?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The current work demonstrates the fabrication of kaolin supported Au nanoparticles (Au NPs-kaolin) mediated by Thymbra spicata extract as green reductant and capping agent without any toxic reagent. Physicochemical characteristics of the said nanocomposite were elucidated by field emission scanning electron microscopy, transmission electron microscopy, energy-dispersive X-ray spectroscopy, elemental mapping, X-ray diffraction and inductively coupled plasma techniques. The figures of the TEM display the black dots signifying Au NPs being dispersed over the kaolin surface. Size of spherical Au NPs are around 10–15 nm. In the in vivo, we established a rat calvaria defect model using a combination of collagen scaffold and nanoparticle. The experimental group was divided into three classifications: control, collagen matrix and nanoparticle with collagen. Histological analyses showed that nanoparticle increased bone formation activity when used in conjunction with collagen matrix. In the nanoparticle group, grafted materials were still present until 12 weeks after treatment, as evidenced by foreign body reactions showing multinucleated giant cells in chronic inflammatory vascular connective tissue. Other results revealed that the nanoparticle increased bone formation activity when used with collagen matrix. All groups showed almost the same histological findings until 7 weeks. In the experimental groups, new bone formation activity was found continuously up to 12 weeks.
1. Introduction
Bone regeneration is a substantial medical challenge, as insufficient bone healing affects tens of millions of individuals in the United States alone. The two most common clinical scenarios of bone insufficiency are cases of systemic bone loss (i.e. osteoporosis and osteopenia) and local trauma (i.e. fractures) [Citation1–3]. In both cases, the understanding of clinical risk and biological pathology permit the development of treatment and regeneration strategies. Osteoporosis affects almost 10 million Americans, and osteopenia almost 50 million, and the most devastating effect of these bone loss conditions is fragility fractures in the elderly [Citation2–5]. In fact, approximately one in two women and up to one in four men 50 years of age and older will sustain a fragility fracture with substantial subsequent morbidity and mortality [Citation2, Citation4]. Even in these injuries, however, the biological process of repair is that of regeneration and restoration of the original tissue type rather than replacement with inferior scar tissue. It seems the gold nanoparticles are a remedial option for bone disorders [Citation5–7].
Metallic nanoparticles are biocompatible and non-toxic and have also been used as medical fillers, cosmetics and drug carriers [Citation8–13]. Among the special properties of metallic nanoparticles are high chemical stability, low dielectric constant, high catalytic activity, absorption of infrared and ultraviolet light and most importantly its antibacterial properties. If the therapeutic and anticancer effects of these compounds are confirmed, this could be a significant step in advancing cancer therapies [Citation12,Citation13].
Nanoparticles, such as mesoporous silica, gold, magnetic iron oxide, calcium phosphates, layered double hydroxides, poly(lactic-co-glycolic) acid and chitosan are continually explored for their use in targeted delivery and sustainable release of drugs, or as inhibitory factors of signalling pathways for the treatment of bone diseases [Citation14a]. One attractive strategy is to utilize nanoparticles for the treatment of osteoporosis, by developing a drug delivery system that specifically binds to osteoporotic bone. Using BPs as a linker agent between nanoparticles and bone minerals is an alternative option for developing a targeted delivery system for bone, due to the high affinity of BPs for calcium phosphates [Citation14b]. Another strategy is to control the function of bone cells using nanoparticles loaded with specific inhibitors for osteoclasts (bone resorbing cells) and promoters for osteoblasts (bone forming cells) to prevent the progression of osteoporosis. Nanoparticles functionalized with BPs can be developed for targeted drug delivery to metastasized bone, in an attempt to increase the therapeutic efficacy of the drug and to reduce the side effects on non-targeted areas. A large amount of research is being conducted in developing nanoparticles for clinical use, such as poly(lactic-co-glycolic) acid, hyaluronic acid and chitosan, in the targeted delivery of drugs, growth factors or genes to an osteoarthritic joint [Citation14c]. Conjugation of these nanoparticles with cartilage targeting molecules, such as chondroitin sulphate and chondrocyte-affinity peptide, has enabled the targeted delivery of inflammatory inhibitors. One of the effective strategies in treating osteomyelitis is to incorporate drugs with both osteoinductive and antibacterial effects into nanoparticles as a co-delivery configuration incorporation of multiple drugs into nanoparticles can be achieved through the combination of various techniques, including internal encapsulation, chemical binding, surface adsorption and surface coating methods [Citation14a,Citation14b]. The use of nanoparticles can be an effective approach for dual or multiple drug delivery systems to facilitate bone healing. However, limitations of these approaches include the lack of synergistic effects in dual delivery of the drugs, and inability to achieve controlled release for more than one drug which leads to a risk of overdose and toxicity. Therefore, it is imperative to fully optimize a multi-delivery system, by investigating the appropriate combination of drugs or growth factors and using the correct dosage to maximize bone healing [Citation14a–Citation14c].
Kaolinite has the general chemical composition AlSiO(OH)4 and structurally a silicate tetrahedral sheet is linked to an octahedral alumina sheet via oxygen atom linkers. In material science it has got immense implications. Due to rich source of Si and Al species, they have been widely used to synthesize mesoporous aluminosilicates and zeolites which in turn being used as excellent catalysts. In addition, kaolin has also been used as a very good support material in catalyst arena based on its low cost, stability and sustainable ground, being comparable to carbonaceous materials like graphene oxide and carbon nanotube, except the advantage of high surface area in latter [Citation14–18]. In the previous study, the microstructure evolution and physico-mechanical properties of bone china porcelain developed using two selected kaolinite clays from Nigeria were investigated. The results showed that higher densification was observed at 1200 °C while water absorption were detected at <1% and porosity at <4% for both samples containing Ikere and Okpella kaolinite clays respectively. Improvements in terms of wear loss and strength properties were also observed to be better for samples containing Okpella kaolin compared with those containing Ikere kaolin also at 1200 °C. This superior behaviour observed at temperature of 1200 °C can be attributed to maturation phases of anorthite, quartz and β-TCP (tri-calcium phosphate) at the same temperature [Citation19].
Herbal medicines have been used for centuries to treat a variety of diseases and conditions including promotion of fractured bone healing and bone diseases such as osteoporosis. Like aspirin (from leaves of the willow tree, non-steroidal anti-inflammatory drug), Taxol (from the cortex of Taxus brevifolia, chemotherapy drug) and Stillen (from leaves of Artemisia argyi, antiulcerant), several studies using herbal medicine have been and are still being performed [Citation13]. Thymbra spicata L. a wild medicinal plant of Eastern Mediterranean countries; is represented by namely T. spicata var. spicata and T. spicata var. intricata in Turkey [Citation5]. The main component, carvacrol, predominates (>80%) in the essential oil and demonstrated significant level of anti-hypercholesterolaemic and antioxidant activities in mice [Citation20a]. Also carvacrol has been observed with many diverse physiological actions, such as antibacterial [Citation13], anti-inflammatory [Citation20b]. Dried herbal parts of the plant are used as herbal tea, and in folk medicine to treat asthma, colic, bronchitis, coughs, diarrhoea and rheumatism in various parts of Turkey [Citation20c]. It was important for our study that dried T. spicata herb contain 1925.7 mg/kg Ca since experimental evidence clearly indicates the key role of Ca2+ in osteoinduction [Citation20d,Citation20e].
So far, there is not any report about the rat calvaria defect model using a combination of collagen scaffold and herbal nanoparticle. According to the above explanations, we investigated the Au NPs-kaolin nanocomposite containing T. spicata in the cytotoxicity study against common human normal cell line. In the in vivo, we established a rat calvaria defect model using a combination of collagen scaffold and nanoparticle.
2. Experimental
2.1. Green synthesis of Au NPs-kaolin nanocomposite using T. spicata extract
The plant extract was prepared by boiling 0.5 g of the powdered T. spicata species into 50 ml of deionized water for 20 min at 80 °C. It was filtered through Whatman-1 filter paper to remove the unwanted matters and the filtrate was kept at freeze for further use. 0.5 g kaolin powder was dispersed separately in 100 ml DI-H2O by ultrasonication for 30 min. Subsequently, 20 ml of plant extract was added to the suspension and stirred for 2 h. This makes the plant biomolecules to bind on the surface of kaolin. Then, 10 ml of 0.1 M HAuCl4 solution was added to it slowly and the mixture was agitated at 100 °C for another 1 h to synthesize Au NPs in situ and deposit over the matrix. Finally, the Au NPs-kaolin nanocomposite was collected by centrifugation, washed thoroughly with DI water and dried in air. Ag content in the as synthesized material was analysed by ICP-AES technique, to be 0.09 mmol/g.
2.2. Cytotoxicity effect of Au NPs-kaolin nanocomposite
In this study, HUVEC cells were used to evaluate the anticancer effect of Au NPs-kaolin nanocomposite on cell culture.
