?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
This work described the one-pot synthesis of orange pectin encapsulated Fe3O4 nanoparticles (Fe3O4@Pectin NPs) which is prepared by co-precipitation of Fe(II/(III) ions in alkaline solution mediated by pectin. This process led to formation of magnetic nanoparticles within the network of pectin. Physicochemical characterization of the as-synthesized Fe3O4@Pectin NPs was carried out through Fourier transformed infrared spectroscopy (FT-IR), electron microscopy (SEM and TEM), energy dispersive X-ray spectroscopy (EDX), vibrating sample magnetometer (VSM) and X-ray diffraction (XRD). The in vitro cytotoxic and anti-liver cancer effects of biologically synthesized Fe3O4@Pectin NPs against pleomorphic hepatocellular carcinoma (SNU-387), hepatic ductal carcinoma (LMH/2A), morris hepatoma (McA-RH7777), and novikoff hepatoma (N1-S1 Fudr) cancer cell lines were assessed. The anti-liver cancer properties of the Fe3O4@Pectin NPs could significantly remove pleomorphic hepatocellular carcinoma (SNU-387), hepatic ductal carcinoma (LMH/2A), morris hepatoma (McA-RH7777), and novikoff hepatoma (N1-S1 Fudr) cancer cell lines in a time and concentration-dependent manner by MTT assay. The IC50 of the Fe3O4@Pectin NPs were 8, 13, 10, and 7 µg/mL against pleomorphic hepatocellular carcinoma (SNU-387), hepatic ductal carcinoma (LMH/2A), morris hepatoma (McA-RH7777), and novikoff hepatoma (N1-S1 Fudr) cancer cell lines. The antioxidant activity of Fe3O4@Pectin NPs was determined by DPPH method. The Fe3O4@Pectin NPs showed the high antioxidant activity according to the IC50 value. It seems that the anti-human liver cancer effect of recent nanoparticles is due to their antioxidant effects.
1. Introduction
The reasons for the clinical trials' failed to achieve the desired multilayer results are complex and intertwined. Unfortunately, therapeutic agents (chemotherapy, biology, and nanotechnology) are so selective and effective in targeting in vitro cancer cells, and even in proportionate animal specimens [Citation1–4]. This is because the biological distribution of therapeutic agents can be a fundamental factor in these fractures. Inadequate concentration at unwanted concentration and target sites elsewhere, leading to dose-limiting poisoning [Citation2, Citation3]. The biological distribution of drug agents is controlled largely by the drugs ability to penetrate biological barriers. Strategy for Adding Targeting Sections to Therapeutic Nanoparticles to Improve Location Specification to date, despite 30 years of effort in pharmaceutical companies and many laboratories, it has not yet been able to produce clinically approved drugs [Citation3, Citation4]. This failure is because the addition of molecular agents increases the targeting of cognitive characteristics. However, it does so in the face of much greater difficulty in managing biological barriers [Citation5–9].
Nanotechnology is defined in different ways in several countries, which affects the nanodrugs clinical validation. However, what these different definitions have in common is the use of nanoscale structures [Citation5–7]. There are several distinct benefits to using nanotechnology in the diseases treatment. Nanoparticles, especially metal nanoparticles and metal oxides, have been widely used by medical consumers and manufacturers. The mechanism of nanoparticle-induced toxicity against cancer cells is the production of reactive oxygen species (ROS) [Citation8–11]. Excessive production of reactive oxygen species can lead to oxidative stress, disruption of normal physiological maintenance, and oxidation regulation. These effects in turn lead to DNA damage, unregulated cell signaling pathways, changes in cell evolution, cytotoxicity, apoptotic death, and the onset of cell death [Citation12–15]. Critical-deterministic factors can affect the production of reactive oxygen species. These critical-deterministic factors include shape, size, nanoparticle surface area, particle surface baroelectricity, surface-forming groups, Particle solubility, metal ion emission from nanomaterials and nanoparticles, optical activation, model of cell reactions, inflammatory effects and ambient pH [Citation15–19]. Metal nanoparticles and oxides of metal nanoparticles due to their optical properties due to the large active area and high atomic number, amplify the photoelectric and Compton effects of both X-ray and gamma-ray interactions with the adsorbent in the diagnostic and therapeutic range [Citation20–23]. Finally, they can lead to the development of methods for the destruction of tumor cells and reduce their survival with minimal side effects in radiation therapy. As a result, increasing industrial knowledge in the field of scalable nanoparticle synthesis, along with the design of multifunctional nanoparticles, will dramatically change the strategies of microenvironmental preparation and therapeutic-diagnostic nanoparticles for cancer treatment [Citation24–30].
