?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Purpose: The aim of this study was to assess the antibacterial potential and ex vivo skin permeation kinetics of cefixime from bionanocomposite films. Methods: The films were prepared by solvent casting method by using chitosan and starch. The fabricated films were tested for their antibacterial potential against three bacteria i.e. Escherichia coli, Klebsiella pneumonia, and Acetobacter aceti. In vitro permeation studies of cefixime from the films across rat skin was conducted using Franz diffusion cell. Results: The highest antibacterial effect was exhibited by F5 formulation (non-irradiated film) against Escherichia coli and Klebsiella pneumonia; however, antibacterial activity of the films was significantly (p < 0.05) reduced after their irradiation. F5 formulation showed the highest cumulative amount of permeated drug after 24 h, while F1 (100% chitosan) showed the lowest amount of permeated drug. Non-Fickian diffusion (anomalous) was the main mode of drug release from all films. The cross-linking of films by γ-radiations improved their mechanical properties. The percentage swelling ratio was the highest in non-irradiated films having a polymeric blend (50:50). Water uptake of irradiated films was appreciably reduced as compared to non-irradiated films. Conclusion: The synthesized bionanocomposites are promising therapeutic moieties which not only improve drug permeability across but also ameliorates antibacterial potential of cefixime.
1. Introduction
In the recent years, much advancement has been made in the field of drug development to minimize the degradation of drug and loss, to overcome the life-threatening adverse effects and to enhance drug bioavailability and fraction of drug accumulated in the requisite zone. In order to achieve this many new drug delivery systems have been introduced, these systems can hamper solubility problems, defend the drug from photodegradation and pH changes, also reduces the dumping of dose by controlling the release profile [Citation1]. These new drug delivery systems include liposomes, niosomes, microsponges, microemulsion, nanoemulsion, nanohydrogels, dendrimers, composites, and nanocomposites. Composite is formed by combining two or more materials having different properties. Two materials when combined give rise to totally unique properties without dissolving into one another. A new category of composite materials is nanocomposites. The bionanocomposites exist as composite materials which consist of component(s) of the biological source in addition to particles with a minimum one dimension in the array of 1–100 nm [Citation2]. Bionanocomposites are synthesized by incorporation of small amounts (less than 10% wt) of nano-sized fillers into a polymer matrix, so they are novel materials with considerably enhanced properties. Bionanocomposites are incredibly promising substances as they have shown improved properties such as preservation of the enclosed material, biodegradability and they are without eco-toxicity [Citation3]. Such dosage forms give improved properties by enhancing the efficiency and duration of the drug, with retention of biodegradability, suitable routes of administration, improved site-specific delivery, reduced adverse effects and without eco-toxicity [Citation4].
Bionanocomposites can also be produced by different processes, from polysaccharides of different natural and renewable sources including starch, cellulose, chitosan, and chitin [Citation5,Citation6]. Solution casting is a versatile and easy way to formulate thin nanocomposite sheets/films on a laboratory scale. In this solution casting, the polymer phase is dissolved in an aqueous or non-aqueous volatile solvent and then mixed with nanometer-sized reinforcement in the same solvent medium prior to casting on a flat surface. The solvent phase is evaporated and dried film is released from the substrate. It is typically a low-temperature procedure and provides films with uniform thickness [Citation7].
Chitosan is a polysaccharide which is obtained by alkaline deacetylation of chitin, obtained from the exoskeleton of crustaceans, has come out as a valuable drug delivery matrix due to its polycationic nature, biocompatibility, much-adhesiveness, and biodegradability. It is made up of β (1→4) linked N-acetyl glucosamine (2-acetamide-2-deoxy-β-d-glucose) with varying degrees of N-acetylation of glucosamine residues [Citation8,Citation9]. Chitin is subjected to alkaline N-deacetylation using concentrated sodium hydroxide (NaOH) solution at high temperature for a long period of time to prepare chitosan [Citation10]. It has a tendency to stick with mucosa giving increased contact time for drug penetration through the skin [Citation11], and thus is widely employed as a drug carrier. Another crucial property of chitosan is its intrinsic antibacterial activity [Citation8], due to its higher affinity for negatively charged membrane and binding specificity with its target site [Citation11]. Previous studies have revealed that combing of chitosan with other polymers (such as alginate and starch) improve its characteristics, including loading efficiency of drug and mechanical property of polymer [Citation9]. Nanocomposites of chitosan with starch are a potential example of polymeric blends that is a very versatile drug delivery tool [Citation12].
Starch is a natural polymer composed of glucose monomers linked with glycosidic bonds, has immense biocompatibility and largely used as essential excipient in pharmaceutical preparations. Starch films developed with biodegradable plasticizers have great potential in controlled drug delivery for wound healing, facial plastic surgery, and treatment of colon [Citation12]. Starch is made of helical and linear amylase units and branched amylopectin units. Starch-based substances are hydrophilic and their mechanical attributes decrease with water intake [Citation13,Citation14]. So, starch films are fragile, absorb humidity and difficult for processing. To overcome these limitations, starch is blended and mixed with numerous natural and synthetic polymers like chitosan, but again the major problem of the starch-chitosan film is its delicacy and hydrophilicity. The delicacy of the films can be minimized by adding plasticizers so that flexibility can be reduced. Glycerol is used as a plasticizer to get elastic and flexible chitosan-starch films [Citation15]. While knowing the advantages and sole properties of starch and chitosan, their blend is used to form a biodegradable film which shows enhanced strength and better flexibility, low water permeability and better antibacterial activity as compared to starch films without chitosan [Citation16].
Cefixime trihydrate is an oral antimicrobial agent of third-generation cephalosporin with bactericidal activity against a broad variety of Gram-negative and Gram-positive bacteria. It is used in the treatment of respiratory tract infections, urinary tract infections, gastrointestinal infections, and typhoid fever. Oral Dosage forms available are tablets, capsules and dry suspension. It is readily absorbed from the enteric tract. The absolute bioavailability of cefixime is in the range of 22% to 54% due to first-pass metabolism [Citation17]. Whereas mean elimination half-life generally lies within the range of 2.5 to 3.8 h. Frequent dosing of cefixime leads to an increased burden on metabolism and may cause gastric problems. Keeping in view all this, a transdermal film loaded with cefixime was formulated to carry the drug to the systemic circulation and to avoid dose-related side effects [Citation11].
Now a day, an advance process of radiation crosslinking of polymers has gained very much commercial interest. Irradiation of the nanocomposite films produces various chemical changes such as chain scission, decomposition, and unsaturation etc. These chemical changes are of great importance as they can cause changes to thermo-mechanical properties of the final products [Citation18]. Use of gamma radiations induced processes has various advantages, i.e. minimum time requirement, nonstop operation, less pollution and remedial at ambient temperatures. Moreover, no catalyst or additives are required to start the reaction, as energy in the form of radiations is absorbed by the polymer backbone and it works to initiate the free radical process [Citation19]. Sepiolite belongs to group of fibrous hydrated magnesium silicate with notational half unit cell formula Si12O30Mg8(OH)4(OH2)4.8H2O (consisting of two tetrahedral silica sheets encircling a central sheet of octahedral magnesia) but the layers lack continuous octahedral sheets which seem similar to 2:1 layered arrangement of montmorillonite [Citation20]. Sepiolite is polar in nature and acts as a better choice for nanocomposites [Citation13].
Keeping in view all of the above revision, in this study the aim and objective were to develop a controlled release nanofilm containing a blend of chitosan and starch loaded with cefixime by using a solvent casting method. These nanocomposite films were then exposed to irradiation of different intensities. Another main goal was to study the effect of radiation dose on the structural organization and mechanical properties of chitosan-starch composite films. Pharmaceutical characterization and Ex-vivo studies including drug content, solubility studies, swelling behavior, erosion studies, permeability studies, and dissolution studies were also performed. Dissolution data were evaluated using DDSolver, recent software designed for kinetic analysis. DDSolver is basically a menu-driven, adds-in program for Microsoft Excel. Antimicrobial activity of these bionanocomposites was also checked against three different strains of bacteria including Escherichia coli, Klebsiella pneumonia, and Acetobacter aceti. Moreover, physical/chemical characterization including structural morphology by using Scanning electron microscopy (SEM), Fourier transformed infrared (FTIR) spectroscopy, thermogravimetric analysis (TGA), energy dispersive x-ray (EDX) analysis were also performed.
2. Methods
2.1. Reagents and chemicals
Chitosan (low molecular weight) was purchased from Int lab, USA. Starch was purchased from Daejung Kosdaq, Korea, having a molecular weight (C6H10O5)n and melting point 256 ̴ 258 °C. Cefixime was gifted by Weatherfolds Pharma, Hattar, Pakistan. Its pka value is 2–2.5 and molecular weight is 453.40 and formula = C16H15N5O7S8.3H2O. Solvents like Glacial acetic acid and Glycerol were purchased from Sigma Aldrich, USA. Glacial acetic acid ≥99.85% having freezing point 16.35 °C was colorless and clear. Glycerol having mol wt. 92.09, density is 1.26 g/ml and melting point is 17.8 °C. Potassium chloride and Sodium hydroxide with the purity of 99% was purchased from Sigma Aldrich, USA. Sodium acetate was purchased from Merck, Germany. Potassium dihydrogen phosphate was purchased from Daejung, Korea. Distilled water was also used as a solvent. Sepiolite was purchased from Int lab, USA.
2.2. Preparation of chitosan-starch films
Starch-chitosan blend bionanocomposites films were prepared by a solvent casting method. Experimentally, starch was dissolved in hot water. A solution of chitosan was obtained by mixing chitosan in 1% aqueous acetic acid solution. After that, 50 ml of aqueous starch solution was added to it with continuous stirring. The chitosan-starch composite was synthesized by adding chitosan and starch solutions in various compositions (both ranging from 0–100%, w/w) followed by the addition of a fixed amount of nanofiller (sepiolite) at room temperature under continuous stirring. After 15 min, cefixime was added to the above mixture. To increase the properties of chitosan-starch films, glycerol was added as plasticizer while stirring the solution. Stirring remained continued for three hours to fully gelatinize the starch. The temperature of the blend was slowly elevated to 60 °C in order to obtain a clear and homogenous solution. Then the mixture was kept away from heat, the foam was disappeared and then cast into Petri-plates. The mixture placed in an oven at 40 °C for about 24 h in order to complete the drying process. After drying, hard smooth plastic type films were obtained. Films were stored in polyethylene bags before use in further studies [Citation21]. Thirty formulations were prepared by changing the quantities of polymers in accordance with .
Table 1. Formulation development design.
2.3. Irradiation of films
Twenty formulations (from F11-F30) were chosen for irradiation and kept in polyethylene bags. Radiation of composite films was carried out with the help of γ-rays produced from a Cobalt-60 source at room temperature, with a dose rate of 40 kGy/hour and 80 kGy/hour in NIFA (Nuclear Institute for Food and Agriculture, Ternab Peshawar, Khyber Pakhtunkhwa, Pakistan) [Citation19].
2.4. Characterization of formulations
The non-radiated, as well as radiation, treated cefixime loaded chitosan/starch bionanocomposite films were subjected to various tests for evaluation of drug-polymer interaction.
2.4.1. Fourier transformed infrared (FTIR) spectroscopy
The Fourier transform infrared (FTIR) spectroscopy was studied to detect the possibility of intermolecular bonding or complexation between cefixime and chitosan/starch blend. The spectrum was obtained by (6700, Thermo Scientific, Nicolet). Samples used for FTIR study were F3, F8, F13, F18, F23, and F28. All these samples were grounded and mixed with potassium bromide thoroughly. The spectrum considered was in the range of wave number 4000–500 cm−1 [Citation22].
2.4.2. Thermogravimetric analysis (TGA)
The thermogravimetric analysis was used to evaluate the decomposing kinetics of cefixime loaded nanocomposite films. Thermal degradation pattern of F3, F8, F13, F18, F23, and F28 was studied by (DTG-60H, Shimadzu, JAPAN). The weight of the sample used was 5 mg and scan temperature ranges from room temperature to 600 °C at a rate of 10 °C/min under nitrogen atmosphere [Citation21].
2.4.3. Scanning electron microscopy (SEM)
The surface morphology of prepared films F8 and F23 was investigated by scanning electron microscope (JSM 6400 F SEM, JEOL, Tokyo, Japan). The film samples were deposited on the aluminum holder with the help of sticky carbon tape and a thin layer of gold was coated on it by a gold stammer. The voltage of 12 kV was used [Citation16].
2.4.4. Energy dispersive x-ray (EDX) analysis
EDX analysis was also performed through a Scanning Electron Microscope (JSM 6400 F SEM, JEOL, Tokyo, Japan). The film samples were deposited on the aluminum holder with the help of sticky carbon tape and a thin layer of gold was coated on it by a gold stammer. The voltage of 20.194 kV was used for EDX analysis. The EDX spectrum of F28 was measured in the spot profile mode by focusing the electron beam onto specific regions of the sheet.
2.5. Preliminary solubility studies of cefixime
The solubility of cefixime in different solvents was carried out at 37 °C. Sufficient amount of cefixime was added to twist capped 30 ml glass vials consist of different solvents. The vials were then mechanically shaken in a shaker and permitted to equilibrate for subsequent 24 h, and centrifuged (Heraeus megafugr 8 R, Thermo Fisher Scientific, lab Centrifuge, Germany) at 5000 rpm for about 10 min, in order to remove any residues. The supernatant of all vials was filtered through filter paper and the specific solvent was used for dilution. The concentration of cefixime was determined spectrophotometrically (O.R.I 3000, UV-VIS spectrophotometer, Germany) at 288 nm using a specific solvent as a blank [Citation23].
2.6. Calibration curve of cefixime in phosphate buffer of pH 7.4
Calibration curve of cefixime was prepared in 0.05 M phosphate buffer pH 7.4. The stock solution was obtained by adding 100 mg of cefixime in 100 ml of phosphate buffer 7.4 to obtain a solution of concentration 1 mg/ml. From the above solution, sufficient aliquots were separated and diluted with the phosphate buffer pH 7.4 to get final concentration ranging from 1–10 µg/ml. All concentrations were scanned through UV-spectrophotometer (O.R.I 3000, UV-VIS Spectrophotometer, Germany) and absorbance was obtained at a wavelength of 288 nm against a blank of phosphate buffer of pH 7.4 [Citation24].
2.7. Drug content uniformity/drug loading efficiency
The desired quantity of film taken from center and proximity was cut into small pieces and put into 100 ml volumetric flask and volume was adjusted up to the mark by using phosphate buffer of pH 7.4 and kept for 24 h. The solution was stirred for the whole night by using magnetic stirrer, so that total release of drug from the films was obtained. Aliquots of the solution were removed and filtered using Whatman-41 filter paper and further diluted up to 50 µg/ml. The cefixime content was estimated by measuring the absorbance at 288 nm by UV-visible spectrophotometer (O.R.I 3000, UV-VIS Spectrophotometer, Germany), with the help of phosphate buffer of pH 7.4 as a blank [Citation25].
2.8. Swelling studies of films
Swelling or water uptake studies were done by the equilibrium weight method. The chitosan and starch γ-irradiated as well as non-irradiated bionanocomposite films containing a varying concentration of polymers, were accurately weighed (W0), and immersed in beakers and left to swell. Each time fresh reweighed sample of films were placed in beakers containing different buffer medias of simulated gastric fluid (pH 1.2), or simulated intestinal fluid (acetate buffer pH 4.5, phosphate buffer pH 6.8), respectively at 37 ± 0.5 °C. After regular time intervals of 15, 30, 60, 90, 120 and 150 min, each film was removed from the beakers and the excess water was removed by blotting with tissue paper, and then each film was reweighed (W1), by using analytical balance (model PA214, Ohaus Corporation, USA), and then kept again in the same medium. The percentage rise in mass because of absorbed fluid or water uptake was assessed every time from the following equation. Each time experiment was conducted in triplicate and for each specific time, new samples were used. The equilibrium swelling ratio was determined by the below-mentioned equation [Citation26].
(1)
(1)
The percentage gain in weight or water content was determined each time by the following equation [Citation27].
(2)
(2)
where Wh = mass of swollen film at time t, and Wd = the primary mass of dry film.
2.9. Erosion studies of films
Film erosion studies were done by equilibrium or gravimetric technique. After performing water uptake studies, the wet films were kept in an oven at 40 °C for 12 h for drying and then cooled down in desiccators. Finally, the films were weighed at regular time interval until a stable weight was obtained (Wt(d)). For each time the experiment was conducted in triplicate [Citation28]. The film erosion at diverse times was calculated from the below-mentioned equation [Citation29].
(3)
(3)
where Wi = initial mass of the sample and Wt(d) = dry mass of sample collected at time t.
2.10. Ex vivo skin permeation studies
The experiments were performed with respect to the guidelines for animal use as mentioned by the Institutional Animal Ethical Committee.
2.10.1. Preparation of rat skin
Fifteen rats of Sprague-Dawle species having same age group of 6–9 weeks and average body weight of 150–200 g were taken from a central animal house located in COMSATS Institute of Information and Technology, Abbottabad and placed under customary laboratory conditions (12 h light/dark cycle) at 25 ± 2 °C [Citation30]. Animals were nutrified with standard animal diet ad-libitum. The animals were allowed free access to drinking water. The study was approved (FA14-R00-020/E.C/M5) by Departmental Ethical Committee, Department of Pharmacy, COMSAT Institute of Information and Technology Abbottabad. This study was conducted in accordance with Good Clinical Practice involving the use of animals in the experimental study [Citation31]. The abdominal skin of Spargue-Dawle rat was used. Hairs on the abdominal region were removed after sacrificing the rats by prolonged chloroform inhalation. Epidermis with stratum corneum and dermis were removed from the shaved abdominal region. The skin was splashed instantly with phosphate buffer saline (PBS), enfolded in aluminum foil and kept at −20 °C for further usage. On experiment day, the rat skin was thawed at room temperature and skin was attached over Franz diffusion cell in such arrangements that dermis side faced receiver compartment while stratum corneum side faced the donor compartment [Citation30].
2.10.2. Permeation study
In vitro permeation studies of cefixime from both γ-irradiated and non-irradiated chitosan-starch films employing rat, the skin was conducted by means of modified Franz diffusion cell with an effective area of 0.685 cm2 having 5 ml cell volume. Thermostat system was used to keep the water warm in order to provide the temperature of skin surface approximately 37 ± 0.5 °C. On top of the donor compartment, 30 mg of the film sample was placed and receptor compartment was packed with 20 ml of phosphate buffer pH 7.4. Receptor fluid was spun in a sonicator to remove the dissolved gases before use. This whole setup was placed on a magnetic stirrer at 600 rpm (Model LMS-1003, Daihan Labtech Company Ltd. Korea) and phosphate buffer was thoroughly stirred in receiver compartment during the whole experiment with the help of magnetic bead. The experiment was conducted for 24 h in case of non-irradiated films, and for 40 h in case of γ-irradiated films. From the receptor compartment, an aliquot of 0.5 ml was taken out and each time substituted with an equal volume of fresh buffer to maintain the sink conditions. The amount of cefixime in the samples was analyzed spectrophotometrically (O.R.I 3000, UV-VIS spectrophotometer, Germany) at 288 nm using phosphate buffer pH 7.4 as a blank [Citation32]. The drug permeated per square centimeter for each time interval was determined and plotted in contradiction of time [Citation33].
2.10.3. Permeation kinetic analysis
The collective quantity of drug permeated was plotted as a function of time through rat skin and flux. The amount of drug [M] which passes through a unit cross-sectional barrier area [S] and appears in receiver solution in time ‘t’ is known as flux ‘J’. Flux calculated from Fick’s law of diffusion by assuming that transport through skin follows the diffusion phenomenon [Citation34].
(4)
(4)
Also,
(5)
(5)
In the above equation, J denotes flux, K denotes partition coefficient, Co represents a concentration of drug that remains persistent in the vehicle, D is diffusion coefficient or diffusivity and h denotes distance of movement perpendicular to the surface of the barrier.
The steady state flux (Jss) was obtained from the slope of the linear plot (in steady state region where drug passed in constant rate) of the collective quantity permeated through unit area against time plot.
(6)
(6)
where Co represents drug amount that remains persistent in the vehicle and ