Abstract
In bionanotechnology, nanoparticles synthesis using biological samples is a broadly used technique as it confers lesser toxicity than chemical methods. Leaf extract of Piper colubrinum Link. was used to synthesise green mediated silver nanoparticles (AgNPs). The confirmation and stability of green synthesised silver nanoparticles by UV–visible spectroscopy showed an absorption peak at 410 nm. The synthesised nanoparticles characterised by Fourier transformed infra-red spectroscopy exhibited possible involvement of various functional groups and bio capping of AgNPs and the crystal structure was confirmed by X-ray diffraction data. Further, the size of synthesised silver nanoparticles was confirmed using transmission electron microscopy, scanning electron microscopy, energy-dispersive X-ray spectroscopy, and atomic force electron microscopy. The spectroscopic and microscopic analysis confirmed the successful synthesis of AgNPs by P. colubrinum extracts acting as strong reducing agents. Our results showed that reducing the silver nitrate with plant extracts formed sphere-shaped AgNPs with distances in the range of 10–50 nm. XRD analysis revealed the crystal-like nature of the nanoparticles with face-centred cubic structure, and the peaks of the XRD pattern were parallel to (111), (200), (220), and (311) planes. The synthesised AgNPs showed the potential antibacterial activity against foodborne pathogens Escherichia coli and Staphylococcus aureus. Spectrophotometric analysis of dye degradation was confirmed the catalytic activity of synthesised AgNPs. The results of the study revealed potential of phyto-synthesised AgNPs act as catalysts and is a simple and cost effective and efficient approach.
1. Introduction
Nanotechnology has played a significant role in developing innovative technology with potential applications in various scientific disciplines, including material science, pharmaceutical science, chemistry, biology, and medicine [Citation1]. Different metals like gold, silver, zinc, copper, etc. are being used to synthesize nanoparticles. Silver is favoured over other metals due to its unique properties, such as higher thermal and electric conductivity. Besides this, silver has potential antibacterial, antioxidant, anticancer, larvicidal, catalytic, wound healing properties [Citation2]. Therefore, silver nanoparticles are used in various applications like drug delivery, biomedicine, agriculture, food industry, water treatment, textile industries, biosensors, and antimicrobial activities [Citation3]. In recent decades, silver nanoparticles (AgNPs) have attained scientist's interest due to their numerous applications in various science realms. In context to their bioactivity, silver nanoparticles have high antibacterial activity against various bacteria and used as potential drug carrier in cancer treatment [Citation4].
Particle size is an integral characteristic of nanoparticles, as the reduction in the particle size leads to an increase in bioactivity and bioavailability of nanoparticles. A vast number of metallic nanoparticle preparations and synthesis methods have been described [Citation5, Citation6] and involves physical and chemical methods that are widely and traditionally used for nanoparticle synthesis. The hazardous chemicals involved in the chemical synthesis of nanoparticles make it unsafe for medical usages. Some of the solid toxic chemicals used as reducing agents are non-biodegradable and are potentially harmful to the environment and biological systems [Citation7, Citation8]. The green synthesis always benefits and prefers the chemical and physical synthesis in order to prevent dangerous by-products [Citation9]. Therefore, developing capable and environment-friendly processes to synthesise silver nanoparticles is one of the most challenging tasks in nanotechnology. Silver nanoparticles are the most familiar example of redox catalysts which require less activation energy and provide a separate path to the electron transfer reaction [Citation10]. Surface plasmon light scattering, surface plasmon resonance (SPR), surface-enhanced Rayleigh scattering, and surface-enhanced Raman scattering (SERS) characteristics of nanoparticles provide them significant benefits over bulk materials [Citation11].
Several reports have been published about the plant-mediated AgNPs production. These studies have shown that plant metabolites are effective in the production of bioactive AgNPs. Although plant-mediated AgNP production has been established, it is crucial to remember that each plant species has its own bioactive component composition [Citation11]. Plants or plant extracts have been developed to play a key role in AgNPs biological synthesis process to overcome the toxicity issue in synthesis and biological applications. Different chemical components/phyto-molecules have protective and reductive actions that are important mainly to reduce the presence of silver ions that adopt natural chemicals and reduction enzyme complexes. In recent years, extracellular AgNPs have been produced as a possible reducing agent with various plant extracts [Citation4]. Previous reports on the biosynthesis of silver nanoparticles involved the use of microorganisms such as bacteria [Citation12], either intracellularly [Citation13] and extracellularly [Citation14], fungi [Citation15, Citation16], and algae [Citation17]. In modern nanoscience and technology, green synthesis (plant assisted) of nanoparticles has captured significant importance due to its flexibility and ecofriendly nature. A few examples of leaves [Citation18–20], seeds [Citation21], fruits [Citation22], root [Citation23], and bark [Citation24] involved in the metal reduction process have been reported in the literature.
