?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
In this study, copper nanoparticles (Cu NPs) were synthesized in an eco-friendly pathway applying Mentha piperita extract as reducing/stabilizing agent. The morphological and physicochemical features of the prepared nanoparticles were determined using several advanced techniques like FE-SEM, EDX, and UV–Vis studies. The as synthesized Cu NPs was explored in the antioxidant and anti-human esophageal cancer tests. The in vitro cytotoxic and anti-esophageal cancer effects of biologically synthesized Cu NPs against human esophageal squamous cell carcinoma (KYSE-270), human caucasian esophageal carcinoma (OE33), and adenocarcinoma of the gastroesophageal junction (ESO26) cancer cell lines were assessed. The anti-esophageal cancer properties of the Cu NPs could significantly remove KYSE-270, OE33, and ESO26 cancer cell lines in a time and concentration-dependent manner by MTT assay. The IC50 of the Cu NPs were 241, 278, and 240 µg/mL against KYSE-270, OE33, and ESO26 cancer cell lines. The antioxidant activity of Cu NPs was determined by DPPH method. The Cu NPs showed the high antioxidant activity according to the IC50 value. It seems that the anti-human esophageal cancer effect of recent nanoparticles is due to their antioxidant effects.
1. Introduction
Esophagus is one part of the gastrointestinal tract and transfers the water and nutritional materials from pharynx to the stomach [Citation1]. The main diseases that affect the normal function of the esophagus are included Zenker's Diverticulum, Nutcracker esophagus, Mallory–Weiss syndrome, Jackhammer esophagus (hypercontractile peristalsis), esophageal varices, diffuse esophageal spasm, Boerhaave syndrome, achalasia, Schatzki's ring, Killian–Jamieson diverticulum, esophageal dysphagia, esophageal atresia and tracheoesophageal fistula, acute esophageal necrosis, neurogenic dysphagia, esophageal web, Chagas disease, Barrett's esophagus, Hiatus hernia, GERD, caustic injury to the esophagus, esophagitis, and esophageal cancer. Among the above diseases, the mortality rate of esophageal cancer is more [Citation1–4]. There are several types of esophageal cancers that the most common esophageal cancers are human esophageal squamous cell carcinoma, human caucasian esophageal carcinoma, adenocarcinoma of the gastroesophageal junction, and distal esophageal adenocarcinoma [Citation3, Citation4]. The common signs of esophageal cancer are weight loss, difficulty in swallowing, vomiting blood, coughing up, a dry cough, a hoarse voice, and enlarged lymph nodes around the collarbone [Citation1]. The main risk factors of esophageal cancer are acid reflux, chewing betel nut, very hot drinks, smoking tobacco, obesity, radiation therapy, alcohol and familial predisposition [Citation2]. The history and physical examination, needle biopsy, blood tests, molecular testing, and medical imaging such as esophageal endoscopic ultrasound, chest X-ray, computerized tomography, low-dose helical computerized tomography scan, magnetic resonance imaging and positron emission tomography are used for diagnosis of esophageal cancer [Citation3, Citation4]. To treat esophageal cancer, radiation therapy, chemotherapy, immunotherapy, targeted therapy and surgery are used. The main anti-esophageal cancer chemotherapeutic drugs are included cisplatin, oxaliplatin, carboplatin, fluorouracil, and capecitabine [Citation5]. According to the high side effects of chemotherapeutic drugs such as mouth sores, weight loss, diarrhoea, vomiting, hair loss, fatigue, and nausea, the formulation of modern chemotherapeutic drugs is necessary [Citation5, Citation6]. Recently, scientists have understood that metallic nanoparticles have excellent anticancer properties [Citation6, Citation7]. In recent decades, the power of nanotechnology has been used in countless fields, including biomedical sciences. Nanoparticles are solid colloidal particles with dimensions of 1–100 nanometers [Citation8–11]. Due to their comparable dimensions to cells, viruses, proteins and genes, they