Cell culture and plating
Cultured human skin fibroblasts (GM5565; ATCC, Manassas, VA, USA) were maintained and cultured in 75 cm2 flasks (Corning Falcon, Corning, NY, USA) in Dulbecco’s Modified Eagle Medium (DMEM), supplemented with GibcoTM 10% (v/v) fetal bovine serum (FBS) (ThermoFisher, Whitby, ON, Canada), in 95% O2:5% CO2 at 37 °C. Because of the negative impacts of antibiotics on mitochondrial function and cell growth [Citation47, Citation48], the media were not supplemented with a penicillin/streptomycin cocktail. Cells were incubated on patterned nanocomposites in two different media, media A containing only the components described above, while media B was supplemented with 5 μM Antimycin A (Sigma-Aldrich, St. Louis, Missouri, USA) at a concentration sufficient to induce mitochondrial dysfunction but not result in immediate apoptosis [Citation21]. Patterned specimens were rinsed and dried with ethanol prior to cell plating. Unless otherwise stated, cells were seeded at a concentration of 1 x 105 cells/mL in 35 mm plates (Falcon, ThermoFisher, Whitby, ON, Canada).
2.3. Cell fixation and staining
After allowing the cells to adhere and proliferate for 72 hours, specimens were rinsed with phosphate-buffered saline (PBS) and fixed with 4% paraformaldehyde for 15 minutes at 37 °C. Cells were subsequently permeabilized by treatment with 0.1% Triton-X 100 solution (Sigma-Aldrich, St. Louis, Missouri, USA) for 5 minutes at room temperature, followed by rinsing with PBS. The F-actin cytoskeletal filaments were then stained by exposure to phalloidin for 45 minutes, using the CytoPainter F-Actin staining kit (ab112127; Abcam, Cambridge, MA, USA). Following multiple rinses with PBS, DNA was subsequently labeled with 4',6-diamidino-2-phenylindole (DAPI) (Life Technologies, Burlington, ON, Canada) by exposure to a 1:25,000 DAPI dilution in PBS for 5 minutes. For labeling mitochondria with MitoTracker® RED (MTR; Life Technologies, Burlington, ON, Canada), cells were washed with PBS and incubated with 100 nM pre-warmed MTR for 20 minutes at 37 °C prior to fixation. Specimens were kept in the dark at 4 °C prior to confocal fluorescence microscopy. Confocal fluorescence microscopy was performed with a Leica TCS SP5 confocal laser-scanning microscope (Leica, Wetzlar, Germany) at the University of Guelph, Ontario, Canada.
2.4. Scanning electron microscopy
Paraformaldehyde-fixed cells were dehydrated with increasing concentrations of ethanol (50%, 75%, 90%, and 100%) for at least 10 minutes each. Samples were dried and kept in a nitrogen box. A Zeiss 1550 field-emission electron microscope (Carl Zeiss AG, Oberkochen, Germany) was used to image cell morphology. The electron gun accelerated voltages were maintained at 7kV and the specimens were not coated with a conductive layer of material.
2.5. Cell orientation characterizations
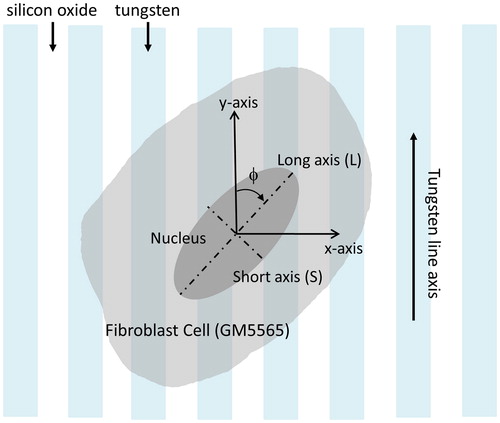
To quantify cell orientations, the angle (ϕ) between the long axis of the elliptically-shaped cell nucleus and the tungsten line axes were measured. This parameter is schematically illustrated in . A cell aligned parallel to the line axes has a ϕ value of 0°. Angular measures were conducted manually by using the Angle Tool of the software ImageJ (U. S. National Institutes of Health, Bethesda, Maryland, USA).
3. Results
3.1. Tungsten – silicon oxide nanocomposites
Representative 70° tilted SEM micrographs of parallel line comb structures and isolated tungsten line are shown in Figure S1. Isolated tungsten lines are separated from the nearest tungsten features by at least 50 μm. Tungsten lines are entrenched at a depth of ∼ 0.5 μm. The comb structures tested in this work had line widths of 0.18 µm, 0.25 µm, 0.5 µm, 1 µm, 2 µm, 5 µm, 10 µm, and 50 µm. The detailed dimension of the test structures is summarized in .
Table 1. Data summary of tungsten/silicon oxide comb structures: physical dimensions, inspected areas, number of cells inspected, cell density, % population of cells with ± 10° of the metal line axes. Two values were reported for the latter four parameters which correspond to results from cells incubated in baseline culture media with no Antimycin A (–ve A) and media with Antimycin A (+ve A), respectively. The media initial cell concentration was 1 × 105 cells/mL. Data spreads correspond to one standard deviation from three independent groups of samples.