Because of the risk of bacterial and chemical contamination, plant-based green synthesis is often preferred over bacterial and chemical methods for synthesising silver nanoparticles. It also requires less energy and has wider with broader implications and easiness. Secondary metabolites of plants include flavonoids, phenolic substances, glycosides and sterols that have decreased silver ions into elemental silver. Since the composition and content of secondary metabolites vary by plant species, their capacity and effectiveness in biosynthesizing metal nanoparticles should be studied for a wide variety of plant species [Citation11]. This study can be utilised to overcome biological restrictions and other difficulties associated with production. Synthesis of Phyto-mediated AgNPs can assist solve the problem of hazardous chemicals.
Dyes are a group of complexes of synthetic organic chemical used for the textile, leather, pharmaceutical, cosmetic, food and plastic industries as colouring agents. Dyes-containing industrial effluents constitute a serious environmental hazard, waste dumping in natural water bodies often leads to environmental contamination and it’s a severe risk to ecosystem. The elimination or degradation of synthetic recalcitrant dyes is a critical environmental concern and a challenge. The removal of synthetic dyes from the wastewater various methods have been applied such as electrolysis, adsorption, oxidation, coagulation, active sludge biochemical methods, membrane filtration, ozonisation and bio-degradation but these methods are incompetent to removal of contaminant to the preferred level with efficacy, due to effective manner of environment-friendly method needs to be applied. In the recent years, researchers are focussed on the study of catalytic and photocatalytic degradation of dyes using various types of metallic nanoparticles [Citation25].
A woody shrub, Piper colubrinum Link, belongs to the family Piperaceae, native to the northern part of South America, and is associated with black pepper Piper nigrum L. It deserves its biotechnological importance as it is resistant to oomycetous fungal pathogen Phytophthora capsici, which is the casual agent of the most severe disease named 'quick wilt' in P. nigrum [Citation26, Citation27], as well as nematodes such as Meloidogyne incognita and Radopholus similis, which leads to major crop loss in the black pepper cultivation [Citation28–30].
There is no report has present about biosynthesis of AgNPs utilising an aqueous leaf extract of Piper colubrinum. In the present study, Piper colubrinum leaves were used for the green biosynthesis of silver nanoparticles. UV–vis spectroscopy, XRD, EDAX, TEM, SEM and AFM were used to characterise biosynthesized silver nanoparticles, and the capping agent of synthesised AgNPs was confirmed by Fourier transform infra-red spectroscopy (FTIR). Moreover, its catalytic efficiency was shown via the reduction of hazardous dye (Methylene blue).AgNPs synthesised using leaf extract were also screened for antibacterial activity against the gram positive and negative food borne pathogens of Escherichia coli and Staphylococcus aureus.
2. Experimental
2.1. Chemicals
All the analytical grade chemicals used during this experiment include: Methylene blue (C16H18ClN3S·xH2O),Bromophenol Blue (C19H10Br4O5S), Coomassie Brilliant Blue(C45H44N3NaO7S2, ≥ 70%),Silver nitrate (AgNO3 ≥ 99.0%), Potassium bromide (KBr, FT-IR grade, ≥99%) and deionised water are purchased from Sigma-Aldrich Ltd.
2.2. Plant materials
The Piper colubrinum leaves were collected from Rajiv Gandhi Center for Biotechnology, Kerala, India. The healthy leaves were used for preparation of extract.
2.3. Preparation of the leaf extract
5 g of freshly collected Piper colubrinum leaves were washed thoroughly with detergent (Teepol solution), finally washed with distilled water 4–5 times, and ground well using sterile mortar and pestle. 100 ml deionized water was poured into the slurry and passed through cheesecloth and later filter using Whatman No. 1 filter paper with a pore size of 25 µm. The freshly filtered extract was stored at 4 °C and was further used for nanoparticle synthesis.
2.4. Synthesis of silver nanoparticles
Aqueous solution of 1 mM silver nitrate (AgNO3) was prepared in double-distilled water and mixed with freshly prepared plant extract9:1 ratio in an Erlenmeyer flask. The flask containing 90 ml of silver nitrate solution was covered with aluminium foil and boiled. 10 ml leaf extract (Piper colubrinum) was added into the silver nitrate solution drop by drop with continuous stirring. A change in colour of the AgNO3 solution was observed due to nanoparticle synthesis. After the colour change (dark brown) occurred, it was stirred until the solution temperature came down to room temperature. The obtained coloured suspensions were subjected to UV–Visible spectroscopic analysis. The remaining portion of the solution was thoroughly centrifuged at 12,000 rpm 10 min along with sterile distilled water for 5 or 6 times and finally washed with ethanol in order to obtain pure silver nanoparticles.
