?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Azo dyes are not efficiently removed in water treatment. So the development of excellent absorbent is significant important for environmental protection. In this study, ceria is successfully immobilized on 40-60 mesh sand by using a simple method of sol–gel. And then research the effect of absorption on azo dyes in waster using immobilized CeO2 (ICe). The ICe absorbent displays high removal efficiency for AB10 (one typical kind of azo dyes) in waste water. In addition, after absorbing, concentration and toxicity of azo dye decreased and the absorbent easily separation from water which indicates that the technique is a promising post-treatment process to improve water quality.
KeyWords:
1. Introduction
The contamination of water by dyes is a very serious environmental problem,[Citation1] approximately half of all known dyes are azo dyes,[Citation2] azo dyes are also the main contamination.[Citation3] Azo dyes usually refer to an organic compound that having one or more azo bonds (-N = N-) in molecule.[Citation4–6] The azo dyes are widely used in the textile, leather, paper and rubber industries due to the properties of simple preparation process, excellent coloring ability and low cost. However, azo dyes are toxic and carcinogenic.[Citation7–10] They could reduce the amount of dissolved oxygen in water, accumulate in aquatic plants and microbes by the process of enrichment. Thus resulted in a serious threat to the growth of aquatic organisms and threaten the nature environment.[Citation10–12] So it is imminent to develop a highly efficient water environmental treatment method to solve these problems.[Citation13]
Up to now, physical,[Citation14] chemical[Citation15, Citation16] and biological[Citation17] treatment techniques have been developed to remove toxic dye from waste water. Advanced oxidation methods could remove azo dyes effectively,[Citation18] but the conditions are more harsh (UV[12], energy consumption, and the complex device, etc.). Because of the waste water quality changes, more kinds, high toxicity, the biological methods are not suitable to treatment the waste water. Although the dye can be removed better by coagulation method.[Citation19] It will produce a large number of precipitation and increase processing cost. Comparison on the treatment methods, adsorption is regarded as the most economical and environmentally friendly treatment method.[Citation20, Citation21] It adsorbs molecules in dye wastewater through adsorbents to achieve the purpose of removal or decolorization.[Citation22] In recent years, the development of nanotechnology make the inorganic nanomaterials with special properties increasing widespread application for the environmental protection, especially in the treatment of waste water.[Citation23, Citation24] However, the commonly adsorbents have no good adsorption property for azo dyes, more costly and uncomfortable recycle limiting their applied. So develop the low-cost adsorbents have become a research direction.[Citation25, Citation26]
CeO2 is one of the most important functional rare earth oxides,[Citation27, Citation28] widely used in catalysis, energy storage, high temperature oxygen sensitive materials, biosensors and electronics[Citation29, Citation30] due to its superior storage and oxygen release function and high temperature rapid oxygen vacancy diffusion ability.[Citation31] CeO2 also plays a significant role in waste water treatment.[Citation32] Di et al[Citation33] prepared CeO2 nanoparticles supported on aligned carbon nanotubes as a novel adsorbent for Cr(VI). Li et al[Citation32] synthesized the stable and crystalline phase of pure nanostructured CeO2 with various morphologies and showed an excellent removal capacity for the organic pollutant CR from waste water. Tomić et al[Citation34] prepared CeO2 nanopowder, which showed high adsorption capability for removal of different dyes (RO 16, MO, MB 9) with fixed initial pH.
Nevertheless, the size of materials with good absorbent usually be controlled in the size of nanometer that is too difficult to recycle and easily lead to secondary pollution. So, the aim of this study is to overcome the recycle difficulty through a simple and low cost method. Firstly, the CeO2 was immobilized on 40-60 mesh sand, then the properties of ICe were studied. Lastly, the results showed that the obtained material has a good absorption for the azo dye of AB10 and easily to recycle and reused.
2. Materials and experimental
2.1. Materials
Materials were purchased from Shanghai Titan Scientific Co., Ltd. (China): cerium nitrate hexahydrate (Ce(NO3)3·6H2O, AR), hydrochloric acid (HCl, AR), ethanol (C2H5OH, AR), ammonia (NH4OH, 25-28%, AR), commercial CeO2 (AR). 40-60 mesh sand and acid black 10 (AB10) suppliers at Alibaba.com. Deionized water (DI) supplied by our university was used in the experiments. All chemicals used were of analytical grade and without further purification.