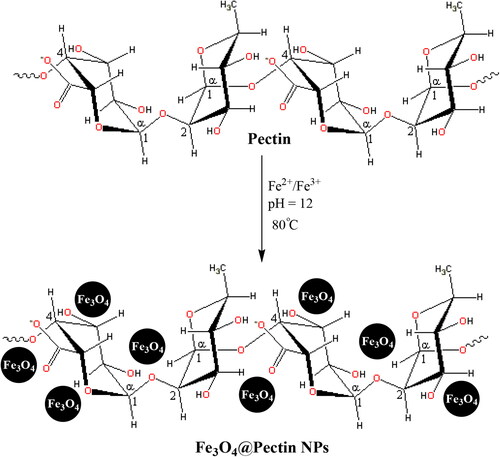
Pectin is a natural biopolymer, which is usually obtained from fruit. Therefore, this polymer, polygalacturonic acid, is a polysaccharide that contains carboxylate groups in each monomer. This fact can cause pectin to have some additional advantages rather to other analogues. Carboxylate groups can act as a covalently chelating agent to Fe3O4 nanoparticles and surrounds it as shell. A general and schematic pathway for one-pot preparation of Fe3O4@Pectin NPs that presented in Scheme 1. In the current research, the properties of Fe3O4@Pectin NPs against common liver cancer cell lines i.e. pleomorphic hepatocellular carcinoma (SNU-387), hepatic ductal carcinoma (LMH/2A), morris hepatoma (McA-RH7777), and novikoff hepatoma (N1-S1 Fudr) were evaluated.
2. Experimental
2.1. Materials and method
The essential chemicals and bio-samples were procured from Fluka and Sigma-Aldrich. All the reagents were used directly without any further purifications. In the structural characterizations, FT-IR was recorded over KBr disc in a Bruker VERTEX 80v spectrophotometer. Morphological and compositional analysis was done over FE-SEM MIRA3 microscope equipped with EDX (TSCAN). The samples were coated with gold atom vapor prior to analysis. A Philips CM10 microscope was used in the TEM analysis, performed at 200 kV operating voltage. The sample was prepared by dispersing it on a carbon coated Cu grid followed by drying. In the analysis of crystallinity, X-ray diffraction study was performed using Co Kα radiation (λ = 1.78897 Å, voltage 40 keV, current 40 mA) in the scanning range of 2θ = 10 to 80°. A STAT FAX 2100, BioTek, Winooski, USA instrument was used in Microplate Reading.
2.2. Preparation of orange pectin coated Fe3O4 nanoparticles (Fe3O4@pectin NPs)
In a typical procedure according to the literature [Citation7], 1.0 g of orange pectin was dissolved in deionized water (100 mL) in a 250-mL three-necked flask equipped with a stirrer and dropping funnel. Then, FeCl3·6H2O (1.67 g) and FeCl2·4H2O (0.67 g) were slowly added into the mixture. The mixture was stirred strongly for 10 min at 80 °C, and then 1 mol/L NaOH aqueous solution was added by dropping into the mixture until the pH increased to 12. The products from this system were magnetized for 30 min by a magnet. The precipitates were cooled, washed and diluted to neutrality by deionized water. The powder of Fe3O4@Pectin NPs was obtained by air drying.