2.5. Characterization of the silver nanoparticles
2.5.1. UV–visible spectrum analysis
The physicochemical characterization of bioreduction of silver ions (Ag+) into silver nanoparticles (Ag0) was monitored in colloidal solution using by UV-visible spectrophotometer (Shimadzu model UV 1800) at a resolution of 1 nm, at 300to 700 nm, in different time intervals.
2.5.2. Fourier-transform IR analysis
The purified silver nanoparticle and plant extract was mixed with potassium bromide (KBr) and pelletised after drying. Later it was subjected to FTIR studies to substantiate the possible role of the various phytochemical found in the plant extracts that are responsible for the surface modification of the biosynthesized AgNPs. The FTIR spectra were recorded on Perkin Elmer at room temperature, and the IR spectra wavelength region of 4000–400 cm−1 were noted. The interactions between silver and bioactive molecules were identified by measuring peak numbers in the FTIR which is used forth formation and stabilisation (capping material) of silver nanoparticles. Further the AgNPs results were conformed with plant extract FTIR results
2.5.3. Transmission electron microscopic analysis
TEM analysis was used for elucidating the nondimensional morphological characters and the size distribution pattern of the synthesised silver nanoparticles. The TEM images of synthesised silver nanoparticles were developed by using JEOL JEM 2100. About 100 µl of colloidal solution (diluted to 1 ml with sterile distilled water) was sonicated 15 min and then filtered with a nylon syringe (0.22 μm) filter. A single drop of the filtered samples was placed on carbon-coated films supported by copper grids and air-dried. ImageJ software was used with a voltage of 200 kV.
2.5.4. X-ray diffraction analysis
The XRD analysis for determining the crystalline nature of biosynthesized AgNPs was carried out using X’pert3 Powder of Malvern-Panalytical (UK) recorded in the 2θ range. The scanning was done in the range between 20 °C and 80 °C of CuKα radiation. The 40 kV voltage was applied, and the energy was 8.04 keV with a wavelength of 1.54. Further, the images obtained were compared with the International Centre for Diffraction Data (ICDD) library to account for the crystalline structure of the particle.
2.5.5. Scanning electron microscopy (SEM) and energy-dispersive X-ray (EDX) analysis
SEM and EDX analysis was performed with the Gemini SEM 300 field emission scanning electron microscope to trace other elementary particle compositions and validate the presence of silver in the particles.
2.5.6. Atomic force microscopy (AFM)
At the nanoscale, atomic force microscopy can provide dimensional Visualisation as well as qualitative and quantitative information on a variety of physical properties, such as size, morphology, surface texture, and roughness. A liquid sample was ultrasonically prepared for AFM analysis. After 60 min to adequately distribute it; the final single drop was uniformly distributed in the centre of the small glass slide. By dissolving AgNPs in the appropriate solvent morphology and coating the sample with thin-film before analysis, the biosynthesized AgNPs were identified using Atomic Force Microscopy (Bruker, Multimode 8, USA). The size and of biosynthesized AgNPs were determined using a line profile and the Nanoscopev software, Scan Asyst.
2.5.7. Catalytic activity of synthesised AgNPs
Different types of commercial available dyes were used to investigate the catalytic activity of synthesised silver nanoparticles. The stock solution was prepared with 10 mg of Methylene Blue (MB), Bromophenol Blue (BPB) and Coomassie Brilliant Blue (CBB) powder, dissolved with 1000 mL of distilled water, and maintained at a concentration of 10 mg/l. To determine the degradation kinetics, 20 ml of MB, BB and CBB solution was taken from the stock solution, and 10 mg silver nanoparticles (concentration 0.5 mg/ml) were added to the solution and stirred for 15 min to check the catalytic activity. MB, BB and CBB dyes degradation was determined at 0, 30, 60, 90, 120 mins time intervals. The OD of dyes was calculated using a UV visible spectrophotometer with 668 nm, 570 nm and 595 nm.
2.5.8. Antibacterial effectiveness of phytosynthesis AgNPs
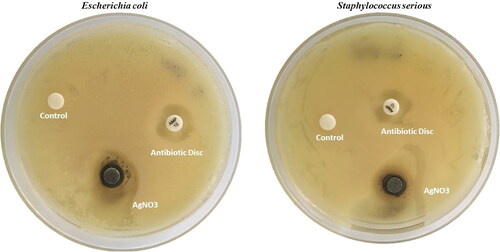
Standard method was used to assess the antibacterial activity of two clinical strains of Escherichia coli and Staphylococcus serious, grew in Luria Bertani Broth (LBB) medium. The bacterium was inoculated in the LBB medium and incubated in a self-regulating orbital shaker (Orbiteh; India) for 12 h at 37 °C at180 rpm. Luria Bertani Agar (LBA) dishes were freshly prepared in triplicate (3.5 g/100 mL). Once the standard culture and dishes were prepared, disc diffusion method was used to study the antibacterial activity. 12 h fresh culture was swabbed on the agar dishes with sterile cotton. Sterile blank antimicrobial susceptibility disc was used in the test. The discs were loaded with 50 μL of solution containing P. colubrinum leaves mediated synthesised AgNPs separately. The disc of Ampicillin (10 μg/disc) used as positive control and distilled water used as negative control for estimation. The plates were incubated for 24 h at 37 °C in temperature controlled incubator. After overnight of incubation, the inhibition zone of bacterial cultures was observed and zone reader was used to measure the inhibition zone of cultures.