The cell viability properties of the synthesized nanoparticles were evaluated using the MTT method. This technique is a calorimetric method for measuring cell vitality and is also used to study the effect of drugs on cell growth, cytotoxicity of medicinal compounds in plant products and other chemicals. The basis of this experiment is based on the tetrazolium yellow crystals reduction by mitochondrial succinate dehydrogenase and the insoluble purple crystals production of formazan. Living cells can convert tetrazolium crystals into formazan crystals. Mitochondrial dehydrogenase enzymes in the respiratory chain and other electron transport systems reduce the MTT salt to form insoluble purple crystals of formazan dye. Formazan crystals are dissolved outside the cell by adding a suitable solvent such as dimethyl sulfoxide (DMSO). The amount of dye produced is measured by spectrophotometry at a wavelength of 500–600 nm. The procedure is as follows: 5 × 103 cells were cultured in each of the wells of a 96-well plate. After reaching the surface dispersion of 80%, the supernatant was replaced with new medium containing different concentrations of nanoparticles synthesized with concentrations of 1–1000 µg/ml. The cells were stored in three separate plates for 24, 48 and 72 h under culture conditions. Each concentration of nanoparticles was considered in three separate replications for each incubation time, so that there are at least three replicates for each concentration. To prepare the solution, 5 mg of yellow MTT powder was dissolved in 1 ml of saline phosphate buffer. The resulting solution was sterilized using a 0.2 μm filter and stored in the dark at −20 °C. For this purpose, 20 μl of MTT solution was added to each house and incubated for 4 h. Therefore, after 4 h of incubation, the MTT medium of each well was removed and 150 μl of DMSO was added. Finally, the optical absorption of the resulting solution at 570 nm was read by the ELISA reader. Cell viability was calculated by the following equation [Citation21]:
2.3. Surgical procedure
Thirty-six Sprague-Dawley male rats, 10 weeks old, weighing 220 g were selected for the study. Rat size increases rapidly during this stage, providing a favourable model for studying normal bone healing. Before the study, the animals were housed in an environmentally controlled animal facility for 1 week for acclimatization.
Surgery was conducted on all rats under sterile conditions. General anaesthesia was induced by intramuscular injection of 10 mg/kg xylazine HCl and 40 mg/kg ketamine HCl and was maintained by injection of the same mixture in half doses. Rat skulls were shaved and sterilized using 70% ethanol. An injection of 2% lidocaine HCl 1.8% containing 1:100,000 epinephrine was administrated locally at the operation site to reduce bleeding. The bone surface of the calvaria was exposed by a 3 cm longitudinal midline incision along the sagittal suture. In each parietal bone, a standardized round defect was created using an 8 mm diameter trephine bur in a low-speed hand piece. To prevent overheating of the bone, the operation site was irrigated continuously with sterile saline solution [Citation21].
The defects were grafted with different materials. In the Au NPs-kaolin nanocomposite group, the defects were filled with a collagen matrix containing Au NPs-kaolin nanocomposite. In the collagen group, the defects were filled with collagen matrix containing saline; in the control group, the defects were left untreated. The graft materials were prepared 15 min before grafting. All experimental areas were closed with 4.0 black surgical silks. Postoperatively, all rats received an intramuscular injection of 25,000 IU/kg penicillin G once daily for 3 days [Citation21].
2.4. Histological preparation
Animals were sacrificed by CO2 inhalation at 5, 7, 10 and 12 weeks after surgery. Defects and surrounding tissues were removed for histological preparation and immediately fixed by immersion in 10% neutral buffered formalin. The specimens was dehydrated in 70% ethanol and then embedded in methacrylate-based resin. Polymerization of the embedding solution occurred in a photopolymerization unit with exposure to daylight for 2 h and to ultraviolet light for 10 h. Each polymerized block was affixed to the vacuum head of the EXAKT macrocutter, and sections were sliced to a thickness of approximately 100 μm. Sections were ground and polished using the EXAKT micro grinder to a thickness of 15 μm, mounted on microscope slides, stained with haematoxylin and eosin, and coverslipped. Bone healing patterns were observed under a light microscope [Citation21].
2.5. Qualitative measurement
At least three independent replications were performed for each data and the result was presented as mean ± SD. Data statistical analysis was done with SPSS software version 19 and ANOVA way one and Duncan’s test. Significance was considered at the level of p ≤ 0.01.
3. Results and discussion
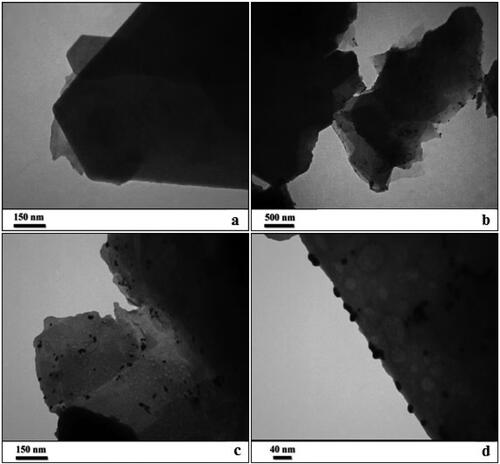
In the recent study, the morphological characteristics of as-prepared Au NPs-kaolin nanocomposite analysed by transmission electron microscopy (TEM) are presented in . The flake like image of bare kaolin is presented in (a). (b–d) displays the black dots signifying Au NPs being dispersed over the kaolin surface ((b)). Size of spherical Au NPs are around 10–15 nm. It is quite evident that there occurs no change in morphology of the kaolin matrix even on surface modifications with plant biomolecules and Au NPs.
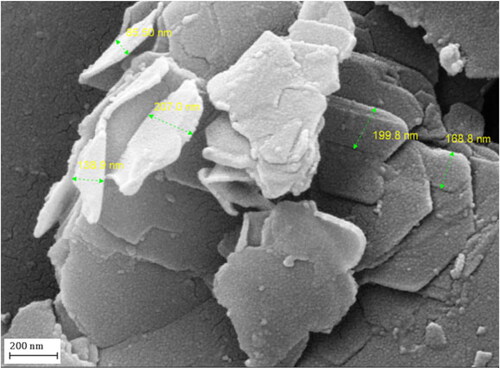
The particle size, shape and surface morphology of Au NPs-kaolin nanocomposite was studied by FE-SEM analysis, as shown in . The flaky nanomaterial reveals a continuously layered and rough surface which is a clear indication of phytochemical functionalization. However, Au NPs are not clear visible in the field emission scanning electron microscopy (FESEM) image, might be due to well-dispersion over the surface.
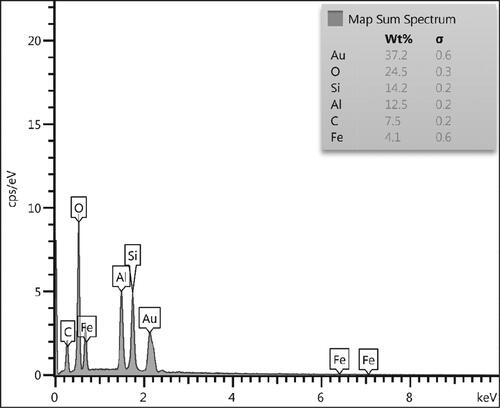
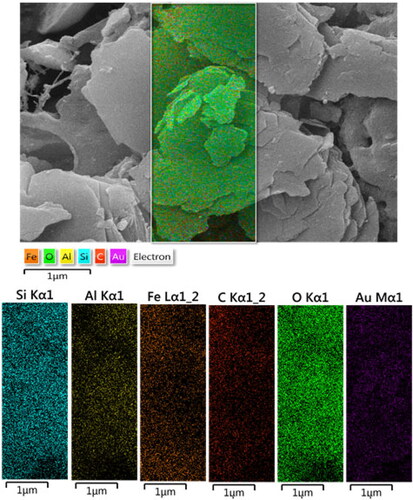
In order to have an idea of chemical composition of the nanocomposite, EDX analysis was carried out and the profile is shown in . The elements Si, Fe and Al are attributed to kaolin matrix. The presence of C and O are the evidence of biomolecular attachments. On the other hand, the occurrence of Au element confirmed the successful synthesis of Au NPs over kaolin surface. The results were further justified by FE-SEM elemental mapping analysis (). The compositional map reveals the Si, Al, C, O and Au species to exist with excellent dispersion throughout the matrix surface.