3.2. Cell adhesion to blanket and patterned surfaces
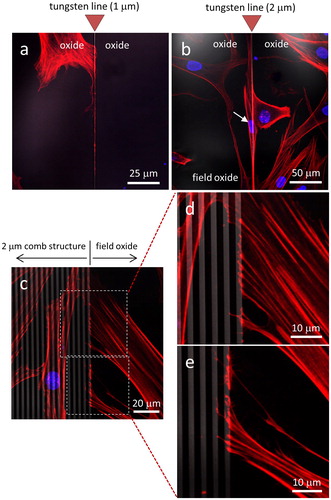
Representative fluorescence confocal micrographs of cells incubated for 72 hours on blanket silicon oxide, in standard cell culture medium lacking Antimycin A, are shown in . Staining of cell nuclei with DAPI (blue) and actin microfilaments with a phalloidin conjugate (red) demonstrate an apparently random orientation of cells adhering to the silicon oxide substratum. These adhesion behaviors were observed at two different cell densities, based on seeding at initial cell concentrations of either 1 x 105 () and 5 x 105 cells/mL (). In contrast, cells appeared to exhibit a preference for adhering to tungsten over silicon oxide when seeded on a substrate with 1 μm wide isolated tungsten lines as shown in . The tungsten line is oriented vertically in the image with no other metal structure nearby and the micrograph shows the actin filaments, originating from a single cell on the field oxide, spreading along the thin metal line but not extending across it. Instead, the filaments appear to be guided downwards along the tungsten line. Similar behavior was observed for cells adhering to substrates containing 2 μm wide isolated tungsten lines, as shown in . The micrograph shows a cell (highlighted with an arrow) self-aligned and elongated, appearing to being in contact with the tungsten. The strong preference for cultured human fibroblasts to adhere to tungsten was also observed at the periphery of comb structures (). The micrographs show the boundary region of the parallel line comb structure with 2 μm line width (tungsten in gray, silicon oxide in black) and the majority of actin filaments originating from cells on the oxide are adhered to the tungsten at the boundary but not spreading further into the comb structure. Only a few filaments were observed to extend into the comb structure.
Figure 2. Fluorescence confocal micrographs of GM5565 human fibroblast cells on blanket silicon oxide surfaces with initial cell concentrations of (a) 1 x 105 cells/mL and (b) 5 x 105 cells/mL. DNA is stained with blue DAPI and filamentous actin filament with red CytoPainter. Cells were incubated in media for 72 hours. Scale bars correspond to 50 μm.
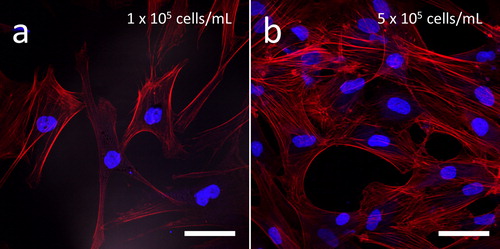
Figure 3. Fluorescence confocal micrographs of cells adhered to isolated tungsten lines with widths of (a) 1 μm and (b) 2 μm. (c) Low magnification images of the 2 μm comb structure boundary. High magnification images (d) and (e) reveal actin filaments spread from a cell on field oxide to tungsten lines with width of 2 μm. DNA is stained with blue DAPI and actin filaments with red CytoPainter. Tungsten lines appear in gray. Scale bars correspond to 50 μm.
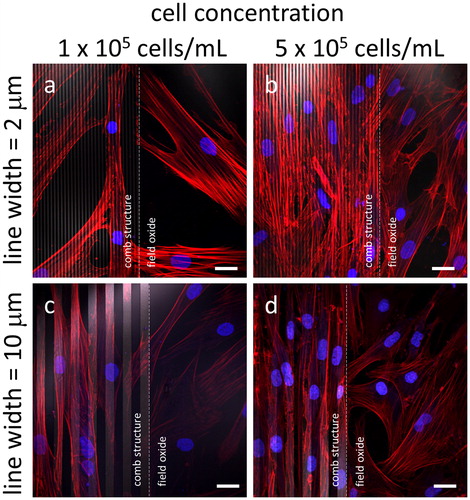
Given these observations, we assessed whether this behavior would also hold for tungsten wires of varying widths. demonstrates that fibroblast adhesion to tungsten leads to elongation of the cells along dense parallel lines, with cells oriented randomly in the field oxide region but aligned in comb structures with line widths of 2 μm () or 10 μm (). This reproducible behavior appears to be independent of the initial cell concentrations - 1 x 105 cells/mL () and 5 x 105 cells/mL (), with the adherent cells on these comb structures aligned with the line axes regardless of the cell concentration in the initial seeding.
Figure 4. Fluorescence confocal micrographs of cells adhered to comb structures with line widths of (a - b) 2 μm and (c - d) 10 μm. Two different cell concentrations were tested (a and c) 1 x 105 cells/mL and (b and d) 5 x 105 cells/mL. White dashed lines represent the boundary between the comb structure and the field oxide area. All cells were incubated in media for 72 hours with DNA stained in blue while actin filaments are in red. Tungsten lines appear in light gray. Scale bars correspond to 25 μm.
3.3. Mitochondrial morphology on tungsten lines
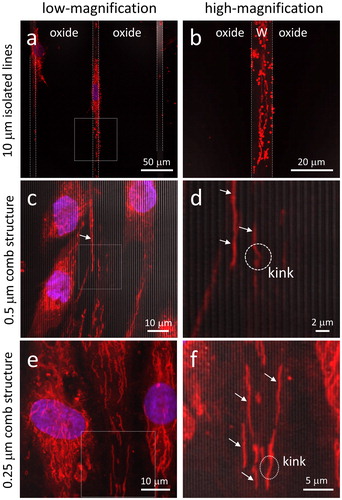
While the results described above show that preferential adhesion to tungsten wires has an impact on overall cell morphology, it was not known how this cell behavior might influence the distribution and morphology of sub-cellular organelles. Because of their charge and polarized state, we analyzed the morphology and the sub-cellular distribution of mitochondria in fibroblasts grown on substrate containing isolated tungsten lines with a width of 10 μm and on dense parallel comb structures with line widths of 0.25 μm. As displayed in , when cells were labeled with the membrane potential-sensitive dye MitoTrackerTM Red (MTR) and examined by confocal microscopy, the mitochondria were observed to align with the tungsten wires. The low magnification image in , in which the boundaries between the 10 μm wide tungsten line and silicon oxide are highlighted with white dash lines, shows the nucleus and the extended mitochondrial network structure being aligned with the tungsten and elongated along the line axes similar to that of the actin filaments described in . We observed both tubular staining that visualizes the extended reticulum and puncta, which arise from individual organelles that are characteristic of the expected mitochondrial distribution. When cells adhered to comb structures with line widths of 0.5 μm (), we observed a similar alignment, with the complete overlap of the MTR signal and the tungsten wire illustrating the effective diameter of the individual tubular segments of the mitochondrial reticulum. Interestingly, there also appear to be significantly fewer mitochondrial puncta under these conditions. Even mitochondria located at least 20 μm away from the nucleus appear to preferentially position themselves on top of the tungsten lines (), but displaying a straight line conformation that is aligned parallel to the line axes. Occasionally, individual mitochondria appear to have a ‘kink’, where the staining crosses to the adjacent line. We further observed similar mitochondrial positioning behavior on line structures with widths significantly smaller than the effective diameter of individual tubular mitochondria. In , micrographs show tubular-shaped mitochondria aligned and positioned above the 0.25 μm wide tungsten lines. Again, kinks form when mitochondrial reticular strands bridge adjacent metal lines. To our knowledge, this is the first observation of the positioning of mitochondria within a live human fibroblast cell that is precise down to the nanometer scale. To demonstrate the reproducibility of these observations regarding mitochondrial morphology and positioning, additional micrographs of cells on comb structures are provided in Figure S2.