3. Results and discussion
3.1. UV spectrum of synthesised AgNPs
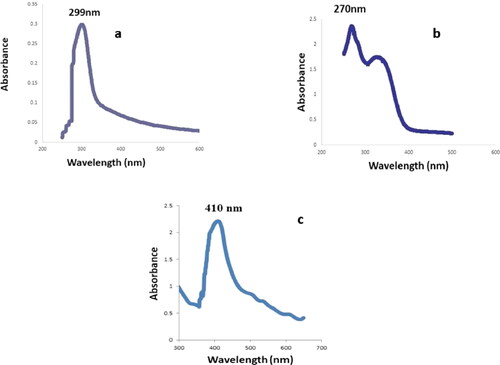
Leaves of Piper colubrinum are shown in . shows the AgNO3 solution, leaf extract, and synthesised colloidal solutions. The pale-yellow colour change that appeared after mixing the leaf extract of Piper colubrinum with silver nitrate solution confirmed silver nanoparticle formation. The colour changes were caused by the excitation of silver nanoparticles surface plasmon resonance (SPR) during the formation phase. The resulting AgNO3, plant extract and synthesised colloidal solutions were scanned with UV–Vis spectroscopy, and the spectrum is shown in c. A sharp band confirmed the 299 nm (AgNO3), 270 nm (plant extract) and synthesis of silver nanoparticles at 410 nm in the UV–Vis spectrum. Augustine et al. [Citation31] discovered similar results in Piper nigrum, where a positive association between colour change and AgNPs synthesis was observed.
3.2. FTIR spectrum
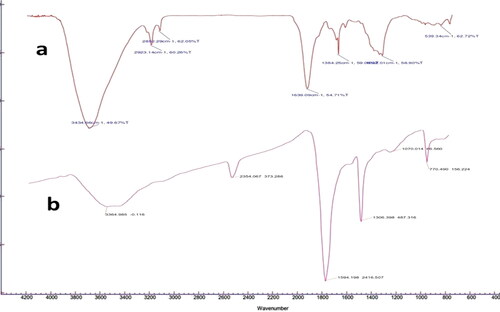
The spectrum analysis was performed in order to determine the potential functional molecules bound with silver nanoparticles. FTIR spectrum of Leaf extract and PCAgNPs shown in () the synthesised silver nanoparticles extracted from the leaf of P. colubrinum showed a weak band at 3,364.9 cm−1 which is a characteristic of O–H stretching secondary alcohols. A clear pattern was determined with the characteristic bands centered at 1594 cm−1 for aromatic skeletal vibrations [Citation32]. The peak at about 1070 cm−1 attributed to C–N stretching of amines [Citation33]. The bands between 900 and 600 cm−1 corresponded to C–H or CH2 groups [Citation34]. The band at 2354 cm−1 corresponded to the carbonyl compounds C-O vibrational stretching [Citation35]. The absorbance peak at 1,306 cm−1 corresponded to α-helix or N-H bending vibration [Citation36]. Hence, plant extract compounds with OH and CO groups played an avital role in reducing and stabilising AgNPs. However, the current mechanism in AgNP synthesis is not fully understood, but is predicted to be involvement free amino groups of plant extract has reduced the silver ions and acting as a capping agent [Citation37].
3.3. X-ray diffraction studies
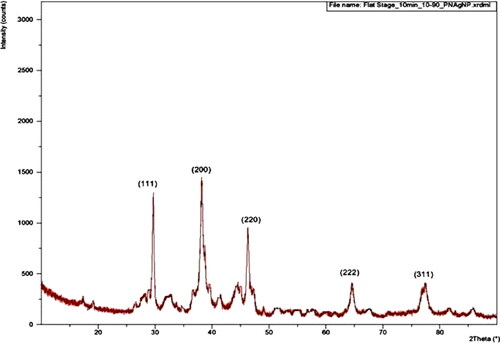
X-ray diffraction pattern (XRD) showed that the obtained biosynthesized silver nanoparticle in the crystalline silver structure in a face-centered cubic form (). In XRD, the substantial numbers of intense peaks have similar strong and sharp diffraction profile with silver at 2θ of 38.12°, 44.3°, 64.45°, 77.42°, and 81.56° corresponding to the planes of (111), (200), (220), (222) and (311). The obtained results were in accordance with the similar results obtained by other researchers [Citation38–40]. The lattice constant calculated for this structure was a = 4.0855 A°, which agrees well with those reported for silver by the ICDD file no. 03-065-2871.The other observed peaks may be due to the leaf extract of P. colubrinum. The obtained Braggs reflections may have resulted from capping agents, stabilising the silver nanoparticles [Citation41, Citation42].