2.2. Synthesis of ICe
ICe was successfully synthesized by a simple method. The process was as follows: first, weighted 10.0 g sand which was pre-treatment with HCl (washed by 20.0 mL 15% HCl and C2H5OH for 10 min, respectively) in a single mouth flask. Second, added 15.0 mL C2H5OH and 0.868 g Ce(NO3)3·6H2O and the mixture materials were stirring at a suitable rate for 1.0 h, then 0.2 mL NH4OH was added and keep stirring for 4.0 h, dried at 70 °C for 4 h. Last, calcined for 1 h at 400 °C and the products were gained.
2.3. Characterization
Materials were characterized by a HITACHI S-3400N scanning electron microscope (SEM) at an accelerating voltage of 15 kV to research the surface morphology. The distributions of elements of the samples were captured by a tracing electron microscope - energy spectrometer (SEM-EDS) (SEM:JSM-5600LV, JEOL, Japan;EDS:IE 300 X, Oxford, UK). A PANalytical X’ Pert PRO X-ray diffractometer with Cu Ka (λ = 0.15418 nm) incident radiation was employed to analyze the crystal structure of the materials. The X-ray diffraction (XRD) patterns were obtained for 2θ angles from 10° to 90° at a scan rate of 0.05°min−1. The absorption spectra of the initial AB10 solution as well as the filtrate after adsorption were monitored by a UV-vis spectrophotometer (Beijing Purkinje General Instrument Production of TU-1900) with a characteristic absorption at the wavelength of 602 nm to quantify the concentration of the AB10 dye.
2.4. Adsorption experiments
The adsorption performance of ICe was measured by the color remove rate of AB 10 solution. 0.902 g ICe and 0.030 g commercial CeO2 (CCe, about 10 μm) were separately added in 50.0 mL AB10 solution (100 mg·L−1) under constant magnetic stirring. 4.0 mL suspension was timely taken out and filtered by membrane in time and then the obtained liquid was measured at 602 nm. The color remove rate (W/%) was calculated according to the following Eqs. (1):
(1)
(1)
Ao and At was the absorbance of AB10 solution before treatment and at the time of T after treatment, respectively.
The adsorption capacity of the adsorbents for AB10 is calculated through the following Eqs. (2) and (3):
(2)
(2)
(3)
(3)
where qt is the adsorption capacity at time t (mg·g−1), and qe is the equilibrium adsorption capacity, V is the volume of the employed AB10 solution (mL), Co, Ct and Ce represent the initial, real-time and equilibrium concentration of AB10 solution (mg·L−1), respectively, and m is the mass of the employed adsorbents of CeO2 (g).
2.5. Recycling performance of the ICe as adsorbents
After adsorption, the adsorbent with the adsorbed AB10 was collected by auto-precipitation and washed for three times by using CH5OH as the desorbent to regenerate. Then reused for the next time of adsorption to evaluate the recyclability and reusability of the ICe adsorbent.
3. Results and discussion
3.1. Characteristics of the materials
3.1.1. SEM images
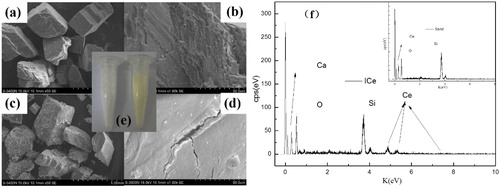
In order to characterize the morphology of samples, SEM-EDS and iphone 6s are used. and are the images of sand, compare with the images of ICe ( and ), it is easily to find that CeO2 has been immobilized on the surface of sand.[Citation35] Before immobilized, the surface of sand is smooth at low expansion, however it is rough at high expansion, which is conducive to attach materials. After immobilized, there is some substance on the surface of sand due to the surface and color of sand is changed. and shows that the surface of ICe is rough at low expansion and smooth at high expansion that is opposed to sand. Iphone image () is obviously find that the color of sand is white and it is pale yellow after immobilized. As well as the color of CCe is pale yellow and there are no other materials added in the preparation process of ICe. So CeO2 is immobilized on the surface of sand. represents the elements which are contained in materials. As shown in the , the main elements of sand are Ca, O and Si, that is fitted the main elements of SiO2 and CaO in sand. Moreover, the element of Ce is found in ICe instead of sand and no other elements are found in ICe, which indicates that the ICe is purity.
