?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Calendula officinalis is known as a popular plant with various uses as pharmaceutical agent or food additive around the world. In this study, cooper nanoparticles were green synthesized using the aqueous extract of Calendula officinalis as the stabilizing, reducing, and capping agents. The formation of CuNPs@C. officinalis was screened using different chemical technique such as FT-IR spectroscopy, XRD, SEM, and Energy EDS. The antioxidant and anti-cancer activity of CuNPs@C. officinalis was studied using common assay including free radical scavenging and MTT assays. The results confirm the green synthesized of CuNPs@C. officinalis with aspherical morphology in the range size of 19.64 to 39.15 nm. In the antioxidant test, the IC50 of CuNPs@C. officinalis and BHT against DPPH free radicals were 193 and 154 µg/mL, respectively. In the cellular and molecular part of the recent study, the treated cells with CuNPs@C. officinalis were assessed by MTT assay for 24, 48, and 72 h about the cytotoxicity and anti-human lung adenocarcinoma properties on normal (HUVEC) and lung adenocarcinoma cell lines, i.e. lung moderately differentiated adenocarcinoma (LC-2/ad), lung poorly differentiated adenocarcinoma (PC-14), and lung well-differentiated bronchogenic adenocarcinoma (HLC-1). The IC50s of CuNPs@C. officinalis were 297, 328, and 514 µg/mL against PC-14, LC-2/ad, and HLC-1 cell lines, respectively. The viability of malignant lung cell line reduced dose-dependently in the presence of CuNPs@C. officinalis. It seems that the anti-human lung adenocarcinoma effect of recent nanoparticles is due to their antioxidant effects.
1. Introduction
The field of synthetic nanotechnology has developed the self-sufficiency of materials with specific sizes and shapes at the nanometer scale environmentally friendly [Citation1–4]. Nanomaterials can be created from the engineering of multiple or single molecules which are engineered and have several functional groups. Biocompatible nanocarriers include peptides, engineered antibodies, micelles, polymers, and liposomes [Citation5–9]. Nanomaterials can be designed to gain the desired chemical and physical characteristics and purposefully direct drugs to dynamic tumour environment with high therapeutic effects and low toxicity. Features to be controlled when designing nanoparticles are surface to volume ratio, shape, size, molecular charge, and drug release. For example, nanoparticles, after binding to functional chemical components such as antibodies and ligands, bind to the surface receptors of the cancer cell and prevent its clearance by the blood [Citation5–7].
Today, nanoparticle technology has made great strides in the production of many drugs, and the production of nanoparticles is one of the hopes in the effective treatment and diagnosis of many diseases, including cancer [Citation4–8]. Metallic nanoparticles have long been considered as a candidate for cancer treatment. Because natural metal oxides are present in large quantities in nature, the processing and synthesis of these nanoparticles can be one of the least expensive synthesis protocols [Citation6–11]. Metallic nanoparticles are one of the new types of widely used mineral particles that have been considered by researchers due to their suitable physical and chemical properties and at the same time, it has more adsorption power than other metallic nanoparticles-containing compounds [Citation10–13]. Metallic nanoparticles are one of the therapeutic compounds recognized by the US Department of Food and Drug Administration as a safe substance. Metallic nanoparticles are biocompatible and non-toxic and have also been used as medical fillers, cosmetics and drug carriers [Citation10–13]. Among the special properties of metallic nanoparticles are high chemical stability, low dielectric constant, high catalytic activity, absorption of infrared and ultraviolet light and most importantly its antibacterial properties. If the therapeutic and anticancer effects of these compounds are confirmed, this could be a significant step in advancing cancer therapies [Citation8–13].
