?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
A fast and facile method was employed for the synthesis of magnetic graphene oxide (MGO). The synthesized MGO is characterized by different techniques and used as a solid-phase adsorbent for the extraction of β-sitosterol from rapeseed oil deodorizer distillates (RODD) followed by gas chromatography-mass spectrometry analysis. Several parameters affecting the extraction efficiency, including the amount of adsorbent, extraction time and temperature, desorption solvent and desorption time, were investigated. The procedure exhibited desirable extraction efficiency within 30 min at 35 °C. Recoveries higher than 80% were obtained with acetone as eluent and the method was successfully applied to concentrate the β-sitosterol in RODD CO2-supercritical extract. Compared to C18-silica and graphitic carbon, the composite showed satisfactory results for the extraction of β-sitosterol from oil samples.
1. Introduction
Deodorisation is the last step of the refining process responsible for the elimination of the volatile compounds that produce undesirable odor, color and flavor in the edible oils. The deodorizer distillate (DOD) achieved in this stage is the principal by-product of edible oil refining industries [Citation1]. A complex mixture of different constituents is determined in DODs such as free fatty acids (FFAs), glyceride esters of fatty acids (predominantly mono and diglyceride esters), terpenic and aliphatic alcohols, waxes, carotenoid pigments, squalene, tocopherols, free phytosterols and phytosterol esters of fatty acids [Citation2]
The composition of DODs depends on the quality of crude oil, deodorizer type, processing parameters mainly temperature, time, vacuum, and the volume of stripping steam. According to previous reports, rapeseed oil deodorizer distillate (RODD) contain a large portion of FFAs (3–50 wt%), phytosterols (7–15 wt%), and tocopherols (5–8.20 wt%). Phytosterols are the most abundant constituent (21.27–25.53 wt%) in the unsaponified part of different canola DODs) [Citation3].
Phytosterols are one of the main groups of steroid alcohol phytochemicals that naturally exist in various types of vegetables and fruits. They have a similar backbone with slight differences in the number of carbon atoms, and also the location of a double bond in the side chain [Citation4]. Considering the low quantity in natural resources and also nearly the same physical characteristics, separation of phytosterols is considerably difficult. Stigmasterol, β-sitosterol, and campesterol are the main dietary phytosterols thet play a vital role in reducing blood cholesterol and cardiovascular diseases in humans. The health benefits of phytosterols lead to their use in functional foods [Citation5].
Commercially available phytosterols are expensive and have inadequate purity. The synthesis of phytosterols usually needs carcinogenic chemicals. The process includes reactions with low selectivity and generates side products; require extra purification. It is possible to purify sterols from a natural mixture or implement a physical/chemical process for this purpose, with practical difficulties due to their similar structure [Citation6]. Generally, a few reports are available on the isolation and purification of individual sterols from a natural source.
Solid-phase extraction (SPE) and liquid–liquid extraction are conventional methods for the extraction of a variety of analytes, such as sterols [Citation7]. Isolation of β-sitosterol from the unsaponifiable portion of a crude sterol mixture was previously reported [Citation8]. The sample treatment was conducted using a blend of fragrant hydrocarbons, a solvent, a polar organic and water. A recovery of over 70%, and a purity of 93% (original content of β-sitosterol was 65%) was reported for The β-sitosterol. Xu et al. [Citation6], achieved satisfying results for the isolation of stigmasterol (7% recovery and 90% purity) and β-sitosterol (39% recovery and 88% purity) by utilising a 5-stage and a 3-stage solvent crystallisation, respectively. Hammann and Veter [Citation9] determined the amount of free and esterified sterols in button mushrooms with solvent extraction followed by gas chromatography-mass spectrometry. The supercritical carbon dioxide was also used for the extraction of phytosterols from edible oil waste [Citation10].
Solid-phase extraction (SPE) is a sample preparation technique with a low solvent requirement and high extraction efficiency. SPE found widespread applications for the extraction of desired analytes in environmental [Citation11], food [Citation12], and biological matrices [Citation13]. Also, the isolation of phytosterols described with silica gel [Citation14] and molecularly imprinted polymers [Citation15] as SPE adsorbents. Although, samples with significant amounts of phytosterols mostly have grease nature and can affect the extraction efficiency of traditional C18 SPE-adsorbents [Citation16]. Therefore, the development of new adsorbent materials is necessary for the purification and enrichment of phytosterols.
Ferrous oxide (Fe3O4) nanoparticles (NPs) are extensively studied due to their biocompatibility, superparamagnetic property, catalytic activity, low toxicity, and low cost. Today, a facile, economical, and effective separation method is magnetic separation using the superparamagnetic Fe3O4 [Citation17] but the aggregation of Fe3O4-NPs and weak cyclic stability limit their application. Several attempts have been made to include Fe3O4 nanoparticles into other structures such as chitosan [Citation18], carbon nanotube [Citation19], graphene [Citation12, Citation20], core-shell structured Fe3O4 [Citation21] to improve extraction capacity and selectivity. graphene oxides (GO) for the immobilisation of lipase for biodiesel production [Citation22], core–shell structured magnetic composites for manufacturing of trans-free plastic fats [Citation23] and core–shell structured Fe3O4/SiO2 for the transformation of low-cost oils to biodiesel [Citation24].