3.1.2. XRD pattern
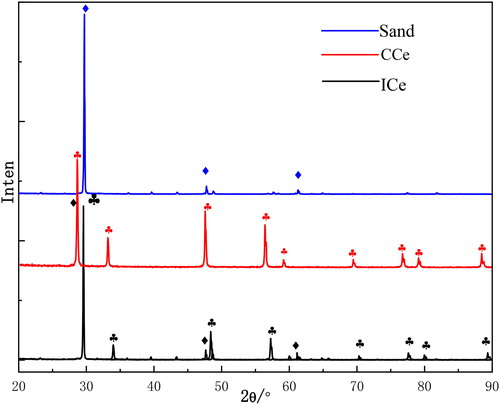
The XRD is used to analysis the phase purity of samples. is the XRD patterns of sand, CCe and ICe. As can be seen, all the typical diffraction peaks of cerium oxide (JCPDF34-0394) are found in CCe and ICe samples and all the typical diffraction peaks of sand are displayed in ICe. The strong and sharp peaks reveal that the products synthesized by sand could also keep a good crystal structure of sand. No diffraction peaks from impurities are observed, that indicates there are no other materials during the preparation of samples. It can be seen from the that the XRD pattern of sand is correspond with ICe. In addition, the elements of Si is found in ICe from the data of EDS. Contrast with the pattern of CCe, the characteristic peaks of cerium oxide are obviously displayed in ICe, that demonstrate CeO2 is immobilized on the surface of sand and the as-obtained ICe has a good crystallization and high purity.
3.1.3. Schematic illustration of ICe
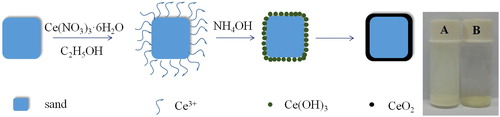
Based on above discussions, the formation mechanism of ICe is described in . Firstly, the pre-treatment sand is mixed in C2H5OH solution of Ce(NO3)3·6H2O and the Ce3+ ions are absorbed on the surface of sand.[Citation36] Secondly, when NH4OH solution is added gradually, the OH- ions combine with Ce3+ ions and form the Ce(OH)3 precipitate on the surface of sand. Lastly, after calcining, amorphous Ce(OH)3 transfer to crystalline CeO2 structure and the ICe is obtained. In order to analysis the performance of separation from water, a simple method is tested: ICe and CCe are mixed with water and standing for two min, ICe () shows excellent separation property than CCe (. The property is convenient for recycle and avoid the secondary pollution of adsorbents which indicate that ICe has a potential applied in wastewater treatment.
3.2. Absorbent test
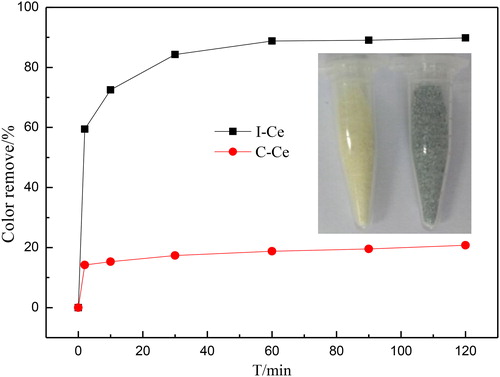
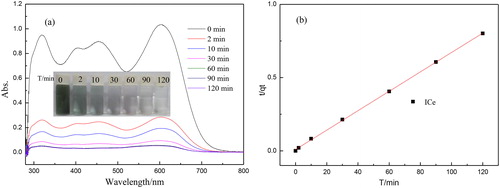
display the adsorbent performance of ICe and CCe for AB10 and the color change of ICe used before and after. It could be noted that the synthesized samples exhibit excellent adsorbent performance for AB10 due to the conversion effect between Ce3+ and Ce4+ and the bigger surface area of ICe.[Citation32, Citation37] Two minutes later, the ICe have removed over 60% of AB10, however the CCe just removed about 12%. Obviously, the adsorption amount of the two materials has increased with time. After two hours, the color of AB10 was removed about 89.1% and 20.6% by ICe and CCe, respectively. From the iphone photo in , it is obviously find that the color of ICe is changed from pale yellow to grey-black after absorbing. That demonstrates AB10 is adsorbed by ICe. The distance for adsorbent AB10 between ICe and CCe may be attributed to the preparation method of material and result in the different properties. As can be seen, the remove of AB10 was mostly occurred during the first two minutes and ended after one hour, that indicated the treatment time have a significant influence on the adsorption process and showed the ICe as adsorbent has great significance.