3.4. TEM report
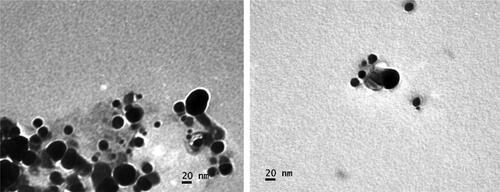
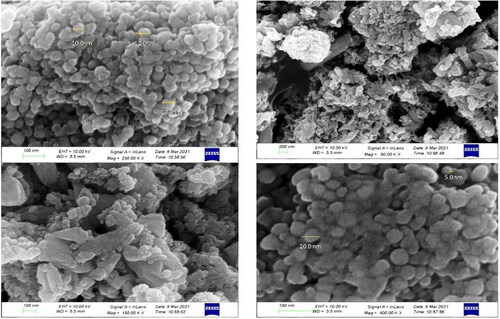
Transmission electron microscopy examined the microstructure and crystallinity of green synthesised silver nanoparticles. It was evident from the excessive-decision TEM image of AgNPs that they have spherical morphology (), and the synthesised nanoparticles own round form with a median diameter of almost 10–40 nm. We effectively synthesised small-sized AgNPs with a narrow size distribution ranging from 10 to 50 nm in the current study. The TEM also revealed that all nanoparticles are well-differentiated and do not clump together. Similar studies also reported analyzing the morphological structure of silver nanoparticles using TEM [Citation43, Citation44].
3.5. SEM and EDX analysis
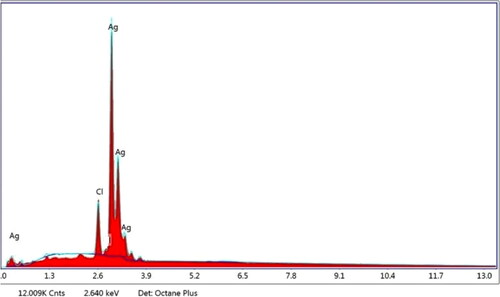
SEM and EDX analysis were used to examine the morphology and elemental composition of synthesised silver nanoparticles as shown in and .The scanning electron micrograph determined a spherical shape with a smooth surface and closely arranged particles [Citation45]. The size of synthesised AgNPs using aqueous leaf extract of P. colubrinum was about 10.0 nm to 50.0 nm, respectively, which is consistent with the crystallite size as determined by XRD. The elemental composition showed a strong signal for silver, as shown by EDX spectra.
3.6. Atomic force microscopy (AFM) image
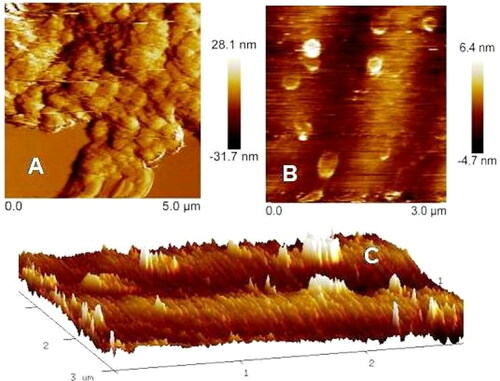
The topology of biosynthesized silver nanoparticles (size and shape) was determined using Atomic Force Microscopy (AFM. A three-dimensional image obtained from AFM () showed that the silver nanoparticles were more or less homogenous, monodispersed, and spherical. Nanoscopev analysis software showed that their typical size ranged between 10 and 36 nm, which was also confirmed using TEM measurement of the silver nanoparticles. The structural elucidation specificity of AFM analysis for silver nanoparticles was reported by Dimkpa et al. [Citation46].