can interact with basic biological processes. In recent years, much attention has been paid to synthesizing different types of nanoparticles as nanomedical materials. Among them, copper nanoparticles have special properties such as high surface-to-volume ratio and high magnetic properties, allowing for potential manipulation by an external magnetic field [Citation12–14]. In recent decades, much research has been done on synthesizing copper nanoparticles and many reports have described efficient synthesis approaches for the production of controlled, stable, biocompatible, and integrated copper nanoparticles [Citation10–13]. The most common methods such as co-precipitation, hydrothermal synthesis, microemulsion, sonochemical synthesis can lead to the high-quality synthesis of copper nanoparticles nanoparticles. In addition, these nanoparticles can be prepared by other methods such as electrochemical synthesis, laser pyrolysis technique, synthesis with microorganisms or bacteria (especially magneto-tactical bacteria and copper-reducing bacteria) [Citation12–17]. Low toxicity and high biocompatibility of copper nanoparticles have led to the expansion of copper nanoparticles in targeted drug delivery. Using an external copper field, the nanoparticles can be directed to the target tissue and release the drug at the target site [Citation15–19]. Targeted drug delivery reduces the side effects of the drug to surrounding healthy tissues and reduces the dose of drug required. To increase the biocompatibility of copper nanoparticles for use in the field of drug delivery, copper nanoparticles are modified by organic or inorganic coatings [Citation15–18]. Coating copper nanoparticles with the right combination can control the loading, delivery and release of the drug. In addition, suitable coatings can reduce the toxicity of nanoparticles and increase their biocompatibility [Citation13–15]. Many cancer drugs are loaded onto magnetic drug carriers through various interactions. Coating copper nanoparticles with polymers is an ideal method of drug delivery; because in addition to reducing carrier toxicity, it also prevents copper nanoparticles from clotting [Citation19–21]. Recently, the copper nanoparticles have been used due to the anticancer effects. The studies have indicated when the copper nanoparticles are combined by the ethno medicinal plants, their anticancer effects increase [Citation26–30]. In the recent study, the copper nanoparticles were green-synthesized by Mentha piperita aqueous extract for killing the esophageal cancer cell lines.
2. Methods and materials
2.1. Materials
Phosphate buffer solution (PBS), Sabouraud Dextrose Agar, Sabouraud Dextrose Medium, Muller Hinton Agar, Mueller Hinton Medium, carbazole reagent, 4-(dimethylamino)benzaldehyde, Dulbecco's-modified Eagle Medium (DMED), Ehrlich solution, dimethyl sulfoxide (DMSO), hydrolysate, decamplmaneh foetal bovine serum, borax-sulphuric acid mixture, 2,2-diphenyl-1-picrylhydrazyl (DPPH), and antimitotic antibiotic solution all were achieved from Sigma-Aldrich company of USA.
2.2. Preparation and extraction of aqueous extract
The aqueous extract of M. piperita was prepared according to the following procedure. First, 100 g of the dried leaves of the plant were powdered and macerated in boiling water for 3 h. After filtration, the extract was evaporated. Finally, the concerted crud extract was put in a freeze drier to obtain the powder extract in brown colour.
2.3. Green synthesis of CuNPs
The CuNPs were green synthesized based on an earlier report [Citation22]. A 30 mL of the aqueous solution of M. piperita extract (40 mg/mL) was added into 30 mL of 0.3 M Cu(NO3)2.3H2O. Then, the reaction mixture was stirred at 60 °C for 24 h. Next, the produced precipitation was washed with water for three times and centrifuged at 15,000 rpm for 10 min subsequently. After that, the remaining precipitate was placed in an oven at 55 °C. The dried nanoparticles in dark brown colour were kept in a vial. In the recent research, the solvent of the CuNPs was distilled water.