2.3. DPPH assay protocol
DPPH is a free radical that changes color in the presence of substances with antioxidant properties and captures electrons. Yellow to purple color change is the basis of antioxidant properties. Solutions with different concentrations (10 to 1000 μg/ml) were prepared from phenolic powder and BHT synthetic antioxidant in methanol solvent. 1 ml of DPPH methanol solution was added to 1 ml of concentrate to 3 ml of nanoparticles and the resulting mixture was stirred vigorously. The test tubes were placed in a dark place for 30 min. After this period, the absorbance at the wavelength of 517 nm was read. It should be noted that in the control sample, the nanoparticles was replaced with 3 ml of methanol. Finally, the DPPH radical's inhibition percentage was calculated with this formula [Citation31–35]:
2.4. MTT assay protocol
In this study, the anticancer effects of Fe3O4@Pectin NPs and levatinib (Positive control drug) samples against the liver cancer cells (pleomorphic hepatocellular carcinoma (SNU-387), hepatic ductal carcinoma (LMH/2A), morris hepatoma (McA-RH7777), and novikoff hepatoma (N1-S1 Fudr)) were investigated.
These cells in DMEM culture medium (Gibco, USA) with 10% FBS (Gibco, USA) and penicillin/streptomycin (100 μL/100 μg/ml) in an incubator containing 5% Carbon dioxide with 90% humidity was stored at 37 °C. Then, when about 80% of the flask was filled, cell passage was performed and about 5 × 104 cells (per square centimeter) were placed in 24 house bacterial petri dishes in the usual environment. The cells were treated with different concentrations of nanoparticles 24 h later and kept in this condition for 3 days. The survival rate of cultured cells was prepared with different concentrations. In this experiment, cells were cultured at 3 × 104 cells/well in 24-well plates and kept in an incubator at 37 °C for 24 h. Then the old culture medium was taken out of the wells and the cells were treated with different concentrations of nanoxidro. This test was performed on the first, second and third days after exposing the cells to the compounds; thus, at the appropriate time after culturing the cells in plates of 24 cells, the culture medium was removed and about 300 μl of fresh medium containing 30 μl of MTT solution was added to each cell. After 3-4 h of incubation at 37 °C, MTT solution is removed and 200 μl (Dimethyl Sulfoxide, Merck, USA, 100%) DMSO is added to each house. Then the sample absorption was read at 570 wavelengths using ELISA rider (Expert 96, Asys Hitch, Ec Austria). This experiment was repeated 3 times and each time, four wells were considered for each nano oxide concentration. Cell survival percentage was evaluated by the following formula [Citation36]:
The results were evaluated as Mean ± SE using SPSS software version 22 and statistical tests of variance of completely randomized block design. Drawing graphs in Excel software was performed and the significance level of the differences was considered p < 0.01.
3. Results and discussion
3.1. Catalyst characterization data analysis
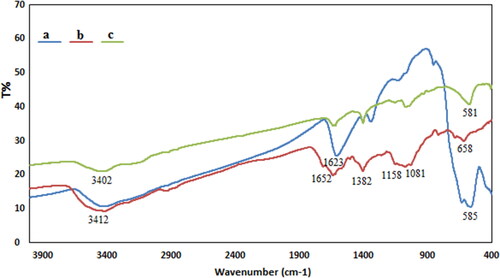
We have used co-precipitation of Fe(II) and Fe(III) in the presence of orange pectin leading to formation of Fe3O4@Pectin NPs in one-pot (Scheme 1). The structural, morphological and physicochemical properties of the Fe3O4@Pectin NPs were determined by various analytical techniques including FT-IR, SEM, EDX, TEM, VSM and XRD study. presented a comparative FT-IR spectrum of Fe3O4, Pectin and Fe3O4@Pectin NPs in order to confirm the successful preparation of the final material. Markedly, a characteristic strong absorption peak is observed at 585 cm−1 due to Fe–O–Fe stretching in the spectrum of Fe3O4 () [Citation6]. shows the featured peaks of pectin, namely, O-H bending, C–O stretching, C–O–C asymmetric stretching and C–C stretching, being appeared at 1652, 1382, 1158 and 1081 cm−1 respectively [Citation7].
For the FTIR spectrum of Fe3O4@Pectin NPs, the characteristic peak of Fe–O–Fe shifted to 581 cm−1 (), all characteristic peaks of Fe3O4 nanoparticles and pectin were presented in the spectrum, indicating that Fe3O4 nanoparticles were successfully coated with pectin. These results are consistent with literatures [Citation7].