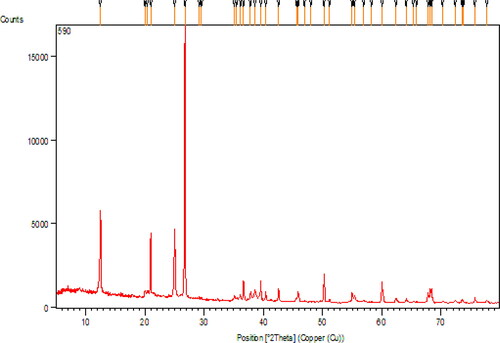
Crystalline phases and the diffraction planes of the nanocomposite were determined by X-ray diffraction (XRD) study, as presented in . The major peak obtained at 2θ = 26.5° represents the quartz structure of kaolin. Two more diffraction peaks observed at 2θ = 45.8° and 21.0° are also contributed from kaolin structure [Citation18]. Due to lower concentration of Au, the peak intensities of Au NPs are also low [Citation19]. Still, the peaks obtained at 2θ = 38.5°, 42.5°, 65.1° and 77.8° correspond to the (1 1 1), (2 0 0), (2 2 0) and (3 1 1) planes of fcc structure of Au crystal (JCPDS No. 04-0783). These XRD results confirmed that the crystallized Au NPs could be successfully immobilized on the surface of kaolin [Citation20].
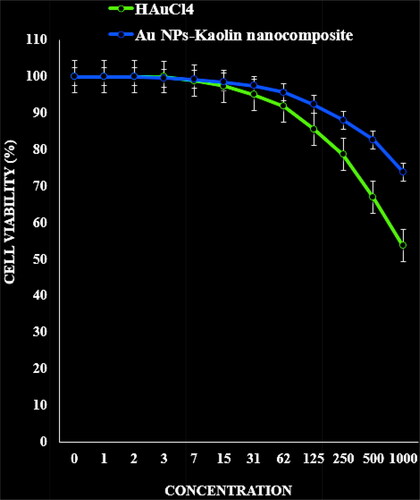
In this experiment, the treated cells with different concentrations of the present Au NPs-kaolin nanocomposite were assessed by MTT assay for 48 h about the cytotoxicity properties on normal (HUVEC) cell line.
Gold nanoparticles are one of the most widely used nanoparticles. One of the reasons for the great attention paid to gold nanoparticles and their use for biological and medical purposes is their quick and easy synthesis method [Citation22–26]. Various methods for the preparation of gold nanoparticles, all of which are based on the reduction of salts (Au (III), the most important of which is the hydrogen tetrachloroate salt with the chemical formula (HAuC14) [Citation27–30]. In fact, to make gold nanoparticles, we must have Au ions suspended in the solvent. Various laboratory and industrial methods for preparing gold nanoparticles; these include chemical reduction methods, electrochemical methods, chemical sound methods and biochemical light methods. In all the mentioned methods, soluble polymer, surfactants and various ligands are used as stabilizing agents [Citation27–30]. The inertia of gold and its resistance to surface oxidation are important properties of this metal. The optical properties of gold at the nanoscale are also very significant. It has now been proven that gold nanoparticles have been used as catalysts in a number of important commercial reactions and have excellent surface chemistry. Based on this unique feature, new applications of nanotechnology using gold are expanding [Citation31–37]. At the nanoscale, gold exhibits properties that make it an important metal for future nanotechnology products. This nanoparticle has applications in pharmacy, medicine, agriculture, electronics, coating, paints and catalysts. It is widely used in biosensors today to detect nucleic acids and proteins [Citation25–30].
Another advantage of gold nanoparticles is their ability to detect microorganisms, cancerous tissues, etc., both in vivo and in vitro. Gold nanoparticles coated with anti-cancer antibodies can effectively bind to cancer cells [Citation33–36]. Many cancer cells have a protein on their surface called the epidermal growth factor receptor (EFGR). This protein is not found mainly in healthy cells of the body. By attaching gold nanoparticles to the EFGR antibody (known as anti-EFGR), researchers have attached these nanoparticles to cancer cells. Gold nanoparticles are an excellent carrier for immunotherapy because, like other nanoparticles, they can easily accumulate inside immune cells [Citation29–33]. Gold nanoparticles can transport several drug molecules, recombinant proteins, vaccines or nucleotides into the target cell. It can also control the release or release of drugs. Conjugation of gold nanoparticles with drug molecules plays an important role in intracellular diseases [Citation34–37]. Anti-biotics and other drugs can bind directly to gold nanoparticles with the help of ion or covalent bonds. The surface of gold nanoparticles can be easily changed to bind drug molecules. Changing the level of gold nanoparticles with polymers plays an important role in its conjugation with the drug. With this strategy, many therapeutic drugs can be successfully transferred, such as doxorubicin and tamoxifen [Citation33–36]. An important challenge in cancer is the delivery of water-soluble drugs, which used hydrophobic polymers to coat the nanoparticles; the drug is encapsulated by a hydrophobic coating. Methotrexate is used as an anti-cancer drug. It is a folic acid analogue that can inhibit the growth and proliferation of cancer cells [Citation29,Citation30]. The carboxylic group on the drug can bind to the surface of gold nanoparticles and exert its effect on cancer cells. Polyethylene glycol increases the adsorption of gold nanoparticles. The hydrophilic properties of PEG prevent the opsonization and purification of nanoparticles in the body. It can also be used as conjugation of gold nanoparticles and drug molecules [Citation34–37].