2.4. Antioxidant assay protocol
In this method, 1 mL of different concentrations of the nanoparticles (0–1000 µg/mL) (with 1 mL of DPPH (300 µmol/l) combined and then the final volume of the combination with methanol reached 4000 µL. The falcons were then vertexed and kept in the dark for 60 min. The absorbance was read at 517 nm. The DPPH radical inhibition percentage was calculated using the following equation [23]:
2.5. MTT assay protocol
In cell culture medium, cells are prepared that are in the process of differentiation and differentiate into new cells with hormones and growth factors. By cell culture, identical cells are produced and intracellular functions such as DNA replication, DNA transcription, RNA and protein synthesis, and cell metabolism are examined. After the molecule binds to its membrane receptor, intracellular reactions such as complexes, intracellular messages, and message transmission are evaluated. The cultured cells can be stored at a very low temperature. The low temperature maintained the growth rate or genetic composition of the cells. Cells can be used when needed, for example, a month later. This method prevents cell aging. In studies with animals such as rats and rabbits, animal homeostasis and experimental stress should be considered. However, this is not the case in cell culture. Standardizing laboratory tests is easier than studying animals; in the laboratory, it is easier to control physicochemical factors, cell environments such as acidity, heat, osmotic pressure, and oxygen and carbon dioxide pressures [Citation23].
In this study, the anticancer effects of Cu NPs samples against the esophageal cancer (human esophageal squamous cell carcinoma (KYSE-270), human Caucasian esophageal carcinoma (OE33), and adenocarcinoma of the gastroesophageal junction (ESO26)) cells were investigated.
These cells in DMEM culture medium (Gibco, USA) with 10% FBS (Gibco, USA) and penicillin/streptomycin (100 μL/100 μg/mL) in an incubator containing 5% Carbon dioxide with 90% humidity was stored at 37 °C. Then, when about 80% of the flask was filled, cell passage was performed and about 5 × 104 cells (per square centimeter) were placed in 24 house bacterial petri dishes in the usual environment. The cells were treated with different concentrations of nanoparticles 24 h later and kept in this condition for 3 days. The survival rate of cultured cells was prepared with different concentrations. In this experiment, cells were cultured at 3 × 104 cells/well in 24-well plates and kept in an incubator at 37 °C for 24 h. Then the old culture medium was taken out of the wells and the cells were treated with different concentrations of nanoxidro. This test was performed on the first, second and third days after exposing the cells to the compounds; thus, at the appropriate time after culturing the cells in plates of 24 cells, the culture medium was removed and about 300 μl of fresh medium containing 30 μl of MTT solution was added to each cell. After 3–4 h of incubation at 37 °C, MTT solution is removed and 200 μl (Dimethyl Sulfoxide, Merck, USA, 100%) DMSO is added to each house. Then the sample absorption was read at 570 wavelengths using ELISA rider (Expert 96, Asys Hitch, Ec Austria). This experiment was repeated 3 times and each time, four wells were considered for each nano oxide concentration. Cell survival percentage was evaluated by the following formula [Citation23]:
2.6. Qualitative measurement
The obtained results were analysed by SPSS (version 20) software using one-way ANOVA, followed by Duncan post-hoc test (p ≤ 0.01).
3. Results and discussion
3.1. Chemical characterization of Cu NPs
FE-SEM Analysis: FE-SEM images of Cu NPs is shown in . The images show a spherical morphology for Cu NPs. This morphology for the green synthesized of cooper nanoparticles using plants extract has been reported previously [Citation24–26]. Despite the homogeneity and well dispersing of the nanoparticles, they show a tendency to aggregate such as the others metallic nanoparticles [Citation26–28]. The particle size diameters of Cu NPs were in range of 13.42–39.85 nm. The previous studies on the synthesized of copper nanoparticles using herbal extract have reported various size for the NPs from 5 to 100 nm. In our review of literature, different sizes have been reported for the biosynthesized Cu NPs using plants extracts [Citation29–33].