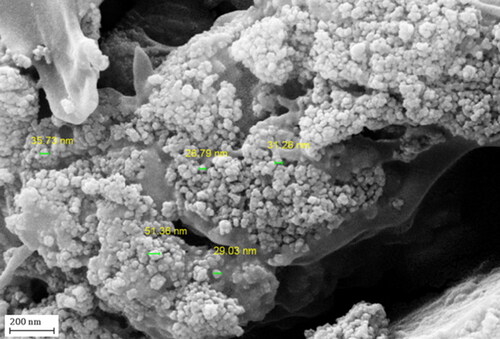
In order to have an idea of structural morphology, shape and size of Fe3O4@Pectin NPs nanocomposite, the electronic microscopic analysis (TEM and SEM) were performed ( and ).The particles are of globular shape. The grey and black particles represent the Fe3O4 NPs that encapsulated in the pectin matrix. The homogeneous growth in the form of a thin layer of pectin polymer can be detected by close observation ( and ). The pectin also stabilizes the corresponding NPs not to agglomerate together. A closer image was obtained in HR-TEM analysis which reveals the exact topography of the nanocomposite (). The ferrite NPs are of perfect round shape. They are highly crystalline as seen from the Braggs planes (220) with a d = 0.29 nm [Citation6].
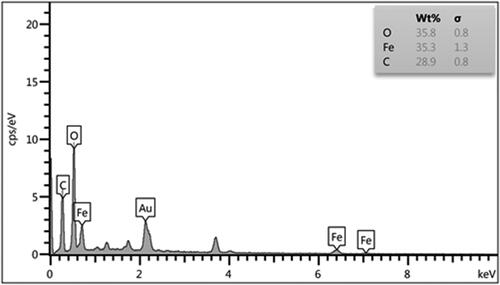
Chemical composition of the nanocomposite was assessed from EDX analysis and the profile is shown in . It represents Fe, O, and C as elemental components. The occurrence of carbon confirmed the presence of pectin and successful fabrication of Fe3O4@Pectin NPs.
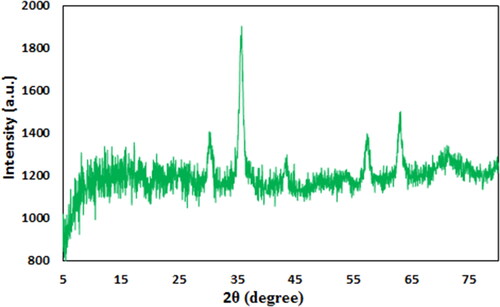
Crystalline phases and the diffraction planes of the Fe3O4@Pectin NPs was ascertained by XRD study, that shown in . It represents a single phase profile indicating a united entity of the assembled counterparts. The typical diffraction peaks due to Fe3O4 are observed at 2θ = 30.1, 35.4, 43.3, 53.6, 57.1, 62.7° corresponding to (220), (311), (400), (422), (511) and (440) Bragg reflection planes respectively (JCPDS file, PDF No. 65-3107). Three additional broad diffraction peaks observed at 2θ = 10-20° are contributed from pectin polymers.
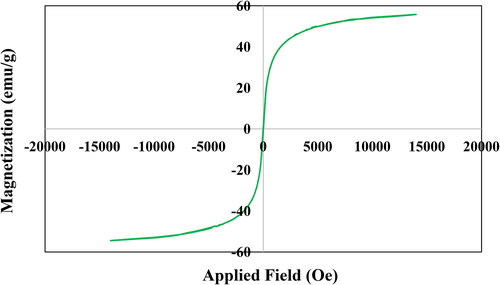
VSM study was carried out to determine the magnetic properties of the Fe3O4@Pectin NPs. On passing an external magnetic field of -20kOe to +20kOe, a magnetic hysteresis curve is obtained and the corresponding saturation magnetization (Ms) value of Fe3O4@Pectin NPs was 52.5 emu/g (). It clearly reveals the material to be superparamagnetic in nature.