Drug delivery by nanoparticles using biodegradable polymers solves many problems. In 1975, a drug-conjugated model of the polymer was proposed by Ringdorf, which increased drug delivery to the cancer cell site [Citation10]. She suggested that the conjugated pharmacological properties of drugs and polymers could be manipulated by altering the chemical and physical properties of the polymer. For example, a water-insoluble drug can be soluble by adding solvent components to a polymer. Therefore, it increases both bioavailability and biodegradability. For cytotoxic drugs to be efficiently delivered to cancer cell tissue, first, cytotoxic agents must be removed from the bloodstream and after leaving the extracellular space, the cancerous tissue must pass through the membrane surface and be targeted inside the cancer cell [Citation11–13]. The ideal properties of nano foundations that should be considered when designing are: 1- Continuous drug release 2- Inactive accumulation of the drug in cancerous tissue 3- Targeting antigens or surface receptors of cancer cells with a controlling effect on endosomal uptake and membrane destruction. 4- Drug release into the cytoplasm and protection from enzymatic degradation [Citation14–17].
Calendula officinalis is a well-known medicinal plant, which belongs Asteraceae family [Citation18]. European Medicines Agency introduced the plant as an herbal medicinal product since 2008 [Citation19]. The plant is used as remedy to cure several diseases in different traditional medicine around the world. C. officinalis is used as sufficient agent in treatment of anti-inflammatory, diaphoretic, analgesic, antiseptic, healing wounds, minor burns [Citation19,Citation20]. This ability of the plant to treat of various illness is contributed to the presence of diverse secondary metabolites in the plant. So far, the existence of phenolic, flavonoids, saponins, carotenes, and mucilage have been reported for the plant [Citation21]. In the recent study, the properties of CuNPs green-medicated by Calendula officinalis leaf aqueous extract against common lung adenocarcinoma cell lines, i.e. lung moderately differentiated adenocarcinoma (LC-2/ad), lung poorly differentiated adenocarcinoma (PC-14), and lung well-differentiated bronchogenic adenocarcinoma (HLC-1) were evaluated.
2. Experimental
2.1. Materials
Phosphate buffer solution (PBS), Sabouraud Dextrose Agar, Sabouraud Dextrose Medium, Muller Hinton Agar, Mueller Hinton Medium, carbazole reagent, 4-(Dimethylamino)benzaldehyde, Dulbecco's Modified Eagle Medium (DMED), Ehrlich solution, dimethyl sulfoxide (DMSO), hydrolysate, decamplmaneh fetal bovine serum, borax-sulphuric acid mixture, 2,2-diphenyl-1-picrylhydrazyl (DPPH), and antimitotic antibiotic solution all were achieved from Sigma-Aldrich company of USA.
2.2. Preparation and extraction of aqueous extract
The aqueous extract of C. officinalis was prepared according to the following procedure. First, 100 g of the dried leaves of the plant were powdered and macerated in boiling water for 3 h. After filtration, the extract was evaporated. Finally, the concerted crud extract was put in a freeze drier to obtain the powder extract in brown colour.
2.3. Green synthesis of CuNPs@C. officinalis
The CuNPs@C. officinalis were green synthesized based on an earlier report [Citation22]. A 30 mL of the aqueous solution of C. officinalis extract (40 mg/mL) was added into 30 mL of 0.3 M Cu(NO3)2.3H2O. Then, the reaction mixture was stirred at 60 °C for 24 h. Next, the produced precipitation was washed with water for three times and centrifuged at 15000 rpm for 10 min subsequently. After that, the remaining precipitate was placed in an oven at 55 °C. The dried nanoparticles in dark brown colour were kept in a vial. In the recent research, the solvent of the CuNPs@C. officinalis was distilled water.
2.4. Antioxidant activities of CuNPs@C. officinalis
In this method, DPPH was used as the standard as a stable radical compound. Thus, 50 µl of different concentrations of 1-1000 μg/ml of nanoparticle was added to 5 ml of 0.004% DPPH solution in methanol. After 30 min of incubation at room temperature, the samples were read against Blank at 517 nm. The percentage of inhibition of DPPH free radicals was calculated using the following formula [Citation23]:
In this test, the ability of hydrogen or electron atoms to be given by nanoparticle is measured by the amount of decolourization of purple solutions 2 and 2-diphenyl-1-picryl hydrazyl or DPPH in methanol. Then the concentration of the nanoparticle with a radical inhibition percentage of 50 was calculated by the graph. Obviously, the smaller this number, the greater the antioxidant power or inhibition of free radicals. In this experiment, the synthetic antioxidant butylated hydroxytoluene was used as a positive control and all experiments were repeated three times [Citation23].