3.7. The catalytic activity of AgNPs
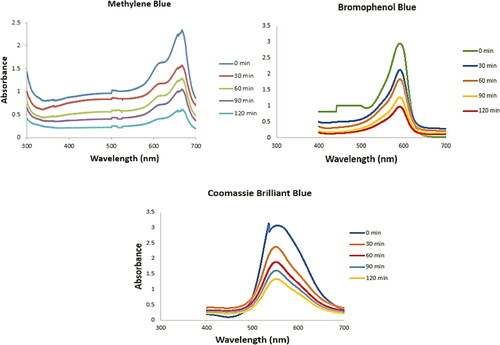
Methylene Blue (MB), Bromophenol Blue (BPB) and Coomassie Brilliant Blue (CBB)was used to determine the catalytic function of biosynthesized AgNPs. Within the electron transfer step, there could also be the restriction of the passage of electrons when there is an outsized redox electric potential between acceptor and donor. When an effective catalyst has a potential redox value intermediate between acceptor and donor, electron transfer becomes easy [Citation47]. The synthesised AgNPs from aqueous leaf extract of P. colubrinum was used to reduce the MB, BB and CBB. Spectrophotometry was used to evaluate the catalytic activity of biosynthesized AgNPs to degrade the MB, BPB and CBB, and the findings were described in . The absorbance value of dyes was measured at 668 nm, 570 nm and 595 nm. It was discovered that after 30 min of mixing silver nanoparticles into dyes, the absorbance peak of dyes steadily decreased and moved to a higher wavelength (Citation48). The reaction containing dyes, AgNPs, and extract, on the other hand, showed a significant decrease in absorbance peak after 30 to 120 min. This observation where the absorbance peak has shrunk indicated that AgNPs have the ability to degrade MB, BB and CBB. AgNPs are often found to serve as a redox catalyst, accepting electrons from the leaf extracts and donating electrons to dyes.
3.8. Antibacterial effectiveness
Agar well diffusion technique was used to examined the antibacterial activity of synthesised AgNPs with against foodborne pathogens of Escherichia coli and Staphylococcus serious [Citation49] (). The results indicate strong growth inhibitory activity of AgNPs against E. coli and S. serious the activity is significantly high compared with standard of Ampicillin (antibiotic disc). The results reveal that the inhibition area of synthesised AgNPs has30mm (E. coli) and 28 mm (S. serious) in diameter while the standard Ampicillin area has a 24 mm. Previous studies confirmed that, AgNPs revealed strong antibacterial activity against pathogenic bacteria like S. aureus, K. pneumoniae, E. coli, V. cholerae, P. aeruginosa, and S. typhi. The bacterial cell membrane is damaged by reactive oxygen species produced by AgNPs and penetrate the intracellular region, totally damaging the cell. With its self-cleaning, anti-polluting and anti-bacterial performance, AgNPs may be regarded as eco-friendly [Citation50].
4. Conclusion
Rapid green phytosynthesis of silver nanoparticles using P. colubrinum leaf extract is an effective biosynthesis strategy. Silver nanoparticles formed in 10 to 15 min, which was confirmed spectrophotometrically with the absorbance peak at 410 nm. The crystalline nature of synthesised silver nanoparticles was confirmed by XRD EDX analysis was used to assess the presence of silver. The size of the green synthesised silver nanoparticles was determined by TEM, which was between 10 to 50 nm. Fourier transform infrared study showed that the involvement of plant extracts played a significant role in reducing silver ions. The presence of alcoholic groups with C = O bonds and C–O stretching of carboxylic groups is shown by FTIR. Green synthesised AgNPs have also shown their better degradation ability of different dyes and therefore, can serve as a redox catalyst. Hence, the phytosynthesis of silver nanoparticles can be seen as the most sought-after economically viable method. The phytosynthesized PCAgNPs also exhibit the strong antibacterial activity against the clinical pathogens of E. coli (Gram +) and S. aureus (Gram˗).In future the other biological potentials, like antifungal and antioxidant properties of P. colubrinum mediated silver nanoparticles might be examined.
Acknowledgement
The authors greatly acknowledge the financial support from Department of Science and Technology (DST/TDT/AGRO-02/2019(C), Government of India.
Disclosure statement
The authors declare that there is no conflict of interest.
Additional information
Funding
References
- Abdelghany TM, Al-Rajhi AMH, Al Abboud MA, et al. Recent advances in green synthesis of silver nanoparticles and their applications: about future directions. A review. BioNanoSci. 2018;8(1):5–16.
- Flores‐López LZ, Espinoza‐Gómez H, Somanathan R. Silver nanoparticles: electron transfer, reactive oxygen species, oxidative stress, beneficial and toxicological effects. Mini review. J Appl Toxicol. 2019;39(1):16–26.
- Tarannum N, Divya D, Gautam YK. Facile green synthesis and applications of silver nanoparticles: a state-of-the-art review. RSC Adv. 2019;9(60):34926–34948.
- Mohanta YK, Panda SK, Jayabalan R, et al. Antimicrobial, antioxidant and cytotoxic activity of silver nanoparticles synthesized by leaf extract of Erythrina suberosa (Roxb.). Front Mol Biosci. 2017;4:14.
- Pal A, Shah S, Devi S. Preparation of silver, gold and silver–gold bimetallic nanoparticles in w/o microemulsion containing TritonX-100. Colloids Surf A Physicochem Eng Asp. 2007;302(1–3):483–487.
- Rosemary MJ, Pradeep T. Solvothermal synthesis of silver nanoparticles from thiolates. J Colloid Interface Sci. 2003;268(1):81–84.
- Ouda SM. Antifungal activity of silver and copper nanoparticles on two plant pathogens, Alternaria alternata and Botrytis cinerea. Res J Microbiol. 2014;9(1):34–42.