3.2. The bio application of Fe3O4@pectin NPs
Regarding cancer, efforts have been made to use smart nanomaterials (nanoparticles, nanostructures), which have a greater ability to target cancer cells, to treat such patients. That is, they kill malignant cells by irradiating them, providing a microscopic therapeutic effect within electrons [Citation11–15]. Nanoparticles are programmed to achieve optimal therapeutic efficacy, delivering therapeutic loads to target cells. Studies have also been performed on several nanocarriers based on lipids, polymers, and peptides for delivery to the respiratory system [Citation16–19]. Properties of nanoparticles for targeted delivery of nanoparticles to tumors is the motivation for targeted drug delivery in cancer treatment to kill cancer cells. In a way, that has the least damage to healthy cells [Citation20–24]. One of the nanotechnology goals is to mount drugs on carriers, send them and release them into the target cell, which is called targeted drug delivery. Using nanoparticles, the drug can be intelligently delivered to the desired tissue, and improve the tissue without damaging other tissues [Citation25–30].
In this study, the treated cells with different concentrations of the present Fe3O4@Pectin NPs were assessed by MTT assay for 48 h about the cytotoxicity properties on normal (HUVEC) and liver malignancy cell lines i.e. pleomorphic hepatocellular carcinoma (SNU-387), hepatic ductal carcinoma (LMH/2A), morris hepatoma (McA-RH7777), and novikoff hepatoma (N1-S1 Fudr). The absorbance rate was evaluated at 570 nm, which represented viability on normal cell line (HUVEC) even up to 1000 μg/mL for Fe3O4@Pectin NPs, but the IC50 of lenvatinib was 22 µg/mL against normal cell line ( and and ).
Figure 8. The anti-liver carcinoma properties of Fe3O4@Orange-Pectin nanocomposite and Lenvatinib against pleomorphic hepatocellular carcinoma (SNU-387 (I)), hepatic ductal carcinoma (LMH/2A (II)), morris hepatoma (McA-RH7777 (III)), and novikoff hepatoma (N1-S1 Fudr (IV)) cell lines.
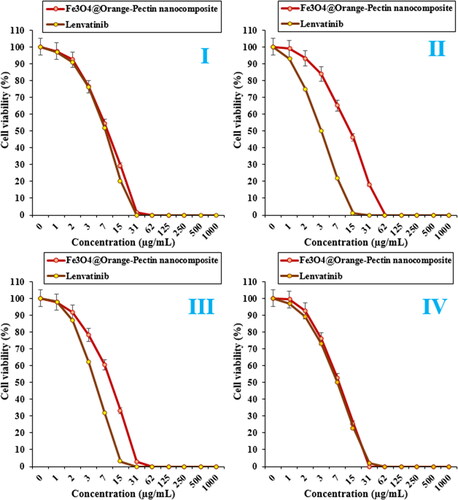
Figure 9. The cytotoxicity effects of Fe3O4@Orange-Pectin nanocomposite and Lenvatinib against normal (HUVEC) cell line.
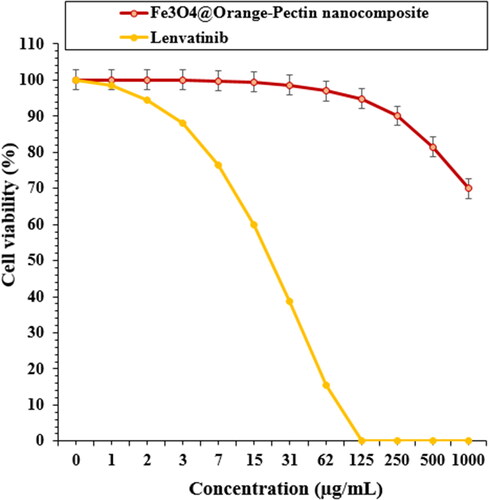
Table 1. The IC50 of Fe3O4@Orange-Pectin nanocomposite and lenvatinib in the anti-liver carcinoma test.
The viability of malignant liver cell line reduced dose-dependently in the presence of Fe3O4@Pectin NPs and lenvatinib. The IC50 of Fe3O4@Pectin NPs were 8, 13, 10, and 7 µg/mL against pleomorphic hepatocellular carcinoma (SNU-387), hepatic ductal carcinoma (LMH/2A), morris hepatoma (McA-RH7777), and novikoff hepatoma (N1-S1 Fudr) cell lines, respectively ( and and ).