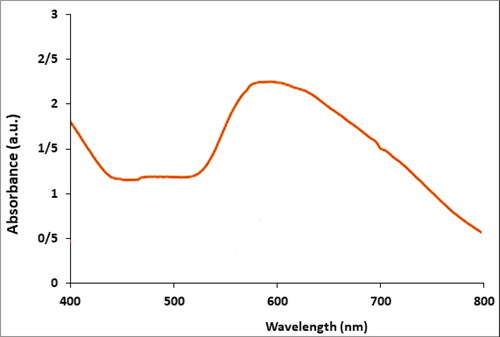
presents the UV–Vis. spectrum of biosynthesized Cu NPs using M. piperita extract. The result of UV–Vis. spectroscopy confirms the formation of Cu NPs. The peak at 586 nm belongs to the biosynthetic Cu NPs. This observation is in good agreement with the previous report of UV–Vis. for a biosynthesized Cu NPs [Citation26].
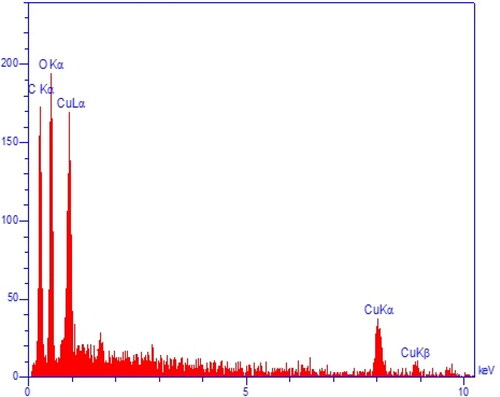
The EDX results approve the green synthesis of copper nanoparticles by the presences of the signals at 0.8 keV (for CuLα), 8.1 keV (for Cu Kα), and 8.9 keV (for Cu Kβ) () [Citation27]. The peak at 0.5 keV for OLα confirm the presences of oxygen in the CuNPs, that shows the nanoparticles are synthesized as copper oxide, previous studies on green synthesis of CuNPs using plants extracts have reported the formation of copper oxide [Citation28].
3.2. Antioxidant effects of Cu NPs
Oxidative stress is caused by an imbalance between the production of free radicals and metabolic reactions, which leads to damage to lipids, proteins and nucleic acids. These damages may be due to low levels of antioxidants or an excessive increase in the production of free radicals in the body [Citation33–35]. In humans, oxidative stress is associated with chronic diseases such as diabetes and cancer. Therefore, the production of synthetic and natural antioxidants is necessary to prevent oxidative stress and its destructive effects. Antioxidants effectively and in various ways reduce the harmful effects of free radicals in the biological and food systems and cause detoxification [34–36]. In this regard, green nanoparticles can be used (using plant substrates to prepare nanomaterials that are environmentally friendly and do not contain any harmful chemicals) that show antioxidant properties. At present, the use of non-toxic substances in synthesizing nanoparticles to prevent biological hazards, especially in medical and pharmaceutical applications is considered [Citation33–38]. Many researchers have focused on bioactive substances derived from plants or other sources such as bacteria, fungi and yeast for synthesizing nanoparticles. The green synthesis method is thought to increase the biocompatibility and performance of metal nanoparticles for biological applications due to removing harmful chemicals [Citation32–35]. During the bioproduction stages of nanoparticles, their extracellular production using plants or their extracts is more beneficial and their production can be adjusted in a controlled way based on size, distribution and shape for different purposes [Citation32–37].
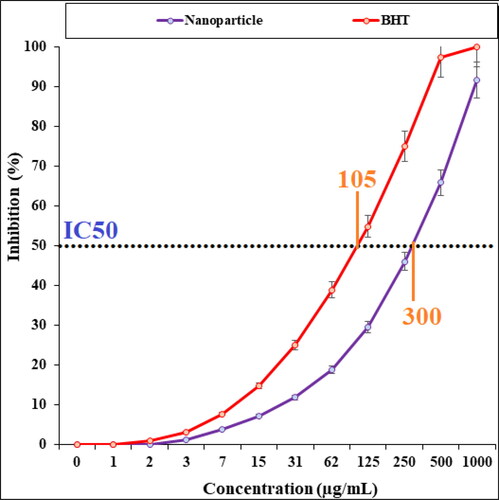
In the recent study, the scavenging capacity of Cu NPs and BHT at different concentrations expressed as percentage inhibition has been indicated in . In the antioxidant test, the IC50 of Cu NPs and BHT against DPPH free radicals were 300 and 105 µg/mL, respectively.