- Wei Y, Ke L, Kong J, et al. Enhanced photoelectrochemical water-splitting effect with a bent ZnO nanorod photo anode decorated with Ag nanoparticles. Nanotechnology. 2012;23(23):235401.
- Biswas K, Mohanta YK, Kumar VB, et al. Nutritional assessment study and role of green silver nanoparticles in shelf-life of coconut endosperm to develop as functional food. Saudi J Biol Sci. 2020;27(5):1280–1288.
- Mallick K, Witcomb M, Scurrell M. Silver nanoparticle catalysed redox reaction: an electron relay effect. Mater Chem Phys. 2006;97(2–3):283–287.
- Mohanta YK, Biswas K, Jena SK, et al. Anti-biofilm and antibacterial activities of silver nanoparticles synthesized by the reducing activity of phytoconstituents present in the indian medicinal plants. Front Microbiol. 2020;11:1143.
- Shahverdi AR, Minaeian S, Shahverdi HR, et al. Rapid synthesis of silver nanoparticles using culture supernatants of enterobacteria: a novel biological approach. Process Biochem. 2007;42(5):919–923.
- Kalishwaralal K, Babu RS, Venkataraman D, et al. Biosynthesis of silver nanocrystals by Bacillus licheniformis. Colloids Surf B Biointerfaces. 2008;65(1):150–153.
- Kalishwaralal K, Deepak V, Ramkumarpandian S, et al. Extracellular biosynthesis of silver nanoparticles by the culture supernatant of Bacillus licheniformis. Mater Lett. 2008;62(29):4411–4413.
- Dhanasekaran D, Latha S, Saha S, et al. Extracellular biosynthesis, characterisation and in-vitro antibacterial potential of silver nanoparticles using Agaricus bisporus. J Exp Nanosci. 2013;8(4):579–588.
- Mohanta YK, Nayak D, Biswas K, et al. Silver nanoparticles synthesized using wild mushroom show potential antimicrobial activities against food borne pathogens. Molecules. 2018;23(3):655.
- Vivek M, Kumar PS, Steffi S, et al. Biogenic silver nanoparticles by Gelidiella acerosa extract and their antifungal effects. Avicenna J Med Biotechnol. 2011;3(3):143.
- Samari F, Parkhari P, Eftekhar E, et al. Antioxidant, cytotoxic and catalytic degradation efficiency of controllable phyto-synthesised silver nanoparticles with high stability using cordia myxa extract. J Exp Nanosci. 2019;14(1):141–159.
- Bahrami-Teimoori B, Nikparast Y, Hojatianfar M, et al. Characterisation and antifungal activity of silver nanoparticles biologically synthesised by Amaranthus retroflexus leaf extract. J Exp Nanosci. 2017;12(1):129–139.
- Yadav R, Saini H, Kumar D, et al. Bioengineering of Piper longum L. extract mediated silver nanoparticles and their potential biomedical applications. Mater Sci Eng C Mater Biol Appl. 2019;104:109984.
- Jagtap UB, Bapat VA. Green synthesis of silver nanoparticles using Artocarpus heterophyllus Lam. seed extract and its antibacterial activity. Ind Crops Prod. 2013;46:132–137.
- Masum MMI, Siddiqa MM, Ali KA, et al. Biogenic synthesis of silver nanoparticles using Phyllanthus emblica fruit extract and its inhibitory action against the pathogen Acidovorax oryzae strain RS-2 of rice bacterial brown stripe. Front Microbiol. 2019;10:820.
- Sagadevan S, Vennila S, Muthukrishnan L, et al. Exploring the therapeutic potentials of phyto-mediated silver nanoparticles formed via Calotropis procera (Ait.) R. Br. root extract. J Exp Nanosci. 2020;15(1):217–231.
- Sathishkumar M, Sneha K, Won SW, et al. Cinnamon zeylanicum bark extract and powder mediated green synthesis of nano-crystalline silver particles and its bactericidal activity. Colloids Surf B Biointerfaces. 2009;73(2):332–338. 15
- Menon S, Agarwal H, Shanmugam VK. Catalytical degradation of industrial dyes using biosynthesized selenium nanoparticles and evaluating its antimicrobial activities. Sustain Environ Res. 2021;31(1):1–12.
- Purseglove JW, Brown EG, Green CL, et al. Spices. Vol. 2. Longman Group Ltd. New York; 1981; pp. 447–813.
- Ravindran P, Remashree AB. Anatomy of Piper colubrinum Link. J Spices Aromatic Crops. 1998;7(2):111–123.
- Nambiar KK, Sarma YR. Wilt diseases of black pepper [India]. J Plant Crops. 1977;5:2–103.
- Ramana KV, Mohandas C. Plant parasitic nematodes associated with black pepper (Piper nigrum L.) in Kerela. Indian J Nematol. 1987;17(1):62–66.