One of the best candidates for the preparation of novel composite materials is graphene. With unique physicochemical features including significant surface area, huge reversible specific capacity, and remarkable conductivity, graphene has a diversity of applications in multiple fields of technology [Citation25]. Nonetheless, pristine graphene sheets have a tendency for agglomeration or even restacking form graphite. Hence, the adsorption properties of graphene significantly destroyed due to the reduction of surface area. An effective route to expand the outstanding properties of graphene is chemical modification. Typically, GO has a quasi-two-dimensional structure with high amounts of carboxyl and hydroxyl groups on its slice layer. As an attractive derivative of graphene, it has a structure comparable to that of graphene and comprises a single layer of SP2 carbon atoms [Citation16].
Up to now, GO-Fe3O4 nanocomposites exhibited attractive applications in magnetic resonance imaging [Citation26], drug delivery [Citation27], environmental remediation [Citation20], and as electrode materials and magnetic controlled switches [Citation28]. Cui et al. (2018) developed a composite monolith based on GO to determine ß-sitosterol in food samples. In the same vein, GO sheets were embedded in polymer monolithic columns by Jin et al. [Citation29] to determine carbamate insecticides through HPLC analysis. Also, magnetic graphene nanocomposite was considered as an adsorbent for the extraction of vitamin D2 and vitamin D3 from milk samples [Citation30]. Magnetic SPE based on GO was evaluated by Wu et al. [Citation17] for the determination of lignans in sesame oil.
In this study, a simple strategy used to prepare magnetic GO (MGO). The product was characterized through transmission electron microscopy (TEM), field emission scanning electron microscopy (FE-SEM), X-ray diffraction (XRD), and energy dispersive X-Ray spectroscopy (EDS). The adsorption/desorption behavior of β-sitosterol was investigated in a model system and the as-synthesized nanocomposite used for the separation and purification of phytosterols from the DOD sample.
2. Materials and methods
2.1. Chemicals and samples
β-sitosterol (98% purity) was purchased from Sigma-Aldrich (St. Louis, MO, USA). Graphite powder (50 mesh size), ferrous sulfate (FeSO4·7H2O), sodium hydroxide (NaOH), potassium permanganate (KMNO4), sodium nitrate (NaNO3), ammonium hydroxide (NH4OH), hydrogen peroxide (H2O2, 30%), acetonitrile (MeCN), and other reagents were obtained from Merck (Darmstadt, Germany). All chemicals were of analytical grade and used as received without any purification. RODD was provided by Shadgol Vegetable Oil Factory (Neishaboor, Iran) in August 2015 and stored under refrigeration (4 °C) until the analysis.
2.2. Apparatus
A scanning electron microscope combined with an energy dispersive X-ray probe (FE-SEM: MIRA3 TESCAN-XMU) was used to study the surface morphology and elemental analysis of the nanocomposite. The presence of iron in the prepared MGO was confirmed by transmission electron microscopy (TEM: Philips CM 12, Netherlands). The X-ray diffraction (XRD) analyses were conducted with an Ultima IV (Rigaku) X-ray diffractometer equipped with a Cu-Kα radiation source (λ = 0.1514 nm) operated at a generator voltage and current of 40 kV and 40 mA, respectively. Infrared analyses were conducted with a Nicolet Nexus 470 FTIR spectrometer (Thermo Nicolet, USA) where KBr was used to prepare the sample tablets. The ultraviolet and visible measurements performed on a 2501 PC UV-Vis spectrophotometer (Shimadzu, Japan). Supercritical extractions were conducted on an SFE apparatus (Model CO-960, SFX, Spain) consists of a first extraction column working with a supercritical CO2/feed (S/F) mass ratio of 5 at 313 K and 40 MPa followed by a second extraction column working with an S/F mass ratio of 7 at 313 K and 35 MPa [Citation31].
2.3. Chromatographic analysis
The bioactive compounds were separated and detected by a gas chromatography instrument coupled with a quadrupole mass spectrometer (5977A-Agilent Technology, USA) [Citation10]. Data analysis was performed on the Agilent MassHunter software (Agilent Technologies, USA).
We applied the separation to a HP-5MS capillary column (0.25 μm film × 30 m × 0.25 mm ID thicknesses). The original temperature of oven (190 °C) was maintained for 2 min and then elevated to 250 °C at 15 ˚C min−1. Helium was used as the carrier gas with a flow rate of 0.5 mL min−1. The sample injections were carried out in triplicates with an injection volume of 1 µL and a split-split less ratio of 1:10. The injector and detector were kept at 310 and 250 °C, respectively. The mass detection was implemented with an electron impact (EI) ion source mode at 70 eV in the mass scan range of 50–550 m/z.