The color changed of AB10 solution is demonstrated by phone images which also corresponding with the absorption spectra of AB10 by ICe in . The photo images shows that the color of dye solution is getting lighter and lighter and two hours later, it is almost colorless. That displays the concentration of AB10 solution is less and less and corresponding with the adsorption performance of ICe in . The highest peak at the wavelength of 601 nm in absorption spectra is decreasing with time. And at the time of 90 min and 120 min, the spectra almost the same and the absorption intensity reach to zero, that is corresponded with the color change of AB10. No other peaks appeared in the spectra indicates that the dye of AB10 is stable and no other changes have occurred during the absorb process. In order to illustrate the adsorption mechanism of ICe for the AB10, the absorption data of ICe are analyzed with the pseudo-second-order kinetic model. The liner plots of t/qt vs t is shown in . Obviously, the absorption data is agree with the pseudo-second-order kinetic model well and the fitted correlation coefficient (R2) is 0.998. That indicates the adsorption of ICe for AB10 is belong to chemical adsorption and the excellent adsorption performance may be related to the valence forces by sharing or exchanging electrons.
3.3. Recycling performance of ICe
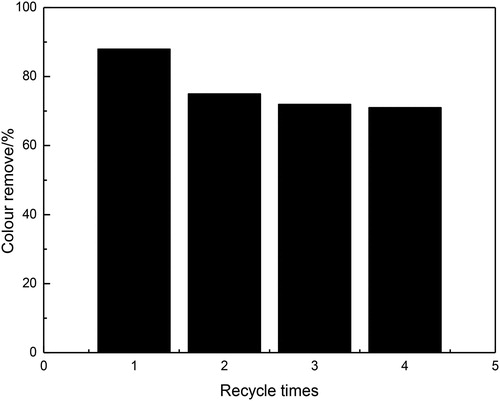
The regeneration and stability of the adsorbents are of extremely significance for practical application. To evaluate the performances of the ICe adsorbent, recycling reactions are performed for the adsorption of AB10 over the ICe adsorbent in . As shown in , the removal rate of AB10 is 89.1% after the first use of ICe. Then the second use of ICe after recycling, the removal rate AB10 is reduced to 76.2% and reduced by12.1%. That may be due to the adsorbed dyes on ICe are difficult to wash completely during the process of regeneration. After four consecutive adsorption cycles, the removal rate of AB10 still keeps at about 71.6%, which reduced 18.5% than that of the first time. These results indicate that the adsorbent of ICe is stable during the adsorption of AB10 and the as-prepared ICe has a promising used in the field of wastewater treatment.
4. Conclusion
In summary, ICe has successfully synthesized with liquid phase deposition method which is simple and low cost. Adsorption experiments show that the removal rate of AB10 could be reached to 89.1% and the adsorption model of ICe for AB10 conforms to pseudo-second-order kinetic, which indicates that the as-obtained ICe adsorbent exhibits excellent adsorption performance for AB10 dye. Moreover, the removal rate of ICe for AB10 still keeps at above 71.6% after four consecutive adsorption cycles, which demonstrates that the outstanding recycling and stability of as-obtained ICe and easily separation from water. Therefore, ICe has a promising candidate as a competitive adsorbents in waste water treatment and this paper provides a better approach for recycling adsorbents to solve the environmental pollution.
References
- Shalla AH, Bhat MA, Yaseen Z. Hydrogels for removal of recalcitrant organic dyes: A Conceptual Overview. Journal of Environmental Chemical Engineering. 2018.
