Abstract
A new 2 D Cu(II)-based coordination polymer (CP) {[Cu5(Hcia)4(dpp)2(H2O)2(μ-OH)2]·2H2O}n (1) was designed and synthesized via using the mixed-ligand strategy, using 1,3-di(4-pyridyl)propane (dpp) colinker with a flexible spacer H3cia (H3cia: 5-(2-carboxybenzyloxy)isophthalic acid). Furthermore, we use the grinding method to downsize the complex 1 to nano-region (denoted as nano 1 hereafter). Furthermore, in order to determine the inhibitory effect of nano 1 on the viability of the BT474 breast cancer cells, the Cell Counting Kit-8 (CCK-8) was performed firstly. Then, the Annexin V-FITC/PI staining method was used to evaluate the BT474 breast cancer cells apoptosis levels after treated with nano 1. Next, the reactive oxygen species (ROS) detection kit was used in this study to determine the ROS level in the cancer cells after nano 1 treatment. By calculating posture scores and docking, the latent binding pattern of compound to receptor DNA were explored.
Introduction
According to the newest research, only in 2019, there were about 1.8 million people in the United States were diagnosed through cancer, and it is estimated that 61 thousand people will die of cancer [Citation1, Citation2]. The incidence and mortality of cancer significantly increased the burden of the families and society. Cancer could be induced by serious of factors, such as age, sex, emotional distress, environmental factors, and so on [Citation3, Citation4]. Thus, in this experiment, the new compound was synthesized for the anti-cancer study.
The design and synthesis of coordination polymers (CPs) have attracted more and more research interest, not only because of their novel topology and structures, but also because of their latent applications in ion exchange, chemical separations and gas storage, heterogeneous catalysis, nonlinear optics as well as microelectronics [Citation5–10]. In general, the structural diversity of CPs often depends on a lot of factors, for example, template, metal ion, metal coordination ratio, counteranion, pH value, as well as the donor atoms of ligands. Within these factors, organic ligands have an important function in controlling CPs diversity [Citation11, Citation12]. CPs construction using O along with N-donor precursors causes the flexible topologies, within it the obtained polymers can have latent application for their distinctive properties of the metal node and the organic ligands [Citation13]. So far, the combination of nitrogen-containing auxiliary ligands along with carboxylate has been a main factor in the generation of a series of polymer frameworks which have interesting preformances. The introduction of nitrogen-containing ligands such as azoles, imines and amines can adjust the dimension of the structure of metal polycarboxylic acids and research self-assembly process mechanism [Citation14]. Furthermore, recent studies have shown that coordination complexes containing Cu(II) have latent anticancer reagents. For instance, copper compounds of Casiopeinas® family [(Cu(II))(4,4′-dimethyl-2,2′-bipyridine)(acetylacetonate)(NO3)(H2O)], which developed via L. Ruiz along with his colleagues, are entering clinical trials first phase [Citation15]. Phosphine copper(I) complexes [Cu(trishydroxymethylphosphine)4][PF6], HydroCuP®, have highly selective anti-proliferation function, which show prospective results in pre-clinical researches [Citation16]. In this study, a new 2 D Cu(II)-MOF {[Cu5(Hcia)4(dpp)2(H2O)2(μ-OH)2]·2H2O}n (1) was designed and synthesized via using the strategy of mixed-ligand, using 1,3-di(4-pyridyl)propane (dpp) with a flexible spacer H3cia (H3cia: 5-(2-carboxybenzyloxy)isophthalic acid). The X-ray of single crystal diffraction research shows that the Cu(II)-MOF has a two-dimensional network, which penetrated into equivalent sets, thus forming a three-dimensional supramolecular network. Furthermore, we use grinding method to downsize the complex 1 to nano-region. In biological research, the inhibitory effect of nano 1 on BT474 breast cancer cell viability was evaluated via CCK-8, the results revealed that nano 1 could decrease cancer cell viability activity in vitro. The outcomes of Annexin V-FITC/PI staining suggested that nano 1 could trigger the apoptosis of BT474 breast cancer cells and exert the anti-cancer activity. In addition to this, the ROS data further explained the protective effect of nano 1 due to it promotes accumulation of ROS within BT474 breast cancer cells. By calculating posture scores and docking, the latent binding pattern of compound to receptor DNA were explored.