Figure 5. Typical fluorescence confocal micrographs of cells adhered to 10 μm wide isolated lines recorded at (a) low-magnification and (b) high-magnification using cells cultured for 72 hours. The boundary between the tungsten and field oxide is labeled with dashed lines. Adherent cells on the 0.5 μm comb structures are shown in (c) and (d). (e) and (f) show cells on 0.25 μm comb structures. White arrows indicate tubular mitochondrial structures positioned directly on top of tungsten lines. DNA is in blue and mitochondria are in red. Cell concentration is 1 x 105 cells/mL.
3.4. Influence of Antimycin A, a mitochondrial respiration inhibitor, on cell alignment
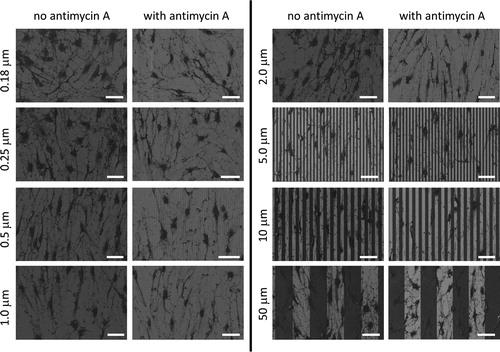
Because we identified a clear tendency for the mitochondrial reticulum within cells to align with the tungsten lines, we posited that the presence of a membrane potential across the inner membrane of these organelles might be exerting an effect on cell adhesion behaviors and mitochondrial alignment. We therefore incubated cells on a variety of substrates for 72 hours, either in the absence or presence of Antimycin A and compared their cellular adherence patterns. In this part of the experiment, cells were plated on eight different comb structures with line widths of 0.18 µm, 0.25 µm, 0.5 µm, 1 µm, 2 µm, 5 µm, 10 µm, and 50 µm. High-resolution SEM technique was chosen to inspect the overall cell morphology of these cells due to its better spatial resolution and capability to highlight the locations of tungsten and silicon oxide lines. As shown in , cells with or without Antimycin A treatment are randomly oriented on dense 0.18 μm comb structures. However, specimens prepared in either medium (i.e. +/- Antimycin A) become increasingly aligned with the line axes as the line width approaches 10 μm; cell alignment is then reduced on substrates with wider lines and the cells appear to be more randomly oriented.
Figure 6. Top-down SEM micrographs of cells incubated on comb structures in media with or without antimycin A. Eight comb structures with line widths of 0.18, 0.25, 0.5, 1, 2, 5, 10, and 50 μm were tested. Tungsten line is in light gray while silicon oxide is in dark gray. Initial cell concentrations were maintained at 1 x 105 cells/mL. All cells were incubated on the engineered surfaces for 72 hours. Scale bars correspond to 50 μm.
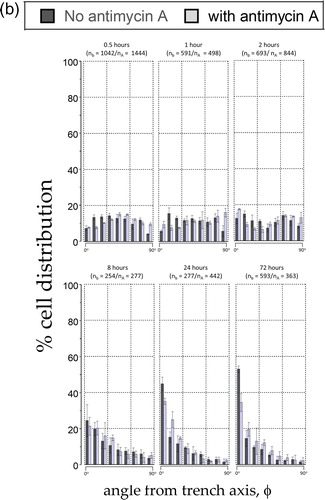
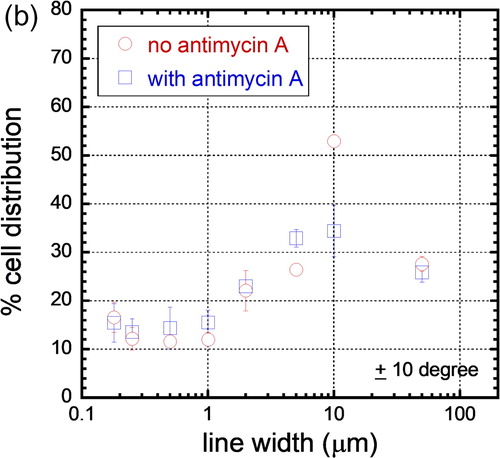
The alignment of cells on these comb structures was quantified by measuring the angular displacement (ϕ) between the elongated cell nucleus and the line axes as described in Section 2.5, and the percentage distribution of cell nuclei on comb structures with different line widths was plotted in . Each bin in the figure corresponds to the population of cell nuclei in 10° increment from the line axes. For example, a cell that is oriented at - 26° relative to the line axis will fall into the cell population of the third bin; error bars correspond to one standard deviation from three independent groups of data. We inspected a minimum of ∼340 cells on each comb structure incubated in either Antimycin A-free (nb) media and Antimycin A-treated (nA) media () and the results show that cells are arbitrarily oriented on the blanket tungsten film with a distribution of cells in each 10° bin that approaches the ideal randomized value of ∼11.1%. As the line width of the comb structures increases, larger proportions of cells are aligned parallel to the line axes and, regardless of the media composition, more than 20% of nuclei on the 2 μm comb structures oriented within ± 10° of the line axes. The alignment to the tungsten increases and reaches a maximum on comb structures with line widths of 10 μm. Not surprisingly, and as previously observed by confocal microscopy, the comb structures with 50 μm line widths display more uniform and random cell orientations. To highlight the influence of Antimycin A on cell alignment, the percentage populations of nuclei that are aligned within ± 10° of the tungsten line axes are plotted in . The figure shows no significant cell orientation difference between cells incubated in media with and without Antimycin A on comb structures with line widths in the range of 0.18 μm and 5 μm. In contrast, the alignment of cells on the 10 μm line comb structures appears to be highly sensitive to decreased mitochondrial respiration – 53.0 ± 1.2% of the cells align within ± 10° of the line axes in Antimycin A-free media, while only 34.4 ± 5.3% of the cells align to this extent in the presence of Antimycin A. These results show that Antimycin A-free media give rise to an ∼ 35% larger population of aligned nuclei than in cells exposed to Antimycin A. The cell alignment on the 50 μm comb structure featured reductions to 27.6 ± 1.5% and 25.9 ± 2.0%, respectively, as would be expected given the random distribution of cells observed previously in .