- Devasahayam S. Insect pests of black pepper. Black Pepper. 2000;7:309–334.
- Augustine R, Kalarikkal N, Thomas S. A facile and rapid method for the black pepper leaf mediated green synthesis of silver nanoparticles and the antimicrobial study. Appl Nanosci. 2014;4(7):809–818.
- Martin JA, Solla A, Woodward S, et al. Fourier transform-infrared spectroscopy as a new method for evaluating host resistance in the Dutch elm disease complex. Tree Physiol. 2005;25(10):1331–1338. 1
- Renuka R, Devi KR, Sivakami M, et al. Solanum torvum mediated synthesis and characterization of silver nanoparticles for antibacterial activities. J Plant Biochem Biotechnol. 2021;2:1–6.
- Santos DI, Neiva Correia MJ, Mateus MM, et al. Fourier transform infrared (FT-IR) spectroscopy as a possible rapid tool to evaluate abiotic stress effects on pineapple by-products. Appl Sci. 2019;9(19):4141.
- Netala VR, Bukke S, Domdi L, et al. Biogenesis of silver nanoparticles using leaf extract of Indigofera hirsuta L. and their potential biomedical applications (3-in-1 system). Artif Cells Nanomed Biotechnol. 2018;46(sup1):1138–1148.
- Song L, Yang K, Jiang W, et al. Adsorption of bovine serum albumin on nano and bulk oxide particles in deionized water. Colloids Surf B Biointerfaces. 2012;94:341–346.
- Mohanta YK, Panda SK, Syed A, et al. Bio-inspired synthesis of silver nanoparticles from leaf extracts of Cleistanthus collinus (Roxb.): its potential antibacterial and anticancer activities. IET Nanobiotechnol. 2018;12(3):343–348.
- Parikh RY, Ramanathan R, Coloe PJ, et al. Genus-wide physicochemical evidence of extracellular crystalline silver nanoparticles biosynthesis by Morganella spp. PLoS One. 2011;6(6):e21401.
- Theivasanthi T, Alagar M. Electrolytic synthesis and characterizations of silver nanopowder. Nano Biomed Eng. 2012;4(2):58–65.
- Martínez-Castañon GA, Nino N, Martínez-Gutiérrez F, et al. Synthesis and antibacterial activity of silver nanoparticles with different sizes. J Nanoparticle Res. 2008;10(8):1343–1348.
- Ibrahim HM. Green synthesis and characterization of silver nanoparticles using banana peel extract and their antimicrobial activity against representative microorganisms. J Radiat Res Appl Sci. 2015;8(3):265–275.
- Roopan SM, Madhumitha G, Rahuman AA, et al. Low-cost and eco-friendly phyto-synthesis of silver nanoparticles using cocos nucifera coir extract and its larvicidal activity. Ind Crops Prod. 2013;43:631–635.
- Ali A, Mohammad S, Khan MA, et al. Silver nanoparticles elicited in vitro callus cultures for accumulation of biomass and secondary metabolites in Caralluma tuberculata. Artif Cells Nanomed Biotechnol. 2019;47(1):715–724.
- Castro-González CG, Sánchez-Segura L, Gómez-Merino FC, et al. Exposure of stevia (Stevia rebaudiana B.) to silver nanoparticles in vitro: transport and accumulation. Sci Rep. 2019;179(1):10372.
- Banerjee P, Satapathy M, Mukhopahayay A, et al. Leaf extract mediated green synthesis of silver nanoparticles from widely available Indian plants: synthesis, characterization, antimicrobial property and toxicity analysis. Bioresour Bioprocess. 2014;1(1):1.
- Dimkpa CO, McLean JE, Martineau N, et al. Silver nanoparticles disrupt wheat (Triticum aestivum L.) growth in a sand matrix. Environ Sci Technol. 2013;547(2):1082–1090.
- Ghorbani HR, Safekordi AA, Attar H, et al. Biological and non-biological methods for silver nanoparticles synthesis. Chem Biochem Eng Q. 2011;25(3):317–326.
- Tripathi RM, Kumar N, Shrivastav A, et al. Catalytic activity of biogenic silver nanoparticles synthesized by Ficus panda leaf extract. J Mol Catal B Enzym. 2013;96:75–80.
- Meethal BN, Panichikkal AF, Jafferali JFM, et al. Shining black nanoscopic ternary zincospiroffite: a panchromatic light harvester for depollution. Mater Des. 2019;165:107600.
- Mohanta YK, Singdevsachan SK, Parida UK, et al. Green synthesis and antimicrobial activity of silver nanoparticles using wild medicinal mushroom Ganoderma applanatum (Pers.) Pat. from Similipal Biosphere Reserve, Odisha, India. IET Nanobiotechnol. 2016;10(4):184–189.