- Vijayaraghavan R, Vedaraman N, Surianarayanan M, Macfarlane DR. Extraction and recovery of azo dyes into an ionic liquid. Talanta. 2006;69:1059–62.
- Sen SK, Raut S, Bandyopadhyay P, Raut S. Fungal decolouration and degradation of azo dyes: A review. Fungal Biology Reviews. 2016;30:112–33.
- Ng IS, Chen T, Lin R, Zhang X, Ni C, Sun D. Decolorization of textile azo dye and Congo red by an isolated strain of the dissimilatory manganese-reducing bacterium Shewanella xiamenensis BC01. Applied Microbiology & Biotechnology. 2014;98:2297–308.
- Solísabbba M. Microbial decolouration of azo dyes: A review. Process Biochemistry. 2012;47:1723–48.
- Işik M, Sponza DT. Fate and toxicity of azo dye metabolites under batch long-term anaerobic incubations. Enzyme & Microbial Technology. 2007;40:934–9.
- Peng N, Hu D, Zeng J, Li Y, Liang L, Chang C. Superabsorbent Cellulose-Clay Nanocomposite Hydrogels for High Efficient Removal of Dye in Water. Acs Sustainable Chemistry & Engineering. 2016;4:7217–24.
- Chung KT, Stevens SE, Cerniglia CE. The reduction of azo dyes by the intestinal microflora. Crc Critical Reviews in Microbiology. 1992;18:175–90.
- Lu L, Zhao M, Liang SC, Zhao LY, Li DB, Zhang BB. Production and synthetic dyes decolourization capacity of a recombinant laccase from Pichia pastoris. Journal of Applied Microbiology. 2010;107:1149–56.
- Robinson T, McMullan G, Marchant R, Nigam P. Remediation of dyes in textile effluent: a critical review on current treatment technologies with a proposed alternative. Bioresource Technology. 2001;77:247–55.
- Fang H, Wenrong H, Yuezhong L. Investigation of isolation and immobilization of a microbial consortium for decoloring of azo dye 4BS. Water Research. 2004;38:3596–604.
- Izgi B, Doll T, Frimmel FH, Gucer S. Photodegradation of azo dyes by simulated solar UV-radiation. Black Sea Basin Conference on Analytical Chemistry. 2005;118.
- Elisangela F, Andrea Z, Fabio DG, Cristiano RDM, Regina DL, Artur CP. Biodegradation of textile azo dyes by a facultative Staphylococcus arlettae strain VN-11 using a sequential microaerophilic/aerobic process. International Biodeterioration & Biodegradation. 2009;63:280–8.
- Akbari A, Remigy JC, Aptel P. Treatment of textile dye effluent using a polyamide-based nanofiltration membrane. Chemical Engineering & Processing Process Intensification. 2002;41:601–9.
- Bouyakoub AZ, Lartiges BS, Ouhib R, Kacha S, El Samrani AG, Ghanbaja J, et al. MnCl2 and MgCl2 for the removal of reactive dye Levafix Brilliant Blue EBRA from synthetic textile wastewaters: an adsorption/aggregation mechanism. Journal of Hazardous Materials. 2011;187:264–73.
- Sun SP, Li CJ, Sun JH, Shi SH, Fan MH, Zhou Q. Decolorization of an azo dye Orange G in aqueous solution by Fenton oxidation process: effect of system parameters and kinetic study. Journal of Hazardous Materials. 2009;161:1052–7.
- Patel Y, Mehta C, Gupte A. Assessment of biological decolorization and degradation of sulfonated di-azo dye Acid Maroon V by isolated bacterial consortium EDPA. International Biodeterioration & Biodegradation. 2012;75:187–93.
- Pratogarcia D, Buitrón G. Solar photoassisted advanced oxidation process of azo dyes. Water Science & Technology A Journal of the International Association on Water Pollution Research. 2009;59:965–72.
- Furlan FR, de Melo da Silva LG, Morgado AF, et al. Removal of reactive dyes from aqueous solutions using combined coagulation/flocculation and adsorption on activated carbon. Resources, Conservation and Recycling. 2010;54:283–90.