The IC50 of Lenvatinib were 7, 3, 4, and 7 µg/mL against pleomorphic hepatocellular carcinoma (SNU-387), hepatic ductal carcinoma (LMH/2A), morris hepatoma (McA-RH7777), and novikoff hepatoma (N1-S1 Fudr) cell lines, respectively ( and and ).
Nanoparticles have several compounds, each with a different structure. The extraction of these compounds depends on several factors, the most important of which are the type of solvent and the extraction method. It will be very difficult to choose a solvent for any group of nanoparticles compounds, because with these compounds, there are other substances that affect the degree of solubility of these substances [Citation20–24]. When extracting nanoparticles, it should be noted that always use a method that has the best performance in the survival of antioxidant compounds. Usually nanoparticles have unique antioxidant effects [Citation26]. Recent studies have shown that when the plant extracts are placed in metal nanoparticles as stabilizing and reducing compounds, they form nanocomposites with extraordinary antioxidant effects. Antioxidants are generally referred to as substances that can delay, slow down, and even stop oxidation processes [Citation35–39]. These compounds can optimally prevent changes in the color and taste of food because of oxidation reactions. The antidote to the mechanism of oxidants is that they prevent the spread of oxidation chain reactions by giving hydrogen atoms to free radicals. In recent years, the synthetic antioxidants use such as BHT, BHA, TBHQ as well as other chemical additives has been limited due to their potential toxicity and carcinogenicity. Today, most research in this area focuses on the use of new and safe antioxidants from plant, animal, microbial and food sources [Citation31–34].
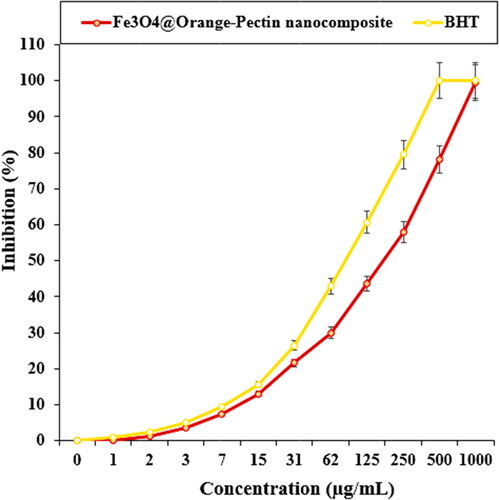
The scavenging capacity of Fe3O4@Pectin NPs and BHT at different concentrations expressed as percentage inhibition has been indicated in and . In the antioxidant test, the IC50 of Fe3O4@Pectin NPs and BHT against DPPH free radicals were 180 and 86 µg/mL, respectively ( and ).
Table 2. The IC50 of Fe3O4@Orange-Pectin nanocomposite and BHT in antioxidant test.
4. Conclusion
In summary, we demonstrate herein a unique biocompatible, natural polymer-pectin modified and magnetically isolable Fe3O4@Pectin NPs being successfully prepared by green method in one-pot. The prepared Fe3O4@Pectin nanocomposite was characterized by a wide range of analytical techniques.
The viability of malignant liver cell lines reduced dose-dependently in the presence of Fe3O4@Pectin nanocomposite. The IC50 of Fe3O4@Pectin nanocomposite were 8, 13, 10, and 7 µg/mL against pleomorphic hepatocellular carcinoma (SNU-387), hepatic ductal carcinoma (LMH/2A), morris hepatoma (McA-RH7777), and novikoff hepatoma (N1-S1 Fudr) cell lines, respectively. The Fe3O4@Pectin nanocomposite showed the best antioxidant activities against DPPH. So, the findings of the recent research show that biologically synthesized Fe3O4@Pectin nanocomposite might be used to cure liver cancer. In addition, the current study offer that Fe3O4@Pectin nanocomposite could be a new potential adjuvant chemopreventive and chemotherapeutic agent against cytotoxic cells. However, it necessitates clinical trial researches to ascertain their effect as anticancer agents.