2.5. Anti-human lung adenocarcinoma properties of CuNPs@C. officinalis
Lung moderately differentiated adenocarcinoma (LC-2/ad), lung poorly differentiated adenocarcinoma (PC-14), and lung well-differentiated bronchogenic adenocarcinoma (HLC-1) cells were used to evaluate the anticancer effect of CuNPs@C. officinalis on cell culture.
These cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) with 10% (w/v) FBS, 100 U/mL penicillin, and 100 µg/mL streptomycin. Then, cells were distributed at 10,000 cells/well in 96-well plates. The cells were grown under a humidified incubator with 5% CO2 at 37 °C until reaching confluency (typically after 24 h). The cells were treated with CuNPs@C. officinalis at concentrations of 0, 1, 2, 3, 7, 15, 31, 62, 125, 250, 500, and 1000 µg/mL and subsequently incubated for 24, 48, and 72 h. CuNPs@C. officinalis were sterilized using UV radiation for 1 h. Finally, the MTT solution (5 mg/mL in PBS) was added to each well and incubated for 4 h at 37 °C. The medium with MTT was removed and the formazan crystals formed in the living cells were dissolved in 100 μL DMSO per well. All tests were run in the triplicates. The relative viability (%) was calculated based on the absorbance at λ = 570 nm determined using a microplate reader [Citation23].
The concentration of the tested compounds that reduced the percentage of cell life by half was considered as IC50 (The half maximal inhibitory concentration).
2.6. Qualitative measurement
The obtained results were analyzed by SPSS (version 20) software using one-way ANOVA, followed by Duncan post-hoc test (p ≤ 0.01).
3. Results and discussion
3.1. Chemical characterization of CuNPs@C. officinalis
3.1.1. EDS analysis
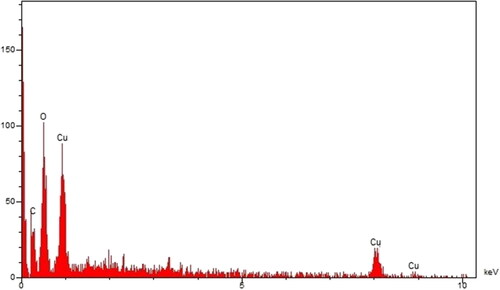
shows the EDS diagram of CuNPs@C. officinalis. The diagram shows the presence of cooper with signals at below of 1 Kev (for CuLα), around 8 Kev (for Cu Kα), and below 9 Kev (for Cu Kβ). These signals for the green synthetic of copper nanoparticles have been reported previously [Citation24]. Furthermore, a single around 0.5 and a peak around 0.3 Kev are related to oxygen and carbon of organic compounds from C. officinalis extract that are linked to the surface of CuNPs@C. officinalis.
3.1.2. SEM analysis
FE-SEM images of CuNPs is shown in . The images show a spherical morphology for CuNPs@C. officinalis. This morphology for the green synthesized of cooper nanoparticles using plants extract has been reported previously [Citation25]. Despite the homogeneity and well dispersing of the nanoparticles, they show a tendency to aggregate such as the others metallic nanoparticles [Citation26–28]. The particle size diameters of CuNPs@C. officinalis were in range of 19.64 to 39.15 nm. The previous studies on the synthesized of cooper nanoparticles using herbal extract have reported various size for the NPs from 5 to 100 nm. In our review of literature, different sizes have been reported for the biosynthesized CuONPs using plants extracts [Citation29–32].