Experimental
Chemicals and measurements
The whole reagents along with chemicals were purchased from Beijing Bailingwei reagent company and Tianjin Guangfu chemical reagent company, and they were utilized without deep purification. The element contents of H, C and N were analyzed via elemental variational trace element analyzer. CP 1's nanostructures morphology along with configuration were characterized via scanning electron microscopy (SEM).
Preparation and characterization for coordination polymer {[Cu5(hcia)4(dpp)2(H2O)2(μ-OH)2]·2H2O}n (1)
With the mixture of H3cia of 0.020 g and 0.06 mmol, 1,3-bis(4-pyridyl)propane (dpp) which is 0.010 g and 0.06 mmol, and Cu(NO3)2·3H2O of 0.056 g and 0.18 mmol within 3 mL water along with 1 M NaOH solution of 0.1 mL, we synthesized Cu(II)-MOF. These ingredients were packaged into the autoclave of Teflon-lined, heated them to 100 °C at high pressure for three days, and then cooled them to the room temperature at 1 °C/min rate. The acquired blue Cu(II)-MOF crystals with block-shaped were filtered and washed repeatedly by using acetone and water and then air-dried (the yield was 64%). Anal. Calcd for C90H72Cu5N4O32: C, 53.01; H, 3.56; N, 2.75%. Found: C, 53.96; H, 3.86; N. 2.51%.
With Oxford Xcalibur E diffractometer to acquire the X-ray data of compound 1. Crysalispro software was used to analyze the strength data and converted them into HKL files [Citation17]. The SHELXS program based on the direct approach was applied to establish the initial structural patterns of compound 1, and modified the SHELXL-2014 program based on least-squares approach [Citation18]. Mixing all the non-H atoms of 1 with the anisotropic parameters. After that we used the AFIX commands to geometrically fix the entire H atoms on C atoms that they are connected to. exhibits the crystallographic parameters along with refinement details of compound 1 in detail.
Table 1. Complex1' s crystallographic parameters along with refinement details.
Cell counting kit-8 detection
To explore the inhibitory effect of nano 1 on BT474 breast cancer cell viability, the Cell Counting Kit-8 (CCK-8) was bought from Beyotime Institute of Biotechnology (Jiangsu, China) and performed in this experiment. This preformation was conducted under the instruction of the protocols with some modifications. In brief, the BT474 breast cells in logarithmic breast cancer were collected and inoculated into plates of 96 well and cultured at 200 µL DMEM culture medium at 37 °C, 5% CO2 environment overnight. After that, nano 1 (1,24,81,02,04,080 μg/mL) diluent was added to the cells for 24 h. Then, added 10% CCK-8 solution (Dojindo Laboratories, Kumamoto, Japan) in fresh medium was added to replace the culture medium for 30 min incubation at 37 °C. Finally, the absorbance of each well at 450 nm was determined via utilizing the microplate reader (ELX808; Bio Tek, USA). The experiment needs repeated three or more times.
Annexin V-FITC/PI staining
After treated with nano 1, the percentage of the apoptotic BT474 breast cancer cell was determined with Annexin V-FITC/PI staining (BD Biosciences, New Jersey, 7 USA) in flow cytometer in accordance to the manufacturer’s instruction. In short, the BT474 breast cancer cells in phase of logarithmic growth were collected and inoculated into plates of 6 well with final destiny of 1 × 105 cells/well. Plates were placed in an incubator at the condition of 37 °C, 5% CO2. Nano 1 of specified consistence was added into wells to treat for 24 h. Subsequently, the cells were washed, harvested as well as re-suspended in 1 × binding buffer. Lastly, the cells were stained in dark for 20 min by 5 μL of Annexin V-FITC and 5 μL propidium iodide (PI) for 20 min, and the absorption of each group was measured in flow cytometry at 488 nm and 525/625 nm. Every experiment was carried out for three times.