Figure 7(a). Percentage of cell distributions aligned at various angular range (f) . Each bin signifies a population of cells within a 10° angular interval. A cell oriented at - 21° to the line axes falls in the third bin from the left. Results from cells without antimycin A are represented by solid bins, while cells incubated with antimycin A are in hatched bins. Cells were incubated for 72 hours.
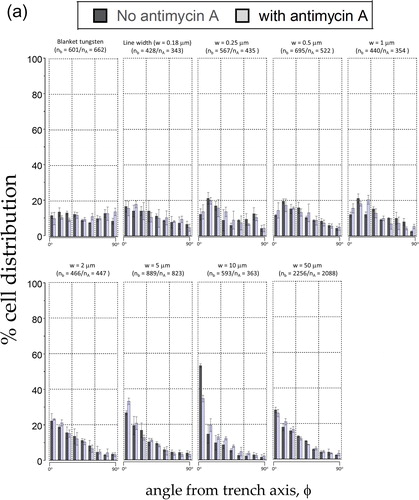
Figure 7(b). Population of cells that is aligned within ± 10° of the line axes of various line widths. Cells adhered to the 10 μm wide lines have the tightest alignment performance.
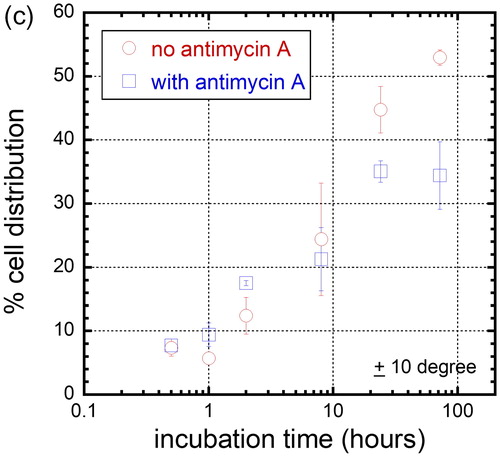
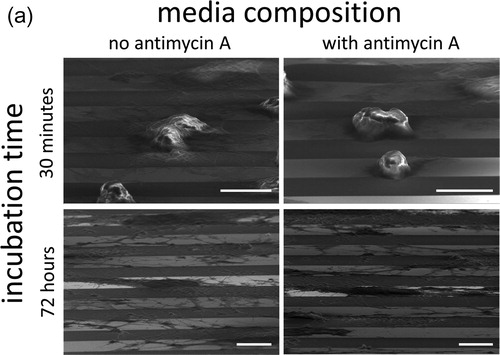
In light of the significant difference in cell alignment we observed in the presence of a mitochondrial inhibitor, and as a preliminary step towards better understanding this novel behavior, we used a time course to interrogate cell dispositions through several population doublings on the 10 μm comb structure. Cells were incubated for 0.5, 1, 2, 8, 24, and 72 hours in either Antimycin A-free culture media or in media containing 5 μM Antimycin A. In , SEM micrographs show cells adherent on the comb structures with line widths of 10 μm maintaining a nearly spherical shape after only 30 minutes in culture, as would be expected for cells that have been lifted from a substrate by trypsin; the cells are fully spread after 72 hours. Cell orientation (ϕ) distribution in percentage after varying incubation time on the 10 μm comb structure is plotted in and the results show that there is little cell alignment to the tungsten lines during the first two hours of culture, regardless of whether mitochondrial respiration is inhibited or not. However, the population of aligned nuclei increased significantly after cells had been in culture for 8 hours. To highlight this temporal effect, the cell population oriented within ± 10° of the line axes is plotted in and shows that there is no significant difference in cell alignment during the initial eight hours of incubation, regardless of the media compositions. The impact of Antimycin A on cell alignment becomes apparent after 24 hours in culture, which fits with the time needed for a complete cell doubling for fibroblasts. The results also show that the maximum population of Antimycin A-treated cells that align within ± 10° of line axes plateaued at ∼ 35% after 24 hours of exposure to this toxin. In contrast, specimens prepared with cells cultured in Antimycin A-free media display a percentage population of aligned cells that continues to increase even after 72 hours in culture.
Figure 8(a). Representative 70° tilted SEM micrographs of cells incubated on comb structures, with line widths of 10 μm, for 30 minutes and 72 hours, respectively, in media with or without antimycin A. Scale bars correspond to 10 μm.