3.1.3. FT-IR analysis
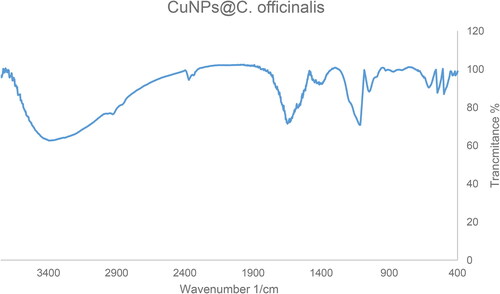
The FT-IR spectra of CuNPs@C. officinalis and C. officinalis extract is shown in . The peaks at 497, 543, and 609 cm−1 belong to vibration band of Cu-O. These bands have been reported for green synthesized of copper previously [Citation33]. The other peaks can be consider as an evidence of the presences of the organic compounds that are linked to the surface of CuNPs@C. officinalis. The different FT-IR bands are related to the presences of various functional groups. For example, peaks at 3417 and 2925 cm−1 for the stretching bands of O-H and aliphatic C-H; the peaks at a range of 1471 to 1662 cm−1 for C = C and C = O stretching; and peak at 1096 cm−1 for C‐O stretching band.
3.1.4. XRD analysis
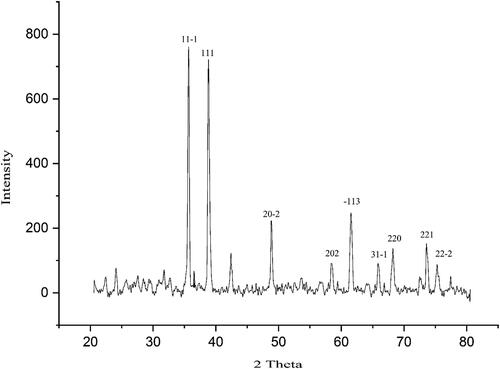
shows the XRD diagram of CuNPs@C. officinalis. The signals at various 2θ values that are related to the specific plans confirm the synthesis of cooper nanoparticles. For example, the peaks at 2θ values of 35.41, 38.64, 48.76, 58.23, 61.59, 66.16, 65.83, 73.42, and 75.13 are indexed as (11-1), (111), (20-2), (202), (-113), (31-1), (220), (221), and (22-2) planes. These peaks are matched as well as to those of standard database ICDD PDF card no. 96-901-6327. A 33.87 nm was calculated for the average crystal size of CuNPs@C. officinalis, that was obtained using Scherrer equation.
3.2. Analysis of the antioxidant properties of CuNPs@C. officinalis
Free radicals are molecules with a free electron ready to react, and oxygen is produced with some molecules. If many of them are suddenly produced in the body, they react with some parts of the cell, such as DNA and cell membranes, and cause cell damage or even death. Normally, the body's defence system neutralizes these harmless free radicals [Citation34–36]. Antioxidants prevent the spread of oxidation chain reactions. Thus, the strength of an antioxidant formed by the contact of an H atom with a free radical is due to the effect of an antioxidant on the ease with which this H atom separates from it [Citation35–38]. Thus, antioxidants can protect cell membranes and various living compounds against oxidants in small amounts. Numerous biochemical and physiological processes may cause the production of free radicals. Reactive oxygen species (ROS) include free radicals and radical-free forms [Citation33–39]. Free radicals include hydrogen peroxide (H2O2), hydroxyl radical (.OH), and superoxide anion radical (O2). When the concentration of ROS increases, it can oxidize macromolecules such as proteins, nucleic acids, and membrane lipids, resulting in cell damage and possibly ‘cell and tissue destruction’. Natural compounds and molecules have two main mechanisms for reducing the concentration of ROS, in other words, natural compounds and molecules reduce the concentration of ROS by producing antioxidants and thus prevent cell damage [Citation37–40]. Recently, many researchers have paid close attention to natural compounds and molecules and their relationship to their antioxidant properties, and many natural compounds and molecules have been studied for their antioxidant activity [Citation36–40].