3.3. Anti-human esophageal cancer effects of the Cu NPs
Despite many advances in disease control and treatment, cancer remains one of the global challenges to human health. The most common treatment for cancer is chemotherapy. An important point in the treatment of cancer with chemotherapy is the acquired resistance of the tumour to drugs, and this has created major problems for the treatment of cancer [Citation4, Citation5]. Because the rate of mutation and genetic instability in cancer cells is very high and genetic changes occur rapidly in them, these cells become resistant to drugs. Therefore, further research on discovering new treatment strategies to overcome the drug resistance of cancers seems necessary [Citation5, Citation6]. Meanwhile, nanotechnology has created a promising field in cancer treatment. Recently, the anti-cancer and anti-angiogenic effects of metallic nanoparticles have been considered and the results have shown that metallic nanoparticles can be considered as a potential anti-cancer agent [Citation7].
Many parameters such as surface functions nature and texture and size are important in the anticancer effects of metallic nanoparticles, of course the efficacy of the size is the main [Citation22–24]. Many reports have been indicated whatever the nanoparticles size is low, their ability in poring and destroying the cancer cells is more. In detail, it has been reported the nanoparticles with the size lower than 50 nm have the best condition for anticancer effects [Citation23, Citation24]. As shown in the FE-SEM figure, the size of nanoparticles is less than 40 nm.
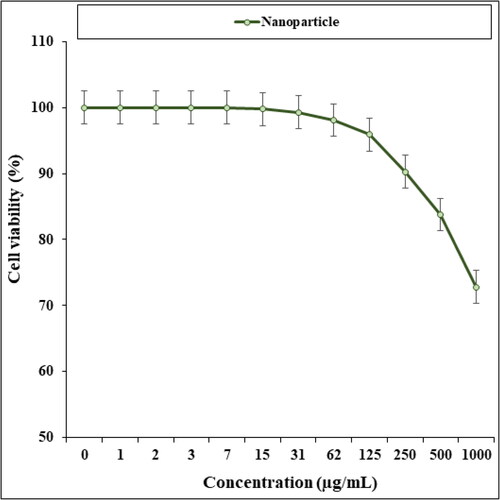
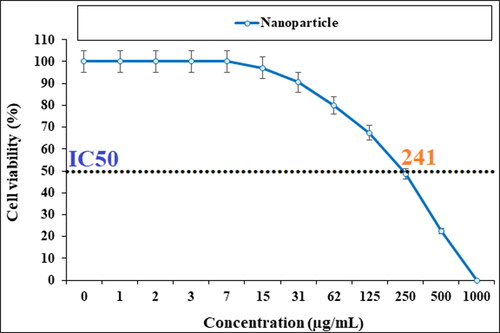
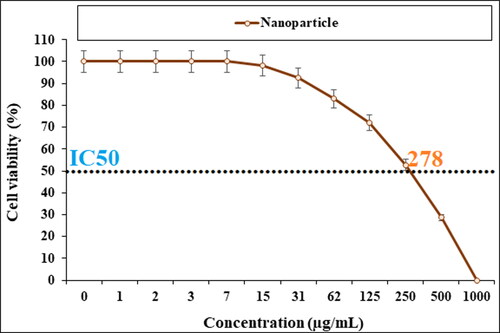
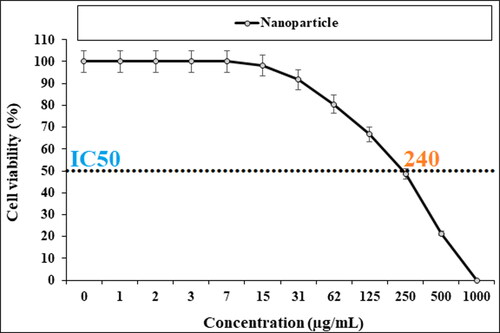
In our research, the treated cell lines with Cu NPs were tested by a well-known cytotoxicity test, i.e. MTT test for 72 h regarding the cytotoxicity activities on normal and esophageal cancer cell lines (). Cu NPs did not show any toxicity on HUVEC cells in the MTT assay.