The absorbance rate was evaluated at 570 nm, which represented viability on normal cell line (HUVEC) even up to 1000 μg/ml for Au NPs-kaolin nanocomposite ().
Figure 6. The cytotoxicity effects of HAuCl4 and Au NPs-kaolin nanocomposite against normal (HUVEC) cell line.
In the recent study, three study groups underwent microscopic evaluation based on the injected biomaterial. Each group was tested at four time intervals, and none of them showed complete filling of the defect with new bone. In all groups, the 5 and 7 week time points showed almost the same histological findings. Bone formation activity was attenuated at different time points in each group. The presence of granulation tissue was characterized by numerous newly formed small blood vessels, an intense inflammatory cell infiltrate, and immature connective tissue with large amounts of fibroblasts and immature collagen fibre bundles in the centre of the bone defect area. Osteocytes were clearly distinguishable at the inner parts of newly formed bone, indicating direct bone repair. Activated osteoblasts rimming newly formed woven bone were observed in the bone repair areas. Grafted biomaterials were resorbed at different time points for each group. In the Au NPs-kaolin nanocomposite groups, grafted materials were present until 12 weeks, as evidenced by a foreign body reaction showing multinucleated giant cells in chronic inflammatory vascular connective tissue. In the collagen group, only one section showed graft material at 12 weeks; most of them totally resorbed in 7 weeks. At 12 weeks, the collagen group showed a defect area filled with fibroblastic dense collagen tissue without inflammation. In the Au NPs-kaolin nanocomposite group, osteoid formation with osteoblasts rimming was found continuously in the trabecular bone surface at the edges and the centre of the defect at 10 and 12 weeks, respectively. In the control group, bone formation was reduced at 6 weeks. Fibroblast and collagen fibre volume density significantly increased in the first 7 weeks, followed by a reduction in the subsequent periods (10 and 12 weeks). In the collagen group, activated osteoblasts rimming newly formed woven bone were seen at 6 weeks, but from 8 weeks on, peripheral osteoid and osteoblastic rimming were markedly reduced. By 12 weeks, a mineralized bone margin was found without osteoblastic rimming (; ).
Figure 7. Representative H&E sections depicting the new bone formation (H&E staining, A-P: ×200, inset: ×1000).
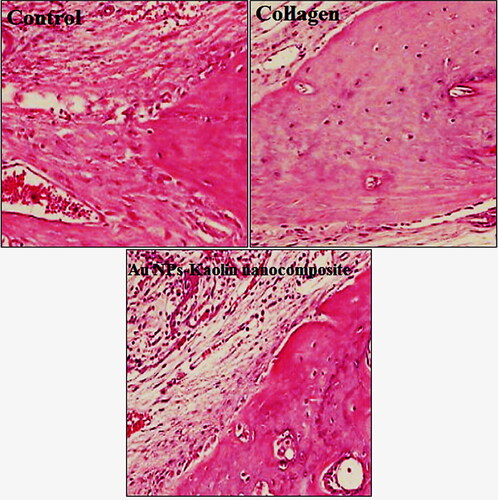
Table 1. Histopathological findings related to the foreign body reaction and osteoblastic rimming at 5, 7, 10 and 12 weeks for Au NPs-kaolin nanocomposite, collagen and control groups.
Table 2. Histopathological findings related to the mineralized bone and osteoid at 5, 7, 10 and 12 weeks for Au NPs-kaolin nanocomposite, collagen and control groups.
Table 3. Histopathological findings related to the granulation tissue and inflammation at 5, 7, 10 and 12 weeks for Au NPs-kaolin nanocomposite, collagen and control groups.
4. Conclusions
In summary, Au NPs-kaolin nanocomposite has been successfully prepared by a facile in situ reduction route by T. spicata extract without additional toxic reductant and stabilizer agent, which was identified by the characteristic analysis of FE-SEM, EDS, TEM, EDX elemental mapping, XRD and ICP. In this in vivo experiment on rat calvarial bone defect, histological analyses showed that Au NPs-kaolin nanocomposite increased bone formation activity when used with collagen matrix. The mechanism of this effect is likely angiogenesis. However, it is possible that this behaviour is simply an inflammation effect.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes on contributors
Dayong Peng
▪▪▪
Bing Han
▪▪▪
Yu Kong
▪▪▪
Meng Chen
▪▪▪
Haoxuan Zhang
▪▪▪
References
- Park JC, Kim YH, Choi HS, et al. The rate and stability of mandibular block bone graft in recent 5 years. Maxillofac Plast Reconstr Surg. 2017;39(1):21.