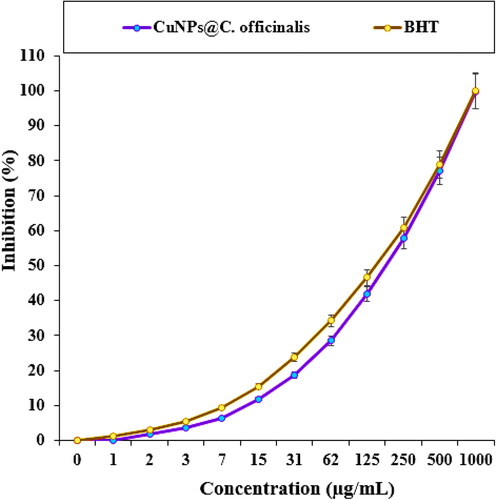
In the recent study, the scavenging capacity of CuNPs@C. officinalis and BHT at different concentrations expressed as percentage inhibition has been indicated in and . In the antioxidant test, the IC50 of CuNPs@C. officinalis and BHT against DPPH free radicals were 193 and 154 µg/mL, respectively ().
Table 1. The IC50 of CuNPs@C. officinalis and BHT in antioxidant test.
3.3. Analysis of the anti-lung adenocarcinoma properties of CuNPs@C. officinalis
The unique physical and chemical properties of copper nanoparticles have attracted the attention of the scientific community due to their high plasmonic properties, heat transfer, chemical stability and antibacterial effects. Using copper is nothing new; it dates back to Hippocrates, who used it as an antibacterial to control wounds [Citation40–42]. Today, copper nanoparticles are used in many commercial products, including soap, food, plastics, catheters, textiles, and bandages. However, their mechanism of action is still unknown. Many factors (shape, size, surface chemistry, morphology, density, charge, and purity) affect the biological activity of copper nanoparticles [Citation43–47]. Another important feature of copper nanoparticles is their role in treating cancer. Cu nanoparticles are a promising tool as an anti-cancer factor in evaluation and diagnosis [Citation48–50]. They have so benefits with strong properties against various cancer cell lines. Their better ability and penetration to detect Cu nanoparticles in the body make them a more effective tool in treating low-risk cancers compared to standard treatments [Citation44–47]. The unique effects of Cu nanoparticles, such as their high surface-to-volume ratio, easy synthesis, and optical properties, make them suitable for treating cancer [Citation49–53]. Cu nanoparticles can also be conjugated to several molecules, including RNA and DNA, to target several cells and polymers or antibodies [Citation45–49]. These important factors are important for increasing the half-life for circulating in vivo, which is very important in drug and gene delivery applications [Citation51–55].
In this investigation, the treated cells with different concentrations of the present CuNPs@C. officinalis were assessed by MTT assay for 48 h about the cytotoxicity properties on normal (HUVEC) and lung malignancy cell lines, i.e. lung moderately differentiated adenocarcinoma (LC-2/ad), lung poorly differentiated adenocarcinoma (PC-14), and lung well-differentiated bronchogenic adenocarcinoma (HLC-1) ().
Figure 6. The cytotoxicity and anti-lung adenocarcinoma properties of CuNPs@C. officinalis against HLC-1 (a), LC-2/ad (b), PC-14 (c), and HUVEC (d) cell lines.
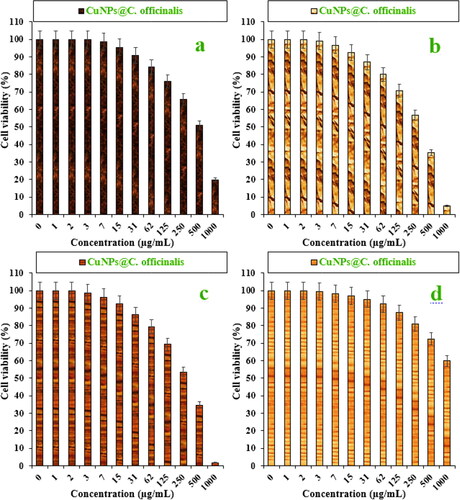
The viability of malignant lung cell line reduced dose-dependently in the presence of CuNPs@C. officinalis. The IC50s of CuNPs@C. officinalis were 297, 328, and 514 µg/mL against PC-14, LC-2/ad, and HLC-1 cell lines, respectively ().
Table 2. The IC50 of CuNPs@C. officinalis in the anti-lung adenocarcinoma test.
The absorbance rate was evaluated at 570 nm, which represented viability on normal cell line (HUVEC) even up to 1000 μg/mL for CuNPs@C. officinalis ( and ).