ROS accumulation assay
In order to determine the accumulation of ROS within the BT474 breast cancer cells after compound treatment, the DCFH-DA detection kit was used in this experiment under the guidance of the instruction with a little modification. Briefly, the BT474 breast cancer cells in phase of logarithmic growth were collected and inoculated into plates of 6 well. After incubated at condition of 37 °C, 5% CO2 for 12 h. Nano 1 of specified consistence was added into wells to treat for 24 h. Then, the DCFH-DA solution was added to wells at 37 °C and incubated for 20 min. Finally, all groups levels of fluorescence were determined via flow cytometer (BD via, New Jersey, USA) and analyzed using FlowJo software. The whole experiments repeat three or more times.
Molecular docking
Currently, two strand DNA is considered as potential targets for anticancer agents, due to multiple gene mutations are generated by mis-transcription or mis-replication process. Having an insight into the possible binding patterns of receptor DNA along with synthesized compound revealed a promising strategy for drug development and drug research. In this field, molecular docking usually has an important function in predicting the possible binding modes. Within the research, we used Discovery Studio 3.0 (Accelrys, Inc) to carry out docking program. Firstly, the X-ray structure of DNA was obtained from protein date bank (PDB: 1AIO) and prepared using “protein prepare” tool. Then, the ligand posture scoring module and Libdock Tool have been utilized to generate the structures of receptor along with ligand for the simulation of molecular docking, grid box length is set at 40, it is enough large to cover the whole docking bag.
Results and discussion
Molecular structures
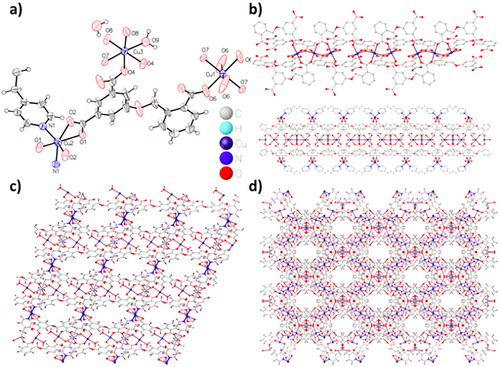
With the concept of pillar-layer architecture in mind, we employed a V-shape organic ligand 5-(2-carboxybenzyloxy)isophthalic acid and a flexible N-donor ligand 1,3-bis(4-pyridyl)propane as the co-ligands to construct the targeted complex 1. Complex 1 was synthesized under hydrothermal conditions. It is worth noting that the addition of NaOH has a main function within forming large crystals of 1 with high crystallinity. The X-ray of single-crystal analysis showed that Cu-MOF 1 crystallizes within the space group of orthorhombic Cccm. The asymmetric unit consists of three different kinds of Cu(II) ions, namely Cu1 accounts for 1/4, Cu2 and Cu3 accounts for half, a bridged hydroxide ion, half dpp ligand and a Hcia2– ligand, a lattice water molecule as well as a coordinated water molecule (. Cu1 coordinates with four O atoms of Hcia2– within the bridging pattern along with two bridged hydroxide (μ2-OH) ions, causing the octahedral geometry of CuO6. Similarly, Cu3 exhibits the geometric structure of CuO6 via coordinating with four O atoms from a single Hcia2– within bridging and double-ring patterns, as well as a water molecule and a μ2-OH ion. In Cu2 case, the distorted octahedral geometry of CuO4N2 is presented by coordinating four O atoms from two distinct Hcia2– with two N atoms from two dpp conjugates. The ligand H3cia shows the species of Hcia2–, among that two carboxylate groups are completely deprototic while one remains intact. The distances of Cu―O are between 