2.4. Synthesis of magnetic graphene oxide
Graphene oxide was synthesized through a modified Hummers method [Citation32]. Briefly, graphite powder (1.0 g) and NaNO3 (0.5 g) mixed with 50 mL of cooled H2SO4 (98 wt%) and under vigorous stirring to avoid agglomeration. When graphite powder was dispersed entirely, KMnO4 (5.0 g) was added gradually under stirring. The temperature was kept below ten degree of centigrade for the next 30 min. The ice bath was then removed, and the mixture stirred at 35 °C (2 h). Then a certain amount of deionized water was added gently, and the diluted suspension stirred at 95 °C for another 30 min. Later, 50 mL of H2O2 (30 wt%) was added, turning the color of the solution from dark brown into bright yellow. The final suspension was centrifuged and repeatedly washed with an HCl aqueous solution (5%v/v) and subsequently neutralized with deionized water. The product (0.7 g) was separated and dispersed in deionized water (1000 mL). The bulk graphite oxide powder was exfoliated into GO sheets by ultrasonication (1 h), and the resulting product was washed with deionized water and finally air-dried.
For the synthesis of MGO, 0.1 g GO was dispersed in 50 mL deionized water through sonication. Then, ferrous sulfate (FeSO4·7H2O, 0.695 g) was added, and the pH was adjusted in the range of 11–12 with ammonium solution (30 wt%). The reaction mixture was transferred into a Teflon-lined stainless steel autoclave and heated to 200 °C and aged at this temperature for 2 h. The precipitate was then cooled naturally to room temperature and separated in the magnetic field. The resulting nanocomposites were washed with deionized water and dried in a vacuum at 50 °C overnight.
2.5. Batch sorption experiments
The synthesized MGO nanocomposite was evaluated as an adsorbent in batch experiments for magnetic solid-phase extraction (MSPE) of β-sitosterol. The RODD supercritical extract (SFE-RODD, 0.1 g) was mixed with 4.0 mL toluene in a test tube. Then, MGO composite (10.0 mg) was added and vortexed intensively under ambient temperature. After a specific time interval, the MGO nanoparticles were separated with an external magnetic field. The residual concentration of sterols was determined by spectrophotometric determination at 640 nm in the linear range of 0.5–2.5 mg/mL [Citation33]. Then, the analyte was desorbed by the addition of acetone (2.0 mL) into the analyte loaded-MGO and mixing for 1.0 min. The adsorbent separated in an external magnetic field, and the desorption solvent was collected. We repeated the desorption procedure twice. After combining the desorption solutions, they were transferred to a 10-mL microtube only to be vaporized to dryness while exposed to a moderate stream of nitrogen. Finally, we re-dissolved the residue with 100 µL acetone, injecting 1 µL into the GC-MS system for further analysis. The adsorption efficiency and recovery were calculated according to the Equaions (1) and (Equation2(2)
(2) ), respectively [Citation34].
(2)
(2)
Where C0 and Ce are the initial and final concentrations of the analyte in the solution (mg/L), respectively. All the experiments were carried out in two replicates and the mean values are reported.
3. Results and discussion
3.1. Characterisation of magnetic nanocomposite
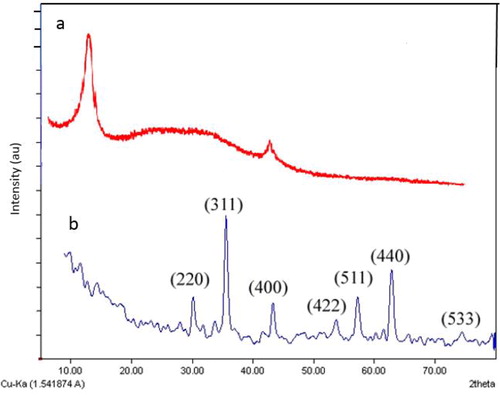
X-ray diffraction (XRD) analysis was performed from 2θ = 1.0°–80.0° to achieve structure and phase information of MGO (). As shown in , the diffraction peak at 2θ = 10.2 can be confidently indexed as the (001) reflection of the GO [Citation27]. There are seven diffraction lines () evident in the characteristic XRD pattern at 2θ = 30.2°, 35.5°, 43.2°, 53.2°, 57.7°, 62.8° and 74.1° which can be indexed to (2 2 0), (3 1 1), (4 0 0), (4 2 2), (5 1 1), (4 4 0) and (5 3 3) planes of the Fe3O4 (JCPDS no. 89-4319) with a face-centered cubic structure [Citation35]. These results suggest the presence of Fe3O4 in the magnetic composite. Moreover, the characteristic diffraction peak of GO (at 11.6°) does not appear () that indicates complete conversion of graphite into reduced GO [Citation36].
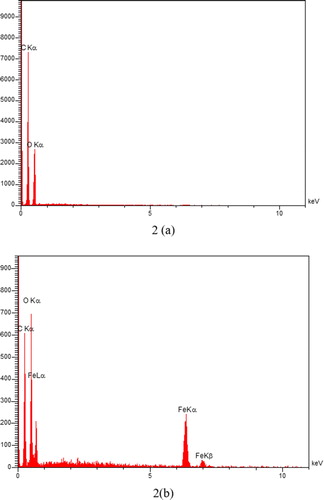
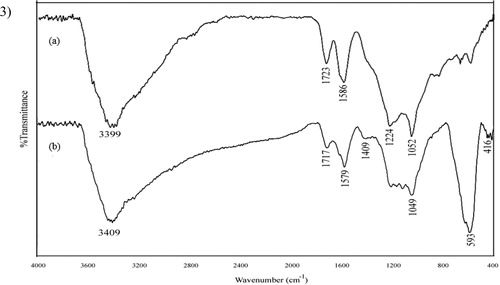
Figure 1. (1) XRD pattern, (2) EDX spectrum, (3) Infrared spectra of (a) GO and (b) MGO.
Energy-dispersive X-ray spectra (EDX) of GO and resulting nanocomposite were also acquired, and the expected elements and embedded NPs recognized. The EDX spectrum of GO showed peaks correspond to the carbon and oxygen (), while the magnetic graphene showed the presence of carbon, oxygen, and iron ().
The FT-IR spectra were recorded in the 4000–400 cm−1 region. In graphite oxide (), the bands at 3399.2 and 1723.6 cm−1 correspond to O–H and C = O stretching vibrations and the band appearing at 1586.1 cm−1 is attributed to OH bending vibration, epoxide groups, and skeletal ring vibrations [Citation37]. The other two vibrational bands located at 1224.5 and 1052.2 cm−1 could be attributed to COO– symmetric vibration, alkoxy C–O and epoxy C–O stretching vibrations, respectively [Citation38]. The typical IR properties of graphite oxide exhibit an abundance of functional groups that contain oxygen, which demonstrates the effective oxidation of graphite. The IR spectrum of MGO, as shown in (), is not the same as graphite oxide, as manifested in vanishing of epoxy C–O and C = O stretching vibrations. The bands located at 1209.1, 1172.3, 1123.2 and 1049.3 cm−1 may be attributed to chemical interaction between carbonyl and hydroxyl groups of GO and magnetic nanoparticles. The absorption band emerging at 1579 cm−1 could be assigned to the graphene sheets’ skeletal vibration. Hence, one may conclude that GO has been reduced to graphene sheets. Besides, the appearance of keen peaks near 593.4 and 632 cm−1 could be attributed to the absorption of iron oxide by lattice, which suggests the successfully grafting of Fe3O4 on GO [Citation37].
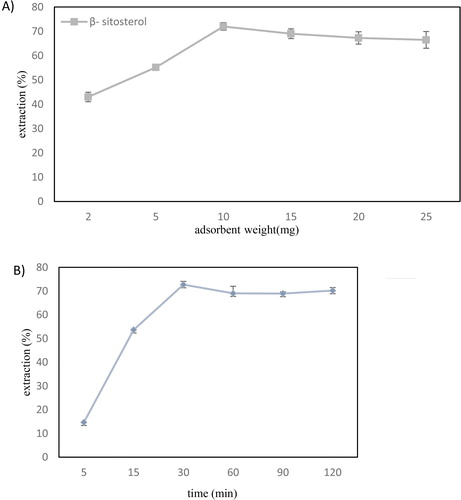
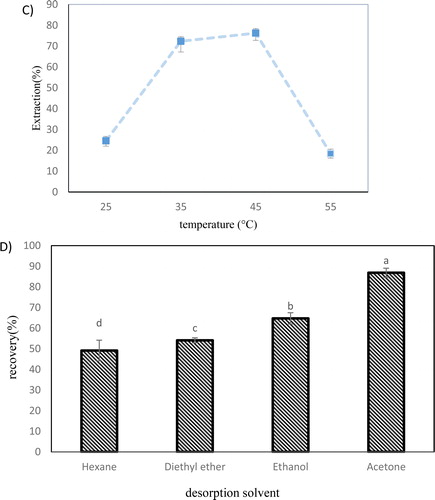
Figure 3. The effect of (a) adsorbent weight, (b) extraction time, (c) temperature on the extraction efficiency of β-sitosterol and (d) represents the desorption efficiency of different solvents and showed quantitative recovery of the β-sitosterol. (Conditions: sample volume = 4 mL, concentration = 5 mg/L, Desorption conditions: solvent volume = 5 mL, time= 1 min, desorption mode: vortex). Error bars show the standard deviations of the means (n = 3).
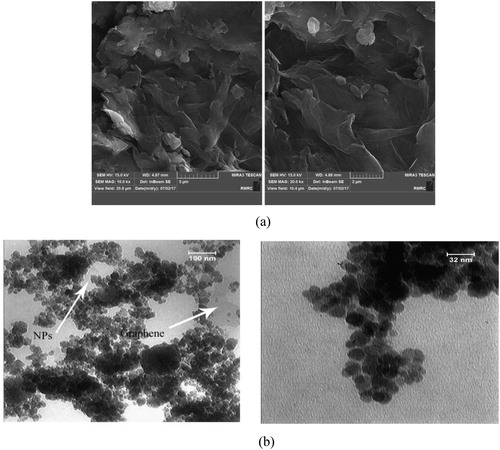
As presented in , SEM images of GO displayed an uneven shape with a flat surface and a number of wrinkles over a vast surface area that provide a considerable number of adsorption sites [Citation36]. The TEM images of MGO (), visually demonstrate the presence of Fe3O4 nanoparticles with an average size of 16 nm. They are inhomogeneously assembled on the surface of GO layers. Lujanien et al. [Citation39], reported the inhomogeneous distribution of NPs on the surface of the GO sheets. A long sonication time for TEM analysis does not affect the Fe3O4 nanoparticles, which indicates a strong interaction between Fe3O4 and GO sheets [Citation37].
3.2. Optimisation of MSPE conditions
The effect of the main parameters like adsorbent weight, elution solvent, adsorption temperature and adsorption time studied through batch SPE experiments.
3.2.1. Effect of amount of MGO
As presented in , the efficiency was significantly improved when the amount of MGO increased, and the maximum adsorption of β-sitosterol achieved for at least 10.0 mg of the adsorbent. With higher amounts of adsorbent, the extraction efficiency remained nearly constant, and 10.0 mg was selected for further studies. An improvement in the extraction efficiency proportional to the adsorbent weight can be attributed to the increases in the adsorbent surface area and also the number of available active sites [Citation19]. These trends are in agreement with previous studies [Citation30].
3.2.2. Effect of extraction time
During an SPE practice, a chemical equilibrium must be established between the analyte and adsorbent surface. Therefore, extraction time is a critical parameter in the evaluation of a new adsorbent. A short extraction time induces incomplete removal of the target substance, while a long extraction time makes the process unnecessarily lengthy [Citation30]. The effect of the extraction time was examined over different time intervals ranging from 5 to 120 min, while other experimental conditions were maintained constant As presented in , the extracted amount of β-sitosterol reached a maximum when the extraction time increased, exhibiting the possibility of obtaining extraction equilibrium in 30 min. Thus, an extraction time of 30 min was chosen for further experiments. More extended extraction times are not effective in the extraction efficiency due to the saturation of active sites [Citation40].
3.2.3. Effect of extraction temperature
The temperature rise can expedite the mass transfer between adsorbents and the solution [Citation30]. Therefore, the extraction experiments were conducted at different temperatures (25–55 °C). The results () showed a notable increase in the extraction efficiency when temperature increased up to 45 °C. But, the extracted amount of β-sitosterol decreased along with the rise of temperature from 45 to 55 °C. The reason could be that sterols are not stable at higher temperatures [Citation41], and extraction at 35 °C is sufficient to extract sterols. Some thermodynamic variables of the adsorption process including enthalpy (ΔH°), Gibbs free energy (ΔG), and entropy (ΔS°) could obtained from the van't Hoff equation (EquationEquation (3)(3)
(3) ) [Citation42]:
(3)
(3)
where R is the ideal gas constant, T is temperature (K) and K is Langmuir's constant. The slope and intercept of the plots of lnKc versus 1/T were used to determine ΔH° and ΔS° (EquationEquation (4)
(4)
(4) ) and Kc (gr/mL) is the ratio of the amount of sterol (mg/g) adsorbed on the adsorbent to its residual content in solution (mg/mL) [Citation43].
(4)
(4)
The negative ΔG value reveals that the adsorption process is spontaneous (). The amplified free energy variation with temperature rise reflects a greater adsorption feasibility at higher temperatures. On the other hand, a positive ΔH value suggests that adsorption is endothermic and that a weak bonding can be forged between adsorbent and adsorbate [Citation42]. The negative value of ΔS indicates a decrease in disorder by increasing the temperature in the solid phase and liquid phase during the absorption process [Citation43].
Table 1. Thermodynamic parameters of β-sitosterol on MGO nanocomposite.
3.2.4. Effect of desorption parameters
A small volume of solvent is recommended for the elution step to attain high enrichment factors. In this study, organic solvents and desorption time were examined to achieve complete desorption of analyte. For the recovery of the bounded analyte, the β-sitosterol-loaded nanocomposite was separated from the initial solution and subsequently vortexed in 5.0 mL of different solvents. Considering the solubility of β-sitosterol in organic solvents, ethanol, hexane, acetone, and diethyl ether were compared in terms of their desorption capability.