4. Conclusion
In the present study, the cooper nanoparticles were green synthesized using the aqueous extract of C. officinalis. The synthesized NPs were characterized using chemical techniques such as FT-IR, FE-SEM, XRD, and EDS. The results revealed a spherical morphology for CuNPs@C. officinalis in the range size of 19.64 to 39.15 nm.
The viability of malignant lung cell line reduced dose-dependently in the presence of CuNPs@C. officinalis. The IC50s of CuNPs@C. officinalis were 297, 328, and 514 µg/mL against PC-14, LC-2/ad, and HLC-1 cell lines, respectively. The CuNPs@C. officinalis showed the best antioxidant activities against DPPH. The IC50 of CuNPs@C. officinalis and BHT against DPPH free radicals were 193 and 154 µg/mL, respectively. After clinical study, CuNPs@C. officinalis can be utilized as an efficient drug in the treatment of lung adenocarcinoma in humans.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Notes on contributors
Jianfa Gu
References
- Arunachalam KD, Annamalai SK, Hari S. One-step green synthesis and characterization of leaf extract-mediated biocompatible silver and gold nanoparticles from Memecylon umbellatum. Int J Nanomed. 2003;8:1307–1315.
- You C, Han C, Wang X, et al. The progress of silver nanoparticles in the antibacterial mechanism, clinical application and cytotoxicity. Mol Biol Rep. 2012;39(9):9193–9201..
- Mao B-H, Tsai J-C, Chen C-W, et al. Mechanisms of silver nanoparticle-induced toxicity and important role of autophagy. Nanotoxicol. 2016;10(8):1021–1040.
- LaVan DA, McGuire T, Langer R. Small-scale systems for in vivo drug delivery. Nat Biotechnol. 2003;21(10):1184–1191.
- Shi J, Xiao Z, Kamaly N, et al. Self-assembled targeted nanoparticles: evolution of technologies and bench to bedside translation. Acc Chem Res. 2011;44(10):1123–1134.
- Langer R. New methods of drug delivery. Science. 1990;249(4976):1527–1533.
- Staroverov SA, Aksinenko NM, Gabalov KP, et al. Effect of gold nanoparticles on the respiratory activity of peritoneal macrophages. Gold Bull. 2009;42(2):153–156.
- Bastus NG, Sanchez-Tillo E, Pujals S, et al. Peptides conjugated to gold nanoparticles induce macrophage activation. Mol Immunol. 2009;46(4):743–748.
- Bao G, Mitragotri S, Tong S. Multifunctional nanoparticles for drug delivery and molecular imaging. Ann Rev Biomed Eng. 2013;15(1):253282.
- Celardo I, Pedersen JZ, Traversa E, et al. Pharmacological potential of cerium oxide nanoparticles. Nanoscale. 2011;3(4):1411–1420.
- De Jong WH, Borm PJ. Drug delivery and nanoparticles:applications and hazards. Int J Nanomed. 2008;3(2):133–149.
- Borm PJ, Robbins D, Haubold S, et al. The potential risks of nanomaterials: a review carried out for ECETOC. Part Fibre Toxicol. 2006;3(1):11.
- Stapleton PA, Nurkiewicz TR. Vascular distribution of nanomaterials. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2014;6(4):338–348.
- Patra JK, Das G, Fraceto LF, et al. Nano based drug delivery systems: Recent developments and future prospects. J Nanobiotechnology. 2018;16(1):71.
- Itani R, Al, Faraj SA. siRNA conjugated Nanoparticles-A next generation strategy to treat lung cancer. IJMS. 2019;20(23):6088.
- Trojer MA, Li Y, Wallin M, et al. Charged microcapsules for controlled release of hydrophobic actives Part II: surface modification by Lbl adsorption and lipid bilayer formation on properly anchored dispersant layers. J Colloid Interface Sci. 2013;409:8–17.
- Liu D, Chen L, Jiang S, et al. Formulation and characterization of hydrophilic drug diclofenac sodium-loaded solid lipid nanoparticles based on phospholipid complexes technology. J Liposome Res. 2014;24(1):17–26.