- Burg KJ, Porter S, Kellam JF. Biomaterial developments for bone tissue engineering. Biomaterials. 2000;21(23):2347–2359.
- Gomes KU, Carlini JL, Biron C, et al. Use of allogeneic bone graft in maxillary reconstruction for installation of dental implants. J Oral Maxillofac Surg. 2008;66(11):2335–2338.
- Peng W, Kim IK, Cho HY, et al. The healing effect of platelet-rich plasma on xenograft in peri-implant bone defects in rabbits. Maxillofac Plast Reconstr Surg. 2016;38:16.
- Lekovic V, Camargo PM, Weinlaender M, et al. A comparison between enamel matrix proteins used alone or in combination with bovine porous bone mineral in the treatment of intrabony periodontal defects in humans. J Periodontol. 2000;71(7):1110–1116.
- Liu D, Chen L, Jiang S, et al. Formulation and characterization of hydrophilic drug diclofenac sodium-loaded solid lipid nanoparticles based on phospholipid complexes technology. J Liposome Res. 2014;24(1):17–26.
- Tong AM, Lu WY, Xu JH, et al. Use of apple seed meal as a new source of β-glucosidase for enzymatic glucosylation of 4-substituted benzyl alcohols and tyrosol in monophasic aqueous-dioxane medium. Bioorg Med Chem Lett. 2004;14:2095–2097.
- Baran T, Sargin I, Kaya M, et al. Design and application of sporopollenin microcapsule supported palladium catalyst: remarkably high turnover frequency and reusability in catalysis of biaryls. J Colloid Interface Sci. 2017;486:194–203.
- Wang X, Hu P, Xue F, et al. Cellulose-supported N-heterocyclic carbene-palladium catalyst: synthesis and its applications in the Suzuki cross-coupling reaction. Carbohydr Polym. 2014;114:476–483.
- Elkhenany H, Abd Elkodous M, Ghoneim NI, et al. Comparison of different uncoated and starch-coated superparamagnetic iron oxide nanoparticles: implications for stem cell tracking. Int J Biol Macromol. 2020;143:763–774.
- Veisi H, Joshani Z, Karmakar B, et al. Ultrasound assisted synthesis of Pd NPs decorated chitosan-starch functionalized Fe3O4 nanocomposite catalyst towards Suzuki-Miyaura coupling and reduction of 4-nitrophenol. Int J Biol Macromol. 2021;172:104–113.
- Budarin VL, Clark JH, Luque R, et al. Palladium nanoparticles on polysaccharide-derived mesoporous materials and their catalytic performance in C–C coupling reactions. Green Chem. 2008;10(4):382–387.
- Wang ZQ, Li JL, Sun YL, et al. Chinese herbal medicine for osteoporosis: a systematic review of randomized controlled trails. Evid Based Complement Alternat Med. 2013;2013:356260.
- (a) Gong T, Xie J, Liao J, et al. Nanomaterials and bone regeneration. Bone Res. 2015;3:15029. (b) Cole LE, Vargo-Gogola T, Roeder RK. Targeted delivery to bone and mineral deposits using bisphosphonate ligands. Adv Drug Deliv Rev. 2016;99:12–27. (c) Holyoak DT, Tian YF, van der Meulen MC, et al. Osteoarthritis: pathology, mouse models, and nanoparticle injectable systems for targeted treatment. Ann Biomed Eng. 2016;44(6):1–14.
- Lin Y, Li L, Hu L, et al. Multifunctional poly(dopamine)-assisted synthesis of silver nano particles/carbon nanotubes nanocomposite: toward electrochemical sensing of hydrogen peroxide with enhanced sensitivity. Sens Actuators B: Chem. 2014;202:527–535.
- Ramprakash Upadhyay P, Srivastava V. Clays: an encouraging catalytic support. Curr Catal. 2016;5(3):162–181.
- He K, Zeng Z, Chen A, et al. Advancement of Ag-graphene based nanocomposites: an overview of synthesis and its applications. Small. 2018;14(32):1800871.