3.5. Influence of Antimycin A on mitochondrial morphology
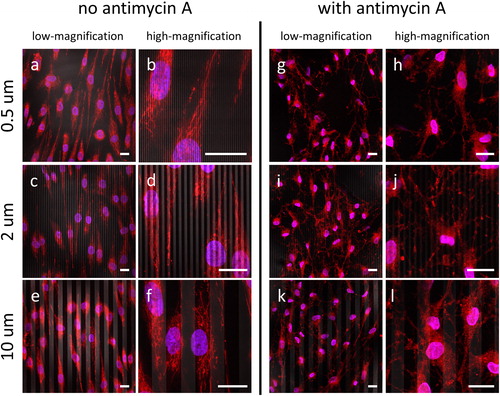
Given that the results shown in clearly demonstrate that the Antimycin A toxin has a negative impact on the cell alignment performance on comb structures during an extended period of exposure, we sought to interrogate the influence of Antimycin A on the mitochondrial morphology of cells grown on comb structures with line widths of 0.5, 2, and 10 μm. As expected and described above, fluorescence confocal micrographs obtained by staining with MTR show that the mitochondrial network morphology is well defined, with elongated geometry and distribution throughout the cells (), when cultured in the absence of Antimycin A. Closer examination also reveals that a majority of the mitochondria in these cells maintain a tubular shape. By contrast, the micrographs of cells cultured in the presence of Antimycin A () reveal a less organized mitochondrial reticulum. Intriguingly, virtually no tubular shaped mitochondria were observed in the Antimycin A-treated cells.
Figure 9. Fluorescence confocal micrographs of cells incubated in baseline media without antimycin A (a-f) and with antimycin A (g-l). Cells were plated on three comb structures: 0.5 μm (a, b, g, h), 2 μm (c, d, I, j), and 10 μm (e, f, k, l). For each structure, images were record at low- (a, c, e, g, i, k) and high-magnification (b, d, f, h, j, l). Initial cell concentrations were maintained at 1 x 105 cells/mL. Cells incubated on the engineered surfaces for 72 hours. DNA is in blue, mitochondria are in red, tungsten lines appeared in gray. Scale bars correspond to 20 μm.
4. Discussions
4.1. Cellular and organellar preferences for adhesion to tungsten
The morphology and spreading characteristics exhibited by the human skin fibroblasts shown in strongly support a preferential adhesion for these cells to tungsten as compared to silicon oxide. Our results show elongated cells that are oriented parallel to the tungsten line axes, likely as a means to increase association with tungsten, and this cell behavior was dependent on line widths (). A previously developed mathematical model [Citation5, Citation28] has shown that such cell alignment pattern dependence is the result of preferential cellular adhesion to tungsten. Given that surface features on a substrate are known to alter the conformation of fibronectin [Citation49], a critical extracellular matrix (ECM) protein that is expressed and secreted by fibroblasts in culture, which may be responsible for mediating the selective adhesion of the fibroblasts to the tungsten. Fibronectin is a well-characterized ECM protein that interacts with integrins in binding to substrates and promoting focal adhesions [Citation49]. Moussa et al [Citation5] have previously shown that monkey kidney epithelial (Vero) cells similarly align to tungsten/silicon oxide nanocomposite when cells are cultured in media containing FBS. However, when cells were cultured in an ultra-low protein medium, this cell alignment behavior degraded significantly, demonstrating that protein adsorption is an important contributing factor to the cell alignment characteristics. The adherence of corneal cells to silicon topographic features was also shown to be dependent on the presence of FBS in the culture media [Citation4]. Ours is the first study to demonstrate that the morphology of human skin fibroblasts can be manipulated by using chemical-mechanical polished tungsten/silicon oxide nanocomposites.
In addition to the overall cell shape and adhesion patterns, the morphology of fibroblast mitochondria also appears to be influenced by the patterned structures on the tungsten silicon-oxide nanocomposite substrates (). Almost all eukaryotic cells contain mitochondria, sub-micron sized organelles that are found throughout the cell and can appear as either an extended reticular network or as distinct puncta in the perinuclear region [Citation50], depending on cell type. As the primary site for cellular energy production, in the form of ATP, these organelles are essential to survival in an aerobic environment and are characterized by a membrane potential of approximately 180 mV that serves as the driving force for ATP synthesis. Since the spatial distribution and shape of mitochondria within a cell are tightly coupled to the local demand for ATP, the distributions identified in suggest that cellular energy consumption is highest at locations directly over top of the tungsten lines, where the presumptive focal adhesions are found. It is also interesting to note that the majority of mitochondria in these cells maintained a tubular conformation over tungsten lines with widths smaller than 0.5 μm, presumably because this confers a physiological advantage to cells adhering to the tungsten wires. This is the first report demonstrating that human fibroblast mitochondrial morphology can be manipulated by engineered surface structures within live cells with lateral resolution on the order of ∼ 250 nm.
4.2. Influence of Antimycin A on cellular and mitochondrial morphology
While bacterial toxins, such as Antimycin A, are known to have a negative influence on cell adhesion [Citation22]–[Citation26] and mitochondrial function [Citation22, Citation51], there is little understanding of how these toxins affect cell behaviors on engineered surfaces. This knowledge is particularly important in the development of surgical implants, as cell alignment on substrates is known to promote cell integration with implant [Citation52, Citation53] and post-surgical bacterial infection remains an obstacle to surgical implant success rates [Citation54]–[Citation57]. Antimycin A specifically inhibits Complex III of the mitochondrial respiratory chain, resulting in reduced mitochondrial membrane potential and ATP synthesis and elevated levels of the ROS, superoxide [Citation58]. In this study, we find () that the adherent cell density on the parallel line comb structures is reduced when cells are exposed to a sub-lethal dose of Antimycin A, which is consistent with experimental observations in other animal cell studies [Citation22]–[Citation24]. However, it should be noted that our study differs in several significant ways from these other works. Firstly, our cells are cultured in medium lacking penicillin and streptomycin, thereby removing the confounding negative impact of these compounds on mitochondrial function and cellular behavior [Citation47, Citation48]. Secondly, we use longer (24 - 72 hrs) exposure times and a higher concentration of antimycin A for our experiments, in contrast to the transient exposures of 15 - 90 minutes typically used in studies modelling sub-lethal ischemia in renal dysfunction [Citation22]–[Citation24]. The influence of antimycin A on cell alignment, when freshly seeding a suspension of fibroblasts, does not become evident until 24 hours, which coincides with the doubling time of normal fibroblasts. Interestingly, the impact of Antimycin A on cell alignment continues to increase with longer incubation times, suggesting that the effects of the toxin on cell alignment are cumulative. Perhaps most importantly, our results suggest a hormetic effect for antimycin A, similar to that recently demonstrated for the mitochondrial Complex I poison, rotenone [Citation59]. Rather than a decrease in membrane potential or reduced ATP synthesis per se, the higher levels of ROS may be the mediators of this phenomenon.