- Sun P, Xu L, Li J, Zhai P, Zhang H, Zhang Z, et al. Hydrothermal synthesis of mesoporous Mg3Si2O5(OH)4 microspheres as high-performance adsorbents for dye removal. Chemical Engineering Journal. 2018;334.
- Gao S, Zhang W, Zhou H, Chen D. Magnetic composite Fe3O4/CeO2 for adsorption of azo dye⋆. Journal of Rare Earths. 2018;36:986–93.
- Gürses A, Doğar Ç, Yalçın M, Açıkyıldız M, Bayrak R, Karaca S. The adsorption kinetics of the cationic dye, methylene blue, onto clay. Journal of Hazardous Materials. 2006;131:217–28.
- Afkhami A, Moosavi R. Adsorptive removal of Congo red, a carcinogenic textile dye, from aqueous solutions by maghemite nanoparticles. Journal of Hazardous Materials. 2010;174:398–403.
- Zhang S, Xu W, Zeng M, Li J, Li J, Xu J, et al. Superior adsorption capacity of hierarchical iron oxide@magnesium silicate magnetic nanorods for fast removal of organic pollutants from aqueous solution. Journal of Materials Chemistry A. 2013;1:11691–7.
- Gupta VK, Carrott PJM, Carrott MMLR, Suhas. Low-Cost Adsorbents: Growing Approach to Wastewater Treatment—a Review. Critical Reviews in Environmental Science & Technology. 2009;39:783–842.
- Mehta MJ, Chorawala KK. Adsorptive Removal Of Dye From Industrial Dye Effluents Using Low-Cost Adsorbents: A Review. International Journal of Engineering Research & Applications. 2014;4: 2248–962240.
- Zhang W, Chen D. Preparation and performance of CeO2 hollow spheres and nanoparticles. Journal of Rare Earths. 2016;34:295–9.
- Ouyang X, Li W, Xie S, Zhai T, Yu M, Gan J, et al. Hierarchical CeO2 nanospheres as highly-efficient adsorbents for dye removal. New Journal of Chemistry. 2013;37:585–8.
- Hatami M, Yazdan PM. Ultrasonic assisted synthesis of nanocomposite materials based on resole resin and surface modified nano CeO2: Chemical and morphological aspects. Ultrasonics Sonochemistry. 2017;39:160–73.
- Hou T F, Lei Y S, Zhang S Y, Zhang J H, Cai W J. Ethanol dry reforming for syngas production over Ir/CeO2 catalyst. Journal of Rare Earths. 2015;33:42–5.
- Lin B, Liu Y, Lan H, Ni J, Lin J, Jiang L. Effect of ceria morphology on the catalytic activity of Co/CeO2 catalyst for ammonia synthesis. Catalysis Communications. 2017;101:15–9.
- Li H, Wang G, Zhang F, Cai Y, Wang Y, Djerdj I. Surfactant-assisted synthesis of CeO2 nanoparticles and their application in wastewater treatment. Rsc Advances. 2012;2:12413–23.
- Di ZC, Ding J, Peng XJ, Li YH, Luan ZK, Liang J. Chromium adsorption by aligned carbon nanotubes supported ceria nanoparticles. Chemosphere. 2006;62:861–5.
- Tomić NM, Dohčevićmitrović ZD, Paunović NM, Mijin DŽ, Radić ND, Grbić BV, et al. Nanocrystalline CeO2-δ as effective adsorbent of azo dyes. Langmuir the Acs Journal of Surfaces & Colloids. 2014;30:11582–90.
- He Y, Sutton NB, Rijnaarts HHH, Langenhoff AAM. Degradation of pharmaceuticals in wastewater using immobilized TiO2 photocatalysis under simulated solar irradiation. Applied Catalysis B Environmental. 2016;189:283-.
- Xia Q, Li Z, Xi H, Xu K. Activation Energy for Dibenzofuran Desorption from Fe3+/TiO2 and Ce3+/TiO2 Photocatalysts Coated onto Glass Fibres. Adsorption Science & Technology. 2005;23:357–66.
- Hu J, Deng W, Chen D. Ceria Hollow Spheres as an Adsorbent for Efficient Removal of Acid Dye. Acs Sustainable Chemistry & Engineering. 2017;5: 3570–82.