The results showed the quantitative recovery achieved with 5.0 mL of acetone (). The previous studies stated that β-sitosterol has a significantly lower solubility in a non-polar solvent like n-hexane compared to acetone and ethanol [Citation8]. However, the solvent polarity is not the absolute factor, and chemical structure also influences the solubility. Given the hydrophobic steroid skeleton moiety of β-sitosterol and its hydroxyl group, the entire steroid molecule is loosely polar. Hence, the acetone interactions are chiefly due to van der Waals forces, which may boost compound dissolution [Citation44].
In the following, the effect of different mixing times studied. The results showed insignificant differences (p < 0.05), and 1.0 min was selected. Wu et al. [Citation17] suggested that 1.5 min is an optimal time for the desorption of Sesamol and Sesamolin in sesame oil from the magnetic absorbent. They also stated that increasing desorption time to 2.0 min, did not significantly affect the recovery of target compounds. Ding et al. [Citation19] also proposed 1.0 min as the optimal time for desorption of estrogen from magnetic absorbent in a milk sample.
3.3. Comparison with other adsorbents
The performance of MGO was compared with some traditional adsorbents and other reported adsorbents for the extraction and purification of phytosterols (). For graphitic carbon, extraction performed at optimum conditions of adsorption/elution similar to MGO. The results revealed low extraction efficiency, mainly due to its large particle size, blank volume, and limited active sites [Citation45]. The presence of hydroxyl groups makes the silica gel surface to be polar, so the adsorption of polar molecules including alcohols, water, amines (derived from hydrogen bonds), phenols as well as unsaturated hydrocarbons (via π–π interactions) is more dominant than non-polar analytes such as saturated hydrocarbons [Citation14]. Satisfying results need higher amounts of silica gel as adsorbent leads to an increase in the analysis cost. The poor recoveries of β-sitosterol by the zeolite may be caused by more operation steps including several crystallisation and adsorption process. Higher recovery is one of the clear advantages of GO over silica gel-C18, graphitic carbon, and zeolite along with easier separation and desorption.
Table 2. Comparison of the performance of grapheme oxide with several other adsorbents.
3.4. Purification of β-sitosterol from RODD-supercritical extract
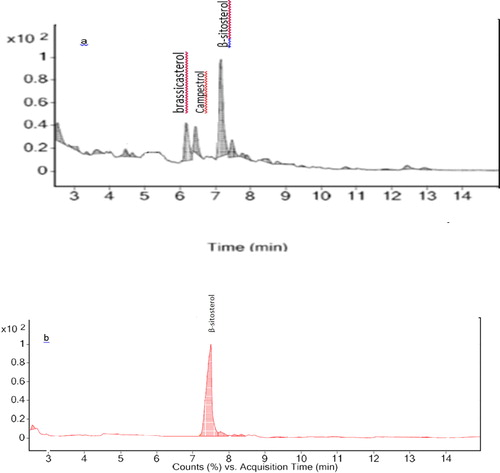
The RODD sample was treated in a CO2 supercritical fluid extraction (SFE) system (the experimental conditions previously described in our previous work [Citation46] followed by GC-MS analysis of the final extract. The results () showed the sterols are purified, and the extract contains 65 wt% phytosterols includes β-sitosterol, campesterol, and brassica sterol. The non-polar nature of carbon dioxide leads phytosterols to transfer to the extract while the polar components remain in the raffinate. Then, the proposed SPE method was performed with SFE extract as the initial sample and under optimized conditions. The results () showed the separation of β-sitosterol by adsorbent with a final recovery of 86%. It demonstrates the feasibility of the suggested method for the purification and determination of β-sitosterol in DOD.
4. Conclusion
In the current study, MGO was synthesized via a straightforward method. It was successfully applied for the separation and purification of β-sitosterol in supercritical CO2 extract of RODD samples. We can conclude that MGO nanocomposite is a good candidate for the extraction of β-sitosterol. It is a cheap adsorbent, easy to separate, and with high extraction efficiency. The recovery achieved for GO was greater than other adsorbents, which could be attributed to the huge surface area and exceptional chemical composition of graphene. More studies are required to corroborate the feasibility of this process in practice. Moreover, the stability and repeatability and reusability of adsorbent is also an important parameter in reducing the costs of this process which has not been investigated in this study.
Acknowledgements
The authors would like to acknowledge all the research laboratories in the research institute of food science and technology (RIFST) for the help of chemical analysis.
Disclosure statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Notes on contributors
Parisa Jafarian-asl
Parisa Jafarian Asl, PhD of food chemistry, research institute of food science and technology, Mashhad, Iran.
Razieh Niazmand
Razieh Niazmand, Associated Professor, food chemistry department, research institute of food science and technology, Mashhad, Iran.