In addition to the observed changes in cell morphology and alignment induced by Antimycin A, fluorescence confocal microscopy revealed striking alterations in mitochondrial morphology. This effect is best seen with untreated cells growing on the 10 μm tungsten wires, in which the mitochondrial network has a primarily tubular appearance (). In contrast, the mitochondria in cells exposed to Antimycin A do not present with the extended reticulum but rather appear as clusters of puncta (). It also seems likely that the relatively weaker fluorescence signal obtained for Antimycin A-treated cells is due to the expected mitochondrial membrane depolarization that occurs as a result of interfering with electron transport and ATP generation. Together with the lack of mitochondria on the silicon oxide portion of the patterned chips, at locations with presumably lower energy demand, our results suggest that mitochondrial morphology may be influenced by surface features on chemical-mechanical polished nanocomposite.
Taken together, our results have obvious implications for medical applications such as tissue regeneration and implants. The use of tungsten in implants has a long history [Citation33]–[Citation36] based primarily on the biocompatibility and materials properties that make it inert and stable. Our results suggest for the first time that some as-yet-unidentified aspect of mitochondrial metabolism or function may contribute to the strong preference that fibroblasts show for aligning with tungsten wire. In light of the significant threat of infections in these surgical interventions, our finding of reduced adhesion and alignment in the presence of a well-characterized bacterial toxin such as antimycin A partially explains how such infections can negatively impact an implant. In this regard, it is interesting to note that a wide variety of bacterial toxins have been shown to adversely impact varying mitochondrial functions [Citation51], suggesting that the phenomenon we have identified herein for Antimycin A might have broader implications in understanding the successes and failures in surgical implants. Given that Antimycin A also serves to increase cellular levels of superoxide, in addition to being a known inhibitor of electron transport in mitochondrial membranes, future experiments will be aimed at characterizing the integrity of the mitochondrial genome (mtDNA), which is known to be sensitive to oxidative stress. Mitochondria have been described as the canaries in the coalmine for their exquisite sensitivity to toxic insults [Citation60] and further investigation of the changes in mitochondrial structure and function that facilitate the alignment patterns we have observed will be needed to elucidate the molecular mechanisms at play in cellular responses to patterned microchip surfaces.
Conclusions
The results presented here demonstrate that cultured human fibroblasts exhibit a clear preference for adhesion to tungsten as compared to silicon oxide. This behavior leads to cell elongation on the chemical-mechanical polished tungsten/silicon oxide nanocomposite surfaces. We find that maximum cell alignment is observed on the 10 μm comb structures and is not affected by the presence of a respiratory inhibitor. However, in the presence of Antimycin A there does appear to be a statistically significant difference in the number of cells that are aligned within ± 10° of the tungsten line axes. A time course study also reveals different alignment performance between cells cultured in media in the presence or absence of Antimycin A, with the variation only becoming significant after cells have been cultured for 24 hours or longer. We also show, for the first time, the morphology of the mitochondrial reticulum from cells incubated in Antimycin A-free media and find that these essential organelles show preferential subcellular localization to the tungsten structures and that a majority of them are maintained in a tubular shape. Perhaps most importantly, this behavior associated with the mitochondrial reticulum is lost upon exposure of the cells to Antimycin A and the organelles acquire a more punctate staining pattern.
Conflicts of Interest
No potential conflict of interest is reported by the authors.
Acknowledgments
The authors would like to acknowledge Dr. Mark O’Neill of Versum Materials, LLC for support of the chemical-mechanical polished specimens. We also thank Seville Scarcello for technical assistance in the early parts of this work. Ting Y. Tsui thanks Canadian NSERC Discovery [RGPIN-355552] for their support of this work.
Additional information
Funding
References
- S. Barr, E. Hill, and a Bayat, “Current implant surface technology: an examination of their nanostructure and their influence on fibroblast alignment and biocompatibility.,” Eplasty, vol. 9, p. e22, 2009.
- R. B. Kato et al., “Nanotopography Directs Mesenchymal Stem Cells to Osteoblast Lineage Through Regulation of microRNA-SMAD-BMP-2 Circuit,” J. Cell. Physiol., vol. 229, no. 11, pp. 1690–1696, 2014.
- L. M. S. Castro-Raucci et al., “Titanium with Nanotopography Induces Osteoblast Differentiation by Regulating Endogenous Bone Morphogenetic Protein Expression and Signaling Pathway,” J. Cell. Biochem., vol. 117, no. 7, pp. 1718–1729, 2016.
- A. I. Teixeira, G. A. Abrams, P. J. Bertics, C. J. Murphy, and P. F. Nealey, “Epithelial contact guidance on well-defined micro- and nanostructured substrates,” J. Cell Sci., vol. 116, pp. 1881–1892, 2003.
- H. I. Moussa et al., “Manipulating mammalian cell morphologies using chemical-mechanical polished integrated circuit chips,” Sci. Technol. Adv. Mater., vol. 18, no. 1, pp. 839–856, 2017.
- B. B. Seo et al., “Mechanical contact characteristics of pc3 human prostate cancer cells on complex-shaped silicon micropillars,” Materials (Basel)., vol. 10, no. 8, 2017.
- Z. Jahed, S. Molladavoodi, B. B. Seo, M. Gorbet, T. Y. Tsui, and M. R. K. Mofrad, “Cell responses to metallic nanostructure arrays with complex geometries.,” Biomaterials, vol. 35, no. 34, pp. 9363–9371, Aug. 2014.