- AshwlayanVD KA, Verma M. Therapeutic potential of Calendula officinalis. Pharm Pharmacol Int J. 2018;6:149–155.
- Cruceriu D, Balacescu O, Rakosy E. Calendula officinalis: potential roles in cancer treatment and palliative care. Integr Cancer Ther. 2018;17(4):1068–1078.
- Shahen MZ, Mahmud S, Rony M, et al. Effect of antibiotic susceptibility and inhibitory activity for the control of growth and survival of microorganisms of extracts of Calendula officinalis. EJMHS. 2019;1(3):1–9.
- Verma PK, Raina R, Agarwal S, et al. Phytochemical ingredients and pharmacological potential of Calendula officinalis Linn. PBR. 2018;4:1–17.
- Kumar PV, Shameem U, Kollu P, et al. Green synthesis of copper oxide nanoparticles using Aloe vera leaf extract and its antibacterial activity against fish bacterial pathogens. BioNanoSci. 2015;5(3):135–139.
- Lu Y, Wan X, Li L, et al. Synthesis of a reusable composite of graphene and silver nanoparticles for catalytic reduction of 4- nitrophenol and performance as anti-colorectal carcinoma. J Mater Res Technol. 2021;12:1832–1843.
- Devi TB, Ahmaruzzaman M. Facile preparation of copper nanaoparticles using Coccinia grandis fruit extract and its application towards the reduction of toxic nitro compound. Mater Today: Proc. 2018;5(1):2098–2104.
- Taghavi Fardood S, Ramazani A. Green synthesis and characterization of copper oxide nanoparticles using coffee powder extract. J Nanostruct. 2016;6:167–171.
- Rao MD, Pennathur G. Green synthesis and characterization of cadmium sulphide nanoparticles from Chlamydomonas reinhardtii and their application as photocatalysts. Mater Res Bullet. 2017;85:64–73.
- Baghayeri M, Mahdavi B, Hosseinpor‐Mohsen Abadi Z, et al. Green synthesis of silver nanoparticles using water extract of Salvia leriifolia: Antibacterial studies and applications as catalysts in the electrochemical detection of nitrite. Appl Organometal Chem. 2018;32(2):e4057.
- Seydi N, Mahdavi B, Paydarfard S, et al. Preparation, characterization, and assessment of cytotoxicity, antioxidant, antibacterial, antifungal, and cutaneous wound healing properties of titanium nanoparticles using aqueous extract of Ziziphora clinopodioides Lam leaves. Appl Organometal Chem. 2019;:e5009. DOI
- Vaidehi D, Bhuvaneshwari V, Bharathi D, et al. Antibacterial and photocatalytic activity of copper oxide nanoparticles synthesized using Solanum lycopersicum leaf extract. Mater Res Express. 2018;5(8):085403.
- Khani R, Roostaei B, Bagherzade G, et al. Green synthesis of copper nanoparticles by fruit extract of Ziziphus spina-christi (L.) Willd.: application for adsorption of triphenylmethane dye and antibacterial assay. J Mol Liquid. 2018;255:541–549.
- Sulaiman GM, Tawfeeq AT, Jaaffer MD. Biogenic synthesis of copper oxide nanoparticles using olea europaea leaf extract and evaluation of their toxicity activities: an in vivo and in vitro study. Biotechnol Prog. 2018;34(1):218–230.
- Kaur P, Thakur R, Chaudhury A. Biogenesis of copper nanoparticles using peel extract of Punica granatum and their antimicrobial activity against opportunistic pathogens. Green Chem Lett Rev. 2016;9(1):33–38.
- Cheirmadurai K, Biswas S, Murali R, et al. Green synthesis of copper nanoparticles and conducting nanobiocomposites using plant and animal sources. RSC Adv. 2014;4(37):19507–19511.
- Davis ME, Chen Z, Shin DM. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat Rev Drug Discov. 2008;7(9):771–782.
- Namvar F, Rahman HS, Mohamad R, et al. Cytotoxic effect of magnetic iron oxide nanoparticles synthesized via seaweed aqueous extract. Int J Nanomed. 2014;9:2479–2488.