2.015(4) and 2.232(5) Å, and the distance of Cu―N is 2.076(5) Å, which is similar to the reported values within the literature [Citation19]. Twelve carboxylic acid along with carboxylic acid groups in six Hcia2– parts are linked together by syn–syn coordination of bicarbonate carboxylic acid group and bridging, Cu1 and Cu3 are connected together to form the cluster of trinuclear Cu, while the chelating carboxylic acid groups coordinated with Cu2 forms a 12.929(2) Å × 15.619(2) Å2 dimensions large channels with the mononuclear manner [the distance denotes the distance between Cu metal ions and Cu, neglecting solvent molecules, . Mononuclear Cu ions and trinuclear copper cluster units are deep connected together via dpp colinkers along with Hcia2– linkers to form a two-dimensional porous framework (. The packing of the 2 D layered network results in a 3 D supramolecular network with a large number of channels between the axis of a and b (. In these channels, pore surface is composed of copper metal ions and μ2-OH as well as O atoms from carboxylic acid group. In addition, the channels are full of disordered water molecules, which could be allocated well from a difference map. The total solvent can enter the void volume is consistent with that calculated by PLATON, which is estimated to be 27.10% (2959.7 out of 10 911 Å3).
Figure 1. (a) 1's basic repetitive unit. (b) The one-dimensional chain-like network within 1. (c) 1's two-dimensional layered network. (d) 1's three-dimensional packing network showing the 1 D channels along the c axis.
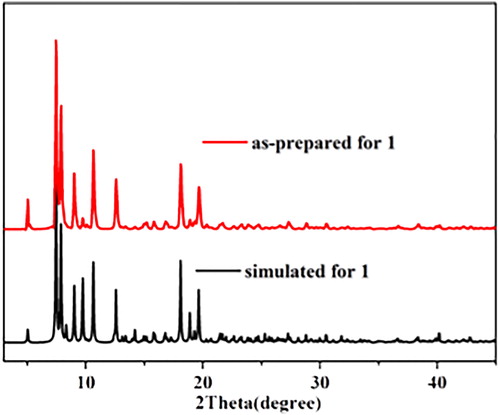
PXRD experiment was carried out on complex 1 in order to establish its crystalline phase purity. As shown in the , the PXRD patterns of the bulk samples were consistent with the simulated patterns based on the single-crystal data, indicating the presence of one crystalline phase in the as-prepared samples of the corresponding complex 1.
Preparation of the nanostructure of 1
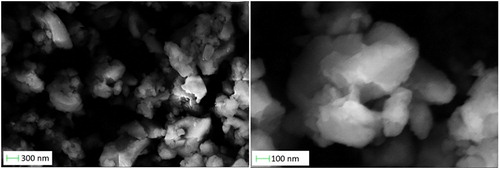
In recent years, nano-scale materials have the characteristics of tailorable surface chemistry, enhanced cell membrane permeability and biocompatibility, high release rate and drug loading, so their clinical application is of great significance. NCP is a hybrid material, which combines the advantages of organic and inorganic nanoparticles. In view of following tests of bioactivity, it virtually reduces 1' s particle size to nano-scale, which stimulates the drug to enter the entire body and then be absorbed via particular tissues via the intravenous injection. Previous researches have revealed that the technology of mechanical grinding is a green along with effective method to establish simple coordination polymer nanostructures. Interestingly, for the single crystal of complex 1, when the single crystal was mechanically ground with a mortar and pestle for about half an hour, complex 1 of crystalline nanoscale (named nano 1 here) was obtained. The formation of nanostructures was deep determined by SEM. The nanoparticles were obtained by droplets of DMSO dispersed on the glass surface. SEM images reveal clusters of nano-1 aggregated sheets, each of which is about 300-500 nanometers in size. The presence of slippery edges and clear facets is a sign of fine crystallinity ().