The cell viability of esophageal cancer cells decreased dose-dependently in the Cu NPs presence.
4. Conclusions
In the present study, the copper nanoparticles were green synthesized using the aqueous extract of M. piperita. The synthesized NPs were characterized using chemical techniques such as EDX, FE-SEM, and UV–Vis. The results revealed a spherical morphology for CuNPs in the range size of 13.42–39.85 nm.
The viability of malignant esophageal cell line reduced dose-dependently in the presence of CuNPs. The IC50s of CuNPs were 241, 278, and 240 µg/mL against KYSE-270, OE33, and ESO26 cell lines, respectively. The CuNPs showed the best antioxidant activities against DPPH. The IC50 of CuNPs and BHT against DPPH free radicals were 300 and 105 µg/mL, respectively. After clinical study, CuNPs can be utilized as an efficient drug in the treatment of esophageal cancer in humans.
Additional information
Funding
References
- Zhang HZ, Jin GF, Shen HB. Epidemiologic differences in esophageal cancer between Asian and Western populations. Chin J Cancer. 2012;31(6):281–286.
- Akhtar S. Areca nut chewing and esophageal squamous-cell carcinoma risk in Asians: a meta-analysis of case-control studies. Cancer Causes Control. 2013;24(2):257–265.
- Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med. 2003;349(23):2241–2252.
- Pennathur A, Gibson MK, Jobe BA, et al. Oesophageal carcinoma. Lancet. 2013;381(9864):400–412.
- Stahl M, Mariette C, Haustermans K, et al. Oesophageal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Annal Oncol. 2013;24( Suppl):51–56.
- Raut RW, Kolekar NS, Lakkakula JR, et al. Extracellular synthesis of silver nanoparticles using dried leaves of Pongamia pinnata (L) pierre. Nano-Micro Lett. 2010;2(2):106–113.
- Warma RS. Extracellular synthesis of silver nanoparticles using dried leaves of Pongamia pinnata (L) pierre. Nano‐Micro Lett. 2010;2:106.
- Harada T, Tanigawa N, Matsuki M, et al. Evaluation of lymph node metastases of breast cancer using ultrasmall superparamagnetic iron oxide-enhanced magnetic resonance imaging. Eur J Radiol. 2007;63(3):401–407.
- Cardoso VF, Francesko A, Ribeiro C, et al. Advances in magnetic nanoparticles for biomedical applications. Adv Healthc Mater. 2018;7:5.
- Khanna L, Verma NK, Tripathi SK. Burgeoning tool of biomedical applications superparamagnetic nanoparticles. J Alloys Compd. 2018;752:332–353.
- Xie W, Guo Z, Gao F, et al. Shape-, size- and structure-controlled synthesis and biocompatibility of iron oxide nanoparticles for magnetic theranostics. Theranostics. 2018;8(12):3284–3307.
- Wu W, He Q, Jiang C. Magnetic iron oxide nanoparticles: synthesis and surface functionalization strategies. Nanoscale Res Lett. 2008;3(11):397–415.
- Pascal C, Pascal JL, Favier F, et al. Payen C electrochemical synthesis for the control of y-Fe2O3 nanoparticle size. Morphology, microstructure, and magnetic behavior. Chem Mater. 1999;11(1):141–147.
- Bomatí-Miguel O, Mazeina L, Navrotsky A, et al. Calorimetric study of maghemite nanoparticles synthesized by laser induced pyrolysis. Chem Mater. 2008;20(2):591–598.
- Bharde AA, Parikh RY, Baidakova M, et al. Bacteria-mediated precursor-dependent biosynthesis of superparamagnetic iron oxide and iron sulfide nanoparticles. Langmuir. 2008;24(11):5787–5794.
- Roh Y, Vali H, Phelps TJ, et al. Moon JW extracellular synthesis of magnetite and metal substituted magnetite nanoparticles. J Nanosci Nanotechnol. 2006;6(11):3517–3520.