- M. M. Gentleman and E. Gentleman, “The role of surface free energy in osteoblast – biomaterial interactions The role of surface free energy in osteoblast – biomaterial interactions,” Int. Mater. Rev., vol. 59, no. 8, pp. 417–429, 2014.
- D. D. Deligianni, N. Katsala, S. Ladas, D. Sotiropoulou, J. Amedee, and Y. F. Missirlis, “Effect of surface roughness of the titanium alloy Ti-6Al-4V on human bone marrow cell response and on protein adsorption,” Biomaterials, vol. 22, pp. 1241–1251, 2001.
- A. A. Khalili and M. R. Ahmad, “A Review of Cell Adhesion Studies for Biomedical and Biological Applications,” Int. J. Mol. Sci., vol. 16, pp. 18149–18184, 2015.
- A. Zareidoost, M. Yousefpour, B. Ghaseme, and A. Amanzadeh, “The relationship of surface roughness and cell response of chemical surface modification of titanium,” J. Mater. Sci. Mater. Med., vol. 23, no. 6, pp. 1479–1488, 2012.
- A. Dolatshahi-Pirouz et al., “Fibronectin Adsorption, Cell Adhesion, and Proliferation on Nanostructured Tantalum Surfaces,” ACS Nano, vol. 4, no. 5, pp. 2874–2882, 2010.
- A. Dolatshahi-Pirouz et al., “The influence of glancing angle deposited nano-rough platinum surfaces on the adsorption of fibrinogen and the proliferation of primary human fibroblasts,” Nanotechnology, vol. 20, p. 095101 (9pp), 2009.
- N. J. Hallab, K. J. Bundy, K. O. Connor, R. L. Moses, and J. J. Jacobs, “Evaluation of Metallic and Polymeric Biomaterial Surface Energy and Surface Roughness Characteristics for Directed Cell Adhesion,” Tissue Eng., vol. 7, no. 1, pp. 55–71, 2001.
- F. Badique et al., “Biomaterials Directing nuclear deformation on micropillared surfaces by substrate geometry and cytoskeleton organization,” Biomaterials, vol. 34, no. 12, pp. 2991–3001, 2013.
- L. Hanson et al., “Vertical nanopillars for in situ probing of nuclear mechanics in adherent cells,” Nat. Nanotechnol., vol. 10, no. 6, pp. 554–562, 2015.
- M. A. Wozniak, K. Modzelewska, L. Kwong, and P. J. Keely, “Focal adhesion regulation of cell behavior,” Biochim. Biophys. Acta - Mol. Cell Res., vol. 1692, no. 2–3, pp. 103–119, 2004.
- C. D. Nobes and A. Hall, “Rho, Rac, and Cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia,” Cell, vol. 81, no. 1, pp. 53–62, 1995.
- A. J. Ridley and A. Hall, “The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors,” Cell, vol. 70, no. 3, pp. 389–399, 1992.
- C. L. Lisdero et al., “The Mitochondrial Interplay of Ubiquinol and Nitric Oxide in Endotoxemia,” Methods Enzymol., vol. 382, no. 1978, pp. 67–81, 2004.
- M. A. King and M. A. Radicchi-Mastroianni, “Antimycin A-induced apoptosis of HL-60 Cells,” Cytometry, vol. 49, no. 3, pp. 106–112, 2002.
- J. Du et al., “ATP depletion-induced actin rearrangement reduces cell adhesion via p38 MAPK-HSP27 signaling in renal proximal tubule cells,” Cell. Physiol. Biochem., vol. 25, no. 4–5, pp. 501–510, 2010.
- D. Sáenz-Morales et al., “Requirements for proximal tubule epithelial cell detachment in response to ischemia: Role of oxidative stress,” Exp. Cell Res., vol. 312, no. 19, pp. 3711–3727, 2006.
- N. Raman and S. J. Atkinson, “Rho controls actin cytoskeletal assembly in renal epithelial cells during ATP depletion and recovery,” Am J Physiol Cell Physiol, vol. 276, no. 6, pp. C1312–1324, 1999.
- P. Prahalad, I. Calvo, H. Waechter, J. B. Matthews, A. Zuk, and K. S. Matlin, “Regulation of MDCK cell-substratum adhesion by RhoA and myosin light chain kinase after ATP depletion,” Am. J. Physiol. Cell Physiol., vol. 286, pp. 693–707, 2004.
- V. M. Kroshian, A. M. Sheridan, and W. Lieberthal, “Functional and cytoskeletal changes induced by sublethal injury in proximal tubular epithelial cells.,” Am. J. Physiol., vol. 266, no. 1 Pt 2, pp. F21–30, 1994.
- H. I. Moussa, M. Logan, K. Wong, Z. Rao, M. G. Aucoin, and T. Y. Tsui, “Nanoscale-Textured Tantalum Surfaces for Mammalian Cell Alignment,” Micromachines, vol. 9, no. 464, pp. 1–18, 2018.
- H. Moussa et al., “Pattern-Dependent Mammalian Cell (Vero) Morphology on Tantalum/Silicon Oxide 3D Nanocomposites,” Materials (Basel)., vol. 11, no. 8, p. 1306, 2018.
- T. Nakamoto, X. Wang, N. Kawazoe, and G. Chen, “Biointerfaces Influence of micropattern width on differentiation of human mesenchymal stem cells to vascular smooth muscle cells,” Colloids Surfaces B Biointerfaces, vol. 122, pp. 316–323, 2014.
- A. I. Teixeira, G. A. McKie, J. D. Foley, P. J. Bertics, P. F. Nealey, and C. J. Murphy, “The effect of environmental factors on the response of human corneal epithelial cells to nanoscale substrate topography,” Biomaterials, vol. 27, no. 21, pp. 3945–3954, 2006.
- X. Zhou, J. Shi, J. Hu, and Y. Chen, “Cells cultured on microgrooves with or without surface coating: Correlation between cell alignment, spreading and local membrane deformation,” Mater. Sci. Eng. C, vol. 33, no. 2, pp. 855–863, 2013.