Additional information
Funding
References
- Stewart B, Wild CP, World Cancer Report 2014. International Agency for Research on Cancer World Health Organization; Lyon, France; 2014. Available online: http://www.iarc.fr/en/publications/books/wcr/wcr-order.php.
- Rasmussen JW, Martinez E, Louka P, et al. Zinc oxide nanoparticles for selective destruction of tumor cells and potential for drug delivery applications. Exp Opin Drug Deliv. 2010;7(9):1063–1077.
- Felice B, Prabhakaran MP, Rodríguez AP, et al. Drug delivery vehicles on a nano-engineering perspective. Mater Sci Eng C. 2014;41:178–195.
- Fernandes E, Ferreira JA, Peixoto A, et al. New trends in guided nanotherapies for digestive cancers: a systemic review. J Control Release. 2015;209:288–307.
- Caputo F, de Nicola M, Ghibelli L. Pharmacological potential of bioactive engineered nanomaterials. Biochem Pharmacol. 2014;92(1):112–130.
- Veisi H, Ozturk T, Karmakar B, et al. In situ decorated Pd NPs on chitosan-encapsulated Fe3O4/SiO2-NH2 as magnetic catalyst in Suzuki-Miyaura coupling and 4-nitrophenol reduction. Carbohydr Polym. 2020;235:115966.
- Doustkhah E, Heidarizadeh M, Rostamnia S, et al. Copper immobilization on carboxylic acid-rich Fe 3 O 4 -Pectin: Cu 2+ @Fe 3 O 4 -Pectin a superparamagnetic nanobiopolymer source for click reaction. Mat Lett. 2018;216:139–143.
- Maier-Hauff K, Ulrich F, Nestler D, et al. Efficacy and safety of intratumoral thermotherapy using magnetic iron-oxide nanoparticles combined with external beam radiotherapy on patients with recurrent glioblastoma multiforme. J Neurooncol. 2011;103(2):317–324.
- Kolosnjaj-Tabi J, di Corato R, Lartigue L, et al. Heat-generating iron oxide nanocubes: Subtle “destructurators” of the tumoral microenvironment. ACS Nano. 2014;8(5):4268–4283.
- Bhattacharyya S, Kudgus RA, Bhattacharya R, et al. Inorganic nanoparticles in cancer therapy. Pharm Res. 2011;28(2):237–259.
- Hilger I, Kaiser WA. Iron oxide-based nanostructures for MRI and magnetic hyperthermia. Nanomedicine (Lond). 2012;7(9):1443–1459.
- Orel V, Shevchenko A, Romanov A, et al. Magnetic properties and antitumor effect of nanocomplexes of iron oxide and doxorubicin. Nanomedicine. 2015;11(1):47–55.
- Van Landeghem FK, Maier-Hauff K, Jordan A, et al. Post-mortem studies in glioblastoma patients treated with thermotherapy using magnetic nanoparticles. Biomaterials. 2009;30(1):52–57.
- Silva AC, Oliveira TR, Mamani JB, et al. Application of hyperthermia induced by superparamagnetic iron oxide nanoparticles in glioma treatment. Int J Nanomed. 2011;6:591–603.
- Johannsen M, Thiesen B, Wust P, et al. Magnetic nanoparticle hyperthermia for prostate cancer. Int J Hyperthermia. 2010;26(8):790–795.
- Bañobre-López M, Teijeiro A, Rivas J. Magnetic nanoparticle-based hyper-thermia for cancer treatment. Rep Pract Oncol Radiother. 2013;18(6):397–400.
- Klein S, Sommer A, Distel LV, et al. Superparamagnetic iron oxide nanoparticles as novel X-ray enhancer for low-dose radiation therapy. J Phys Chem B. 2014;118(23):6159–6166.
- Chatterjee DK, Fong LS, Zhang Y. Nanoparticles in photodynamic therapy: an emerging paradigm. Adv Drug Deliv Rev. 2008;60(15):1627–1637.
- Zhang AP, Sun YP. Photocatalytic killing effect of TiO2 nanoparticles on Ls- 174-t human Colon carcinoma cells. WJG. 2004;10(21):3191–3193.