- Faraji M, Yamini Y, Rezaee M. Magnetic nanoparticles: synthesis, stabilization, functionalization, characterization, and applications. JICS. 2010;7(1):1–37.
- Mou X, Ali Z, Li S, et al. Applications of magnetic nanoparticles in targeted drug delivery system. J Nanosci Nanotechnol. 2015;15(1):54–629.
- Renard P-EL, Buchegger F, Petri-Fink A, et al. Local moderate magnetically induced hyperthermia using an implant formed in situ in a mouse tumor model. Int J Hyperther. 2009;25(3):229–239.
- Schroeder A, Heller DA, Winslow MM, et al. Treating metastatic cancer with nanotechnology. Nat Rev Cancer. 2011;12(1):39–50.
- Gobbo L, Sjaastad K, Radomski MW, et al. Magnetic nanoparticles in cancer theranostics. Theranostics. 2015;5(11):1249–1263.
- Kumar PV, Shameem U, Kollu P, et al. Green synthesis of copper oxide nanoparticles using aloe vera leaf extract and its antibacterial activity against fish bacterial pathogens. BioNanoSci. 2015;5(3):135–139.
- Lu Y, Wan X, Li L, et al. Synthesis of a reusable composite of graphene and silver nanoparticles for catalytic reduction of 4- nitrophenol and performance as anti-colorectal carcinoma. J Mater Res Technol. 2021;12:1832–1843.
- Devi TB, Ahmaruzzaman M. Facile preparation of copper nanaoparticles using Coccinia grandis fruit extract and its application towards the reduction of toxic nitro compound. Mater Today: Proc. 2018;5(1):2098–2104.
- Taghavi Fardood S, Ramazani A. Green synthesis and characterization of copper oxide nanoparticles using coffee powder extract. J Nanostruct. 2016;6:167–171.
- Rao MD, Pennathur G. Green synthesis and characterization of cadmium sulphide nanoparticles from Chlamydomonas reinhardtii and their application as photocatalysts. Mater Res Bull. 2017;85:64–73.
- Baghayeri M, Mahdavi B, Hosseinpor‐Mohsen Abadi Z, et al. Green synthesis of silver nanoparticles using water extract of Salvia leriifolia: Antibacterial studies and applications as catalysts in the electrochemical detection of nitrite. Appl Organometal Chem. 2018;32(2):e4057.
- Seydi N, Mahdavi B, Paydarfard S, et al. Preparation, characterization, and assessment of cytotoxicity, antioxidant, antibacterial, antifungal, and cutaneous wound healing properties of titanium nanoparticles using aqueous extract of Ziziphora clinopodioides lam leaves. Appl Organometal Chem. 2019;e5009.
- Vaidehi D, Bhuvaneshwari V, Bharathi D, et al. Antibacterial and photocatalytic activity of copper oxide nanoparticles synthesized using Solanum lycopersicum leaf extract. Mater Res Express. 2018;5(8):085403.
- Khani R, Roostaei B, Bagherzade G, et al. Green synthesis of copper nanoparticles by fruit extract of Ziziphus spina-christi (L.) wild: application for adsorption of triphenylmethane dye and antibacterial assay. J Mol Liq. 2018;255:541–549.
- Sulaiman GM, Tawfeeq AT, Jaaffer MD. Biogenic synthesis of copper oxide nanoparticles using Olea europaea leaf extract and evaluation of their toxicity activities: an in vivo and in vitro study. Biotechnol Progress. 2018;34(1):218–230.
- Kaur P, Thakur R, Chaudhury A. Biogenesis of copper nanoparticles using peel extract of Punica granatum and their antimicrobial activity against opportunistic pathogens. Green Chem Lett Rev. 2016;9(1):33–38.
- Cheirmadurai K, Biswas S, Murali R, et al. Green synthesis of copper nanoparticles and conducting nanobiocomposites using plant and animal sources. RSC Adv. 2014;4(37):19507–19511.