- I. Poudel, J. S. Lee, L. Tan, and J. Y. Lim, “Micropatterning – retinoic acid co-control of neuronal cell morphology and neurite outgrowth,” Acta Biomater., vol. 9, no. 1, pp. 4592–4598, 2013.
- A. Prasad et al., “Comprehensive characterization of tungsten microwires in chronic neurocortical implants,” in 34th Annual International Conference of the IEEE EMBS, 2012, pp. 755–758.
- A. Weltman, J. Yoo, and E. Meng, “Flexible, Penetrating Brain Probes Enabled by Advances in Polymer Microfabrication,” Micromachines, vol. 7, no. 180, pp. 1–36, 2016.
- M. A. M. Freire et al., “Distribution and Morphology of Calcium- Binding Proteins Immunoreactive Neurons following Chronic Tungsten Multielectrode Implants,” PLoS One, vol. 10, no. 6, p. e0130354, 2015.
- T. Nishida, “Electrode impedance analysis of chronic tungsten microwire neural implants: understanding abiotic vs . biotic contributions,” Front. Neuroeng., vol. 7, no. May, pp. 1–12, 2014.
- F. Baino, S. Fiorilli, and C. Vitale-Brovarone, “Bioactive glass-based materials with hierarchical porosity for medical applications: Review of recent advances,” Acta Biomater., vol. 42, pp. 18–32, 2016.
- P. Naruphontjirakul, A. E. Porter, and J. R. Jones, “In vitro osteogenesis by intracellular uptake of strontium containing bioactive glass nanoparticles,” Acta Biomater., vol. 66, pp. 67–80, 2018.
- K. Hanada et al., “In vitro and in vivo effects of a novel bioactive glass-based cement used as a direct pulp capping agent,” J. Biomed. Mater. Res. - Part B Appl. Biomater., pp. 1–8, 2018.
- R. Doering and Y. Nishi, Handbook of Semiconductor Manufacturing Technology, 2nd ed. New York: CRC Press, Taylor & Francis Group, 2007.
- W.-K. Chen, The VLSI Handbook, 2nd ed. New York: CRC Press, Taylor & Francis Group, 2007.
- Y. Li, Microelectronic Applications of Chemical Mechanical Planarization. Hoboken, New Jersey: John Wiley & Sons Inc., 2007.
- M. R. Oliver, Chemical-Mechanical Planarization of Semi-conductor Materials, 1st ed. New York: Springer Berlin Heidelberg, 2004.
- R. D. Mcconnell and A. M. Hurst, “United States Patent 8,506,831 B2,” 2013.
- R. D. Mcconnell, A. M. Hurst, and X. Shi, “United States Patent 8,858,819,” 2014.
- R. D. Mcconnell and A. M. Hurst, “United States Patent 8,790,521,” 2014.
- S. Kalghatgi et al., “Bactericidal Antibiotics Induce Mitochondrial Dysfunction and Oxidative Damage in Mammalian Cells Sameer,” Sci. Transl. Med., vol. 5, no. 192, pp. 1–11, 2013.
- L. Llobet, J. Montoya, E. Lopez-Gallardo, and R.-P. Eduardo, “Side Effects of Culture Media Antibiotics on Cell Differentiation,” Tissue Eng., vol. 21, no. 11, pp. 1143–1147, 2015.
- B. G. Keselowsky, D. M. Collard, and J. Garcı, “Surface chemistry modulates fibronectin conformation and directs integrin binding and specificity to control cell adhesion,” J. Biomed. Mater. Res. A, vol. 66A, pp. 247–259, 2002.
- I. E. Scheffler, MITOCHONDRIA, First Edit. New York: John Wiley & Sons, Inc., 1999.
- J. H. Jiang, J. Tong, and K. Gabriel, “Hijacking mitochondria: Bacterial toxins that modulate mitochondrial function,” IUBMB Life, vol. 64, no. 5, pp. 397–401, 2012.
- X. Chen, X. Fu, J. Shi, and H. Wang, “Regulation of the osteogenesis of pre-osteoblasts by spatial arrangement of electrospun nanofibers in two- and three-dimensional environments,” Nanomedicine Nanotechnology, Biol. Med., vol. 9, no. 8, pp. 1283–1292, 2013.
- D.-H. Kim, P. P. Provenzano, C. L. Smith, and a. Levchenko, “Matrix nanotopography as a regulator of cell function,” J. Cell Biol., vol. 197, no. 3, pp. 351–360, 2012.
- D. Campoccia, L. Baldassarri, V. Pirini, S. Ravaioli, L. Montanaro, and C. R. Arciola, “Molecular epidemiology of Staphylococcus aureus from implant orthopaedic infections: ribotypes, agr polymorphism, leukocidal toxins and antibiotic resistance.,” Biomaterials, vol. 29, no. 30, pp. 4108–16, Oct. 2008.
- L. G. Harris and R. G. Richards, “Staphylococci and implant surfaces: a review.,” Injury, vol. 37 Suppl 2, pp. S3–S14, May 2006.
- D. Teterycz et al., “Outcome of orthopedic implant infections due to different staphylococci.,” Int. J. Infect. Dis., vol. 14, no. 10, pp. e913–8, Oct. 2010.
- Y. Wu, J. P. Zitelli, K. S. TenHuisen, X. Yu, and M. R. Libera, “Differential response of Staphylococci and osteoblasts to varying titanium surface roughness.,” Biomaterials, vol. 32, no. 4, pp. 951–60, Feb. 2011.
- C. L. Quinlan, A. A. Gerencser, J. R. Treberg, and M. D. Brand, “The mechanism of superoxide production by the antimycin-inhibited mitochondrial Q-cycle,” J. Biol. Chem., vol. 286, no. 36, pp. 31361–31372, 2011.
- S. Marthandan et al., “Hormetic effect of rotenone in primary human fibroblasts,” Immun. Ageing, vol. 12, no. 11, pp. 1–14, 2015.
- R. S. Williams, “Canaries in the coal mine: Mitochondrial DNA and vascular injury from reactive oxygen species,” Circ. Res., vol. 86, no. 9, pp. 915–916, 2000.