- Ma H, Wang B, Wang Y. Application of molybdenum and phosphate modified kaolin in electrochemical treatment of paper mill wastewater. J Hazard Mater. 2007;145(3):417–423.
- Toludare TS, Owoeye SS, Kenneth-Emehige A, et al. Microstructure evolution and physico-mechanical properties of bone china porcelain compositions using two selected kaolinite clays from Nigeria. Sci Afr. 2019;3:e00066.
- (a) Warnke PH, Springer IN, Wiltfang J, et al. Growth and transplantation of a custom vascularized bone graft in a man. Lancet. 2004;364:766–770. (b) Wang X, Wu J, Chiba H, et al. Puerariae radix prevents bone loss in ovariectomized mice. J Bone Miner Metab. 2003;21:268–275. (c) Chueh FS, Chang CP, Chio CC, et al. Puerarin acts through brain serotonergic mechanisms to induce thermal effects. J Pharmacol Sci. 2004;96:420–427. (d) Overstreet DH, Lee YW, Rezvani AH, et al. Suppression of alcohol intake after administration of the chinese herbal medicine, NPI-028, and its derivatives. Alcohol Clin Exp Res. 1996;20:221–227. (e) Wang LY, Zhao AP, Chai XS. Effects of puerarin on cat vascular smooth muscle in vitro. Zhongguo Yao Li Xue Bao. 1994;15:180–182.
- Lee DH, Kim IK, Cho HY, et al. Effect of herbal extracts on bone regeneration in a rat calvaria defect model and screening system. J Korean Assoc Oral Maxillofac Surg. 2018;44(2):79–85. Apr
- Nie S, Xing Y, Kim GJ, et al. Nanotechnology applications in cancer. Annu Rev Biomed Eng. 2007;9:257–288.
- Dykman L, Khlebtsov N. Gold nanoparticles in biomedical applications: recent advances and perspectives. Chem Soc Rev. 2012;41(6):2256–2282.
- Dreaden EC, Alkilany AM, Huang X, et al. The golden age: gold nanoparticles for biomedicine. Chem Soc Rev. 2012;41(7):2740–2779.
- Abraham GE, Himmel PBJ. Management of rheumatoid arthritis: rationale for the use of colloidal metallic gold. J Nutr Environ Med. 1997;7:295–305.
- Khlebtsov N, Dykman L. Biodistribution and toxicity of engineered gold nanoparticles: a review of in vitro and in vivo studies. Chem. Soc. Rev. 2011;40:1647–1671.
- Chen YH, Tsai CY, Huang PY, et al. Methotrexate conjugated to gold nanoparticles inhibits tumor growth in a syngeneic lung tumor model. Mol. Pharmaceutics 2007;4:713–722.
- Brown CL, Whitehouse MW, Tiekink ERT, et al. Colloidal metallic gold is not bio-inert. Inflammopharmacology. 2008;16(3):133–137.
- Paciotti GF, Myer L, Weinreich D, et al. Colloidal gold: a novel nanoparticle vector for tumor directed drug delivery. Drug Deliv. 2004;11(3):169–183.
- Paciotti GF, Kingston DG, Tamarkin L. Colloidal gold nanoparticles: a novel nanoparticle platform for developing multifunctional tumor-targeted drug delivery vectors. Drug Dev Res. 2006;67(1):47–54.
- Park H, Tsutsumi H, Mihara H. Cell penetration and cell-selective drug delivery using α-helix peptides conjugated with gold nanoparticles . Biomaterials. 2013;34(20):4872–4879.
- Dreaden EC, Austin LA, Mackey MA, et al. Size matters: gold nanoparticles in targeted cancer drug delivery. Ther Deliv. 2012;3(4):457–478.
- Remant Bahadur KC. Gold nanoparticle-based gene delivery: promises and challenges. Nanotechnol Rev. 2014;3(3):269–280.
- Mendes R, Fernandes A, Baptista P. Gold nanoparticle approach to the selective delivery of gene silencing in cancer – the case for combined delivery? Genes. 2017;8(3):94.
- Huang X, Jain PK, El-Sayed IH, et al. Plasmonic photothermal therapy (PPTT) using gold nanoparticles. Lasers Med Sci. 2008;23(3):217–228.
- Cruz LJ, Tacken PJ, Rueda F, et al. Targeting nanoparticles to dendritic cells for immunotherapy. Methods Enzymol. 2012;509:143–163.
- Andersson HA, Kim Y-S, O'Neill BE, et al. HSP70 promoter-driven activation of gene expression for immunotherapy using gold nanorods and near infrared light. Vaccines. 2014;2:216–227.