Moslem Jahani
Moslem Jahani, Assistant Professor, food chemistry department, research institute of food science and technology, Mashhad, Iran.
References
- Shoaib H, Mahesar SA, Jafarian P, et al. Quality evaluation of canola oils and deodorizer distillate during industrial processing. J Chem Soc Pak. 2019;41:983–992.
- Estiasih T, Ahmadi K, Widyaningsih TD, et al. Bioactive compounds of Palm Fatty Acid Distillate (PFAD) from several palm oil refineries. Adv J Food Sci Technol. 2013;5(9):1153–1159.
- Sherazi STH, Mahesar SA, Sirajuddin. Vegetable oil deodorizer distillate: a rich source of the natural bioactive components. J Oleo Sci. 2016;65(12):957–966.
- Asl PJ, Niazmand R, Yahyavi F. Extraction of phytosterols and tocopherols from rapeseed oil waste bysupercritical CO2 plus co-solvent: a comparison with conventional solvent extraction. J Heliyon. 2020;6:e03592. doi:
- Naz S, Sherazi S, Talpur F, et al. Chemical characterization of canola and sunflower oil deodorizer distillates. Pol J Food Nutr Sci. 2014;64(2):115–120.
- Xu WL, Huang YB, Qian JH, et al. Separation and purification of stigmasterol and β-sitosterol from phytosterol mixtures by solvent crystallization method. Sep Purif Technol. 2005;41(2):173–178.
- Huang Z, Liu S, Xu J, et al. Porous organic polymers with different pore structures for sensitive solid-phase microextraction of environmental organic pollutants. Anal Chim Acta. 2017;989:21–28.
- Chuang YH, Ju YH, Widjaja A. Separation of campesterol and β-sitosterol from a sterol mixture. Sep Sci Technol. 2006;41(13):3027–3038.
- Hammann S, Vetter W. Method development for the determination of free and esterified sterols in button mushrooms (Agaricus bisporus). J Agric Food Chem. 2016;64(17):3437–3444.
- Jafarian Asl PJ, Niazmand R, Jahani M. Chemical Analysis of composition of raw soybean oil deodorized distillates by GC-MS. Res Innov Food Sci Technol. 2019. doi:10.22101/JRIFST.2019.07.28.e1023.
- Stankovich S, Dikin DA, Dommett GHB, et al. 2006. Graphene-based composite materials. Nature 442:282–286.
- Magnuson BA, Jonaitis TS, Card JW. A brief review of the occurrence, use, and safety of food-related nanomaterials. J Food Sci. 2011;76(6):126–133.
- Zheng Q, Kim J-K. Graphene for transparent conductors. New York: Springer; 2015;
- Gunawan S, Kasim N, Ju YH. Separation and purification of squalene from soybean oil deodorizer distillate. J Sep Purif Technol. 2008;60(2):128–135.
- Kane SN, Mishra A, Dutta AK. Preface: International Conference on Recent Trends in Physics (ICRTP 2016). J Phys Conf Ser. 2016;755(1):011001.
- Cui B, Guo B, Wang H, et al. Graphene oxide-based composite monolith as new sorbent for the on-line solid phase extraction and high performance liquid chromatography determination of ß-sitosterol in food samples. Talanta. 2018;186:200–205.
- Wu L, Yu L, Ding X, et al. Magnetic solid-phase extraction based on graphene oxide for the determination of lignans in sesame oil. Food Chem. 2017;217:320–325.
- Fang CL, Xiong ZC, Qin HQ, et al. One-pot synthesis of magnetic colloidal nanocrystal clusters coated with chitosan for selective enrichment of glycopeptides. Anal Chim Acta. 2014;841:99–105.
- Ding J, Gao Q, Li XS, et al. Magnetic solid-phase extraction based on magnetic carbon nanotube for the determination of estrogens in milk. J Sep Sci. 2011;34(18):2498–2504.
- Ye N, Shi P. Applications of graphene-based materials in solid-phase extraction and solid-phase microextraction. Sep Purif Rev. 2015;21:125–191.
- Wang J-N, Shao R-Q, Zhang Y-L, et al. Biomimetic graphene surfaces with super- hydrophobicity and iridescence. Chem Asian J. 2012; 7(2):301–304.
- Xie W, Huang M. Immobilization of Candida rugosa lipase onto graphene oxide Fe3O4 nanocomposite: Characterization and application for biodiesel production. Energy Convers Manage. 2018;159:42–53.
- Xie W, Zang X. Lipase immobilized on ionic liquid-functionalized magnetic silica composites as a magnetic biocatalyst for production of trans-free plastic fats. Food Chem. 2018;257:15–22.
- Xie W, Wang H. Immobilized polymeric sulfonated ionic liquid on core-shell structured Fe3O4/SiO2 composites: A magnetically recyclable catalyst for simultaneous transesterification and esterifications of low-cost oils to biodiesel. Renew Energy. 2020;145:1709–1719.