- Sankar R, Maheswari R, Karthik S, et al. Anticancer activity of Ficus religiosa engineered copper oxide nanoparticles. Mater Sci Eng C Mater Biol Appl. 2014;44:234–239.
- Katata-Seru L, Moremedi T, Aremu OS, et al. Green synthesis of iron nanoparticles using Moringa oleifera extracts and their applications: Removal of nitrate from water and antibacterial activity against Escherichia coli. J Mol Liq. 2018;256:296–304.
- Sangami S, Manu M. Synthesis of green iron nanoparticles using laterite and their application as a fenton-like catalyst for the degradation of herbicide Ametryn in water. Environ Technol Innov. 2017;8:150–163.
- Beheshtkhoo N, Kouhbanani MAJ, Savardashtaki A, et al. Green synthesis of iron oxide nanoparticles by aqueous leaf extract of Daphne mezereum as a novel dye removing material. Appl Phys A. 2018;124:363–369.
- Radini IA, Hasan N, Malik MA, et al. Biosynthesis of iron nanoparticles using Trigonella foenum-graecum seed extract for photocatalytic methyl orange dye degradation and antibacterial applications. J Photochem Photobiol B. 2018;183:154–163.
- Beyene HD, Werkneh AA, Bezabh HK, et al. Synthesis paradigm and applications of silver nanoparticles (Ag NPs), a review. Sustain Mater Technol. 2017;13:18–23.
- Chen X, Schluesener HJ. Nanosilver: a nanoproduct in medical application. Toxicol Lett. 2008;176(1):1–12.
- Alexander JW. History of the medical use of silver. Surg Infect (Larchmt). 2009;10(3):289–292.
- Bhattacharya S, Zhang Q, Carmichael PL, et al. Toxicity testing in the 21 century: Defining new risk assessment approaches based on perturbation of intracellular toxicity pathways. PLoS One. 2011;6(6):e20887.
- Jo DH, Kim JH, Lee TG, et al. Size, surface charge, and shape determine therapeutic effects of nanoparticles on brain and retinal diseases. Nanomed Nanotechnol Biol Med. 2015;11(7):1603–1611.
- Rai M, Kon K, Ingle A, et al. Broad-spectrum bioactivities of silver nanoparticles: the emerging trends and future prospects. Appl Microbiol Biotechnol. 2014;98(5):1951–1961.
- Riehemann K, Schneider SW, Luger TA, et al. Nanomedicine-challenge and perspectives. Angew Chem Int Ed Engl. 2009;48(5):872–897.
- Huang Y, Fan CQ, Dong H, et al. Current applications and future prospects of nanomaterials in tumor therapy. Int J Nanomedicine. 2017;12:1815–1825.
- Conde J, Doria G, Baptista P. Noble metal nanoparticles applications in cancer. J Drug Deliv. 2012;2012:751075.
- Bhattacharyya S, Kudgus RA, Bhattacharya R, et al. Inorganic nanoparticles in cancer therapy. Pharm Res. 2011;28(2):237–259.
- Sau TK, Rogach AL, Jackel F, et al. Properties and applications of colloidal nonspherical noble metal nanoparticles. Adv Mater. 2010;22(16):1805–1825.
- Schroeder A, Heller DA, Winslow MM, et al. Treating metastatic cancer with nanotechnology. Nat Rev Cancer. 2011;12(1):39–50.
- Gobbo L, Sjaastad K, Radomski MW, et al. Magnetic nanoparticles in cancer theranostics. Theranostics. 2015;5(11):1249–1263.
- Will O, Purkayastha S, Chan C, et al. Diagnostic precision of nanoparticle-enhanced MRI for lymph-node metastases: a meta-analysis. Lancet Oncol. 2006;7(1):52–60.
- Harada T, Tanigawa N, Matsuki M, et al. Evaluation of lymph node metastases of breast cancer using ultrasmall superparamagnetic iron oxide-enhanced magnetic resonance imaging. Eur J Radiol. 2007;63(3):401–407.