- Thevenot P, Cho J, Wavhal D, et al. Surface chemistry influences cancer killing effect of TiO2 nanoparticles. Nanomedicine. 2008;4(3):226–236.
- Colon J, Hsieh N, Ferguson A, et al. Cerium oxide nanoparticles protect gastrointestinal epithelium from radiation-induced damage by reduction of reactive oxygen species and upregulation of superoxide dismutase 2. Nanomedicine. 2010;6(5):698–705.
- Wason MS, Colon J, Das S, et al. Sensitization of pancreatic cancer cells to radiation by cerium oxide nanoparticle-induced ROS production. Nanomedicine. 2013;9(4):558–569.
- Tarnuzzer RW, Colon J, Patil S, et al. Vacancy engineered ceria nanostructures for protection from radiation-induced cellular damage. Nano Lett. 2005;5(12):2573–2577.
- Ali D, Alarifi S, Alkahtani S, et al. Cerium oxide nanoparticles induce oxidative stress and genotoxicity in human skin melanoma cells. Cell Biochem Biophys. 2015;71(3):1643–1651.
- Neri D, Supuran CT. Interfering with pH regulation in tumors as a therapeutic strategy. Nat Rev Drug Discov. 2011;10(10):767–777.
- Seo JW, Chung H, Kim MY, et al. Development of water-soluble single-crystalline TiO2 nanoparticles for photocatalytic cancer-cell treatment . Small. 2007;3(5):850–853.
- Hou Z, Zhang Y, Deng K, et al. UV-emitting upconversion-based TiO2 photosensitizing nanoplatform: near-infrared light mediated in vivo photodynamic therapy via mitochondria-involved apoptosis pathway. ACS Nano. 2015;9(3):2584–2599.
- Cui S, Yin D, Chen Y, et al. In vivo targeted deep-tissue photodynamic therapy based on near-infrared light triggered upconversion nanoconstruct. ACS Nano. 2013;7(1):676–688.
- Lucky SS, Idris NM, Li Z, et al. Titania coated upconversion nanoparticles for near-infrared light triggered photodynamic therapy. ACS Nano. 2015;9(1):191–205.
- Idris NM, Lucky SS, Li Z, et al. Photoactivation of core-shell titania coated upconversion nanoparticles and their effect on cell death. J Mater Chem B. 2014;2(40):7017–7026.
- Chen J, Patil S, Seal S, et al. Rare earth nanoparticles prevent retinal degeneration induced by intracellular peroxides. Nat Nanotechnol. 2006;1(2):142–150.
- Das M, Patil S, Bhargava N, et al. Auto-catalytic ceria nanoparticles offer neuroprotection to adult rat spinal cord neurons. Biomaterials. 2007;28(10):1918–1925.
- Korsvik C, Patil S, Seal S, et al. Superoxide dismutase mimetic properties exhibited by vacancy engineered ceria nanoparticles. Chem Commun. 2007;(10):1056–1058.
- Hong R, Han G, Fernandez JM, et al. Glutathione-mediated delivery and release using monolayer protected nanoparticle carriers. J Am Chem Soc. 2006;128(4):1078–1079.
- Zangeneh MM, Zangeneh A, Pirabbasi E, et al. Appl Organometal Chem. 2019c;33:e5246.
- Jalalvand AR, Zhaleh M, Goorani S, et al. Chemical characterization and antioxidant, cytotoxic, antibacterial, and antifungal properties of ethanolic extract of allium saralicum R.M. Fritsch leaves rich in linolenic acid, methyl ester. J Photochem Photobiol B. 2019;192:103–112.
- Barclay L, Baskin KA, Dakin KA, et al. The antioxidant activities of phenolic antioxidants in free-radical peroxidation of phospholipid-membranes. Can J Chem. 1990;68(12):2258–2269.
- Hamelian M, Varmira K, Veisi H. Green synthesis and characterizations of gold nanoparticles using thyme and survey cytotoxic effect, antibacterial and antioxidant potential. J Photochem Photobiol B Biol. 2018;184:71–79.
- Esumi K, Takei N, Yoshimura T. Antioxidant-potentiality of gold-chitosan nanocomposites. Colloids Surf B Biointerfaces. 2003;32(2):117–123.