- Wang L, Zang X, Chang Q, et al. Determination of triazole fungicides in vegetable samples by magnetic solid-phase extraction with graphene-coated magnetic nanocomposite as adsorbent followed by gas chromatography-mass spectrometry detection. Food Anal Methods. 2014;7(2):318–325.
- Zhu Y, Murali S, Cai W, et al. Graphene and graphene oxide: synthesis, properties, and applications. Adv Mater Weinheim. 2010;22(35):3906–3924.
- Zhang M, Lei D, Du Z, et al. Fast synthesis of SnO2/graphene composites by reducing graphene oxide with stannous ions. J Mater Chem. 2011;21(6):1673–1676.
- Liang J, Xu Y, Sui D, et al. Flexible, magnetic, and electrically conductive graphene/fe3o4 paper and its application for magnetic-controlled switches. J Phys Chem C. 2010;114(41):17465–17471.
- Jin T, Li F, Cheng J, et al. Polymer monolithic column containing embedded graphene oxide sheets for sensitive determination of carbamate insecticides by HPLC. J Microchim Acta. 2016;183(2):543–551.,
- Jiao Z, Jiao S, Guo Z, et al. Determination of Trace Vitamin D in Milk Samples by Graphene-Based Magnetic Solid-Phase Extraction Method Coupled with HPLC. Food Anal Methods. 2017;10(3):820–826.
- Asl PJ, Niazmand R. Modelling and simulation of supercritical CO2 extraction of bioactive compounds from vegetable oil waste. J Food Bioprod Process. 2020;122:311–321.
- Hummers WS, Offeman RE. Preparation of graphitic oxide. J Am Chem Soc. 1958;80(6):1339–1339.
- Sabir SM, Imran H, Ahmed SD. Estimation of sterols in edible fats and oils. Pak J Nutr. 2003;2:130–139.
- Han Q, Wang Z, Xia J, et al. 2013. Short communication graphene as an efficient sorbent for the SPE of organochlorine pesticides in water samples coupled with GC – MS. J Sep Sci. 36:3586–3591.
- Lu X, Niu M, Qiao R, et al. Superdispersible PVP-coated Fe3O4 nanocrystals prepared by a "one-pot" reaction. J Phys Chem B. 2008;112(46):14390–14394.
- Choi Y-J, Kim E, Han J, et al. A novel biomolecule-mediated reduction of graphene oxide: a multifunctional anti-cancer agent. Molecules. 2016;21(3):375–385.
- Yan S, Qi TT, Chen DW, et al. Magnetic solid phase extraction based on magnetite/reduced graphene oxide nanoparticles for determination of trace isocarbophos residues in different matrices. J Chromatogr A. 2014;1347:30–38.
- Ma YX, Li YF, Zhao GH, et al. Preparation and characterization of graphite nanosheets decorated with Fe3O4 nanoparticles used in the immobilization of glucoamylase. Carbon. 2012;50(8):2976–2986.
- Lujanien G, Šemčuk S, Lečinskytė A, et al. Magnetic graphene oxide based nano-composites for removal of radionuclides and metals from contaminated solutions. J Environ Radioact. 2016;23:1–9.
- Kumar E, Bhatnagar A, Kumar U, et al. Defluoridation from aqueous solutions by nano-alumina: characterization and sorption studies. J Hazard Mater. 2011;186(2–3):1042–1049.
- Busch TP, King AJ. Stability of cholesterol, 7-ketocholesterol and β-sitosterol during saponification: ramifications for artifact monitoring of sterol oxide products. J Am Oil Chem Soc. 2010;87(9):955–962.
- Pimentel PM, Melo MAF, Melo DMA, et al. Kinetics and thermodynamics of Cu(II) adsorption on oil shale wastes. Fuel Process Technol. 2008;89(1):62–67.
- Arzu YD. A comparative study on determination of the equilibrium, kinetic and thermodynamic parameters of biosorption of copper(II) and lead(II) ions onto pretreated Aspergillus niger. J Biochem Eng J. 2006;28:187–195.
- Wei D, Wang L, Liu C, et al. Sitosterol solubility in selected organic solvents. J Chem Eng Data. 2010;55(8):2917–2919.
- Zhou QX, Wang WD, Xiao JP, et al. Comparison of the enrichment efficiency of multiwalled carbon nanotubes, C18 silica, and activated carbon as the adsorbents for the solid phase extraction of atrazine and simazine in water samples. Microchim Acta. 2006;152(3–4):215–224.
- Asl PJ, Niazmand R, Jahani M. Theoretical and experimental assessment of supercritical CO2 in the extraction of phytosterols from rapeseed oil deodorizer distillates. J Food Eng. 2019;269:109748.
- Rajan, RG, Krishna A. Simple method for purification of deodorizer distillate from Indian rice (Oryza Sativa) bran oil and preparation of phytosterols. J.Grasas Aceites. 2014;65(4):87–90.