Nano 1 inhibits the viability of BT474 breast cancer cells
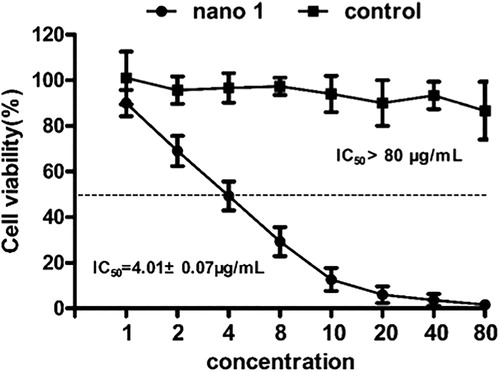
After synthesis of nano 1, the inhibitory effect of the nano 1 on the BT474 breast cancer cells was evaluated using Cell Counting Kit-8, which is reported as the most common method to determine the anti-cancer activity of candidates in vitro. According to the results of , we can find that viability of the BT474 breast cancer cells was significantly reduced after nano 1 treatment, and the inhibitory effect of the nano 1 showed a dose-dependent relationship. The IC50 value of compound on BT474 breast cancer cells is 4.01 ± 0.07 μg/mL. These results suggest that the synthesized compound exhibits excellent anti-cancer activity against BT474 breast cancer cells.
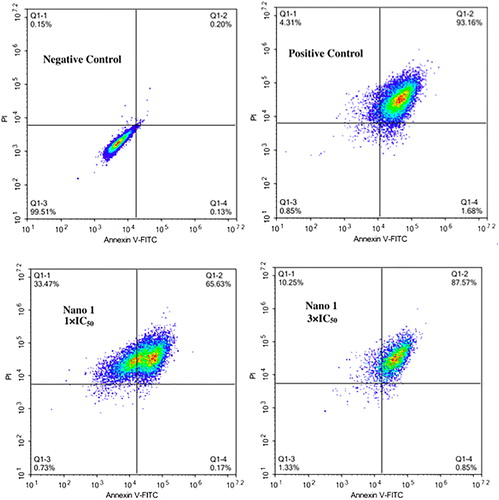
Nano 1 increases the percentage of BT474 breast cancer cell
Within the previous research, we have convinced the excellent inhibitory function of nano 1 on the BT474 breast cancer cells. However, the detain mechanism was still unclear. Thus, percentage of the apoptotic BT474 breast cancer cells was determined with Annexin V-FITC/PI method. From the data in , we got this information, nano 1 could significantly induce the production of the apoptosis in BT474 breast cancer cells, which is different from control groups. The compound induced the apoptosis to 65.63% and 87.57% at the consistence of 1 × IC50 as well as 3 × IC50, respectively. These results suggested the compound could induce the cancer cell apoptosis then exert protective effect in anti-cancer treatment activity.
Figure 5. Increased percentage of the apoptotic BT474 breast cancer cell after nano 1 treatment. The BT474 breast cancer cell were inoculated into plates of 6 well and treated by 1 × IC50 as well as 3 × IC50. The apoptosis of cell was measured via Annexin V-FITC/PI method kit in flow cytometry at 488/525 and 625 mm. This experiment was performed at least three times.
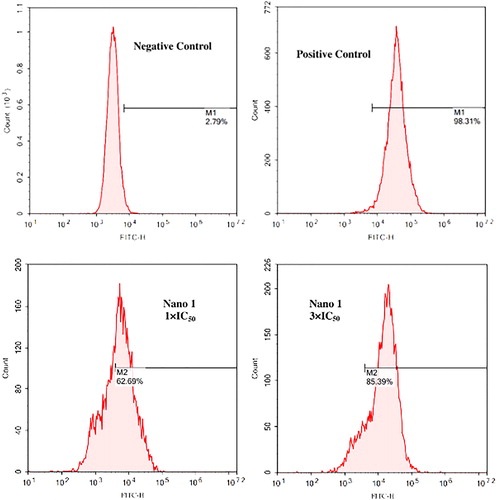
Nano 1 triggers ROS accumulation in BT474 breast cancer cells
ROS also play a vital important role in the cancer cell biological function. It is reported that the ROS could induce the cancer cell apoptosis via damaging the cell DNA. Besides, within the former research, we have demonstrated the excellent induction effect of nano 1 in inducing BT474 breast cancer cells apoptosis. Thus, in this experiment, the ROS accumulation in BT474 breast cancer cells was also measured. As expected, nano 1 treatment dramatically increases intracellular ROS levels in BT474 breast cancer cells with a dose-dependent manner, which was showed in . The nano 1 induce the ROS accumulation level to 62.69% and 85.39% at 1 × IC50 along with 3 × IC50 consistence, respectively. All of those data indicate that nano 1 treatment promotes the accumulation of ROS and then leads to apoptosis in BT474 breast cancer cells. The nano 1 has excellent anti-cancer activity in vitro, and could be further developed into candidates for the clinical anti-cancer treatment.
Figure 6. Increased ROS accumulation in the BT474 breast cancer cells after nano 1 treatment. The BT474 breast cancer cell were inoculated into plates of 6 well and treated by 1 × IC50 as well as 3 × IC50. The levels of ROS within the cancer cells were determined via DCFH-DA with fluorescence method.
Molecular docking
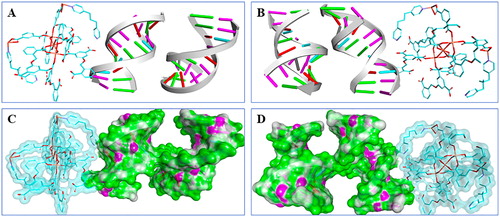
The results of experiment revealed that synthesized compound showed latent binding affinity with receptor DNA, but the potential interaction patterns are still missing. , B reveals possible binding pattern global view, in the view we can find that there is a rod-like appearance of the compound at the docking bag. On the surface, several functional groups of the compound revealed latent capacity for interaction with receptor DNA. The surface binding pattern is exhibited in , which clears showed the binding interaction of receptor and ligand. We can find that compound' s two functional groups revealed latent binding ability with protein sites and helix, which might affect the potency of the receptor DNA. The results are in good agreement with the results of experiment, the compound binds to the receptor DNA and exert anti-breast cancer activity.
Figure 7. Predicted binding pattern of receptor DNA and synthesized compound. (A&B) Binding pattern holistic view reveals a direct binding potential for the compound to DNA. Compound was cyan rod-like and DNA banded. (C&D) The views of surface binding reveal that compound interacts with the DNA through its multiple functional group.
Conclusion
To sum up, we have prepared a novel coordination polymer containing Cu(II) via employment of a mixed-ligand approach. The X-ray of single crystal diffraction research exhibits that the Cu(II)-MOF has a two-dimensional network, which further penetrated into equivalent sets, thus forming a three-dimensional supramolecular network. Furthermore, we use a grinding method to downsize the complex 1 to nano-region. In study of biology, anti-cancer activity of nano 1 against J82 bladder cancer cells was discussed. Firstly, the results of CCK-8 shown that nano 1 could decrease cancer cell activity in vitro. The data of Annexin V-FITC/PI staining suggested that nano 1 could trigger the production of apoptosis within BT474 breast cancer cells and exert the anti-cancer activity. In addition to this, and the ROS data further explained the protective effect of nano 1 was due to it promotes the accumulation of ROS within the BT474 breast cancer cells. The results of ligand scoring module along with molecular docking offered the latent binding patterns of receptor DNA and compound, which shown the possible mechanism for compound to achieve potential therapeutic effects by modulation of DNA.
Acknowledgements
This study was supported by Natural Science Foundation of Shandong Province (No. ZR2017PH032), Higher Educational Science and Technology Program of Shandong Province (No. J17B092), National Natural Science Foundation of China (No. 81302290 and 81700029) and Doctoral Foundation of Shandong Province (No. ZR2017BH061).
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Zhang D, Zhang HQ, Zhao S, et al. Electrochemical impedance spectroscopy evaluation of corrosion protection of X65 carbon steel by halloysite nanotube-filled epoxy composite coatings in 3.5% NaCl solution. Int J Electrochem Sci. 2019;14:4659–4667.
- Muhammad N, Guo Z. Metal-based anticancer chemotherapeutic agents. Curr Opin Chem Biol. 2014; 19:144–153.
- Guo S, Chen R, Li H, et al. Identify severity bug report with distribution imbalance by CR-SMOTE and ELM. Int J Soft Eng Knowl Eng. 2019;29(02):139–175.
- Bergamo A, Sava G. Ruthenium anticancer compounds: myths and realities of the emerging metal-based drugs. Dalton Trans. 2011;40(31):7817–7823.
- Feng X, Feng Y-Q, Chen JJ, et al. Reticular three-dimensional 3d-4f frameworks constructed through substituted imidazoledi carboxylate: syntheses, luminescence and magnetic properties study. Dalton Trans. 2015;44(2):804–816.,
- Feng X, Ma LF, Liu L, et al. A series of heterometallic three dimensional frameworks constructed from imidazole dicarboxylate: structures, luminescence and magnetic property. Cryst Growth Des. 2013;13(10):4469–4479.
- Feng X, Wang LY, Zhao JS, Wang JG, et al. Series of anion-directed lanthanide-rigid-flexible frameworks: syntheses, structures, luminescence and magnetic properties. CrystEngComm 2010;12(3):774–783.
- Feng X, Zhao JS, Liu B, et al. A series of lanthanide-rigid-flexible frameworks based on 2-Propyl-1H-imidazole-4,5-dicarboxylate and oxalate: syntheses, structures, luminescence and magnetic properties. Cryst Growth Des. 2010;10(3):1399–1408.
- Wu H, Chi F, Zhang S, et al. Control of pore chemistry in metal-organic frameworks for selective uranium extraction from seawater. Micropor Mesopor Mat. 2019;288:109567.
- Liu D, Ding C, Chi F, et al. Polymer brushes on graphene oxide for efficient adsorption of heavy metal ions from water. J Appl Polym Sci. 2019;136(43):48156.
- Shen J, Wei Y, Liao P, et al. Unique (3,9)-connected porous coordination polymers constructed by tripodal ligands with bent arms. CrystEngComm 2016;18(22):4115–4120.
- Han Y, Sheng S, Yang F, et al. Size-exclusive and coordination-induced selective dye adsorption in a nanotubular metal–organic framework. J Mater Chem A. 2015;3(24):12804–12809.
- Mondal S, Dastidar P. Mixed ligand coordination polymers for metallogelation and iodine adsorption. Cryst Growth Des. 2019;19(1):470–478.
- Chen DM, Zhang XJ. Stepwise and hysteretic sorption of CO2 in polycatenated metal–organic frameworks. CrystEngComm 2019;21(32):4696–4700.
- Serment-Guerrero J, Cano-Sanchez P, Reyes-Perez E, et al. Genotoxicity of the copper antineoplastic coordination complexes casiopeinas. Toxicol Vitr. 2011;25(7):1376–1384.
- Gandin V, Pellei M, Tisato F, et al. A novel copper complex induces paraptosis in colon cancer cells via the activation of ER stress signaling. J Cell Mol Med.2012;16(1):142–151.
- Rigaku OD. CrysAlis PRO [J]. Rigaku Oxford Diffraction, Yarnton, England, 2015.
- Sheldrick GM. Crystal structure refinement with SHELXL. Acta Crystallogr C Struct Chem. 2015;71(1):3–8.
- Chen DM, Zhang XJ. A polyoxometalate template metal-organic framework with unusual {Cu8(μ4-OH)6}10+ secondary building unit for photocatalytic dye degradation. Inorg Chem Commun. 2019; 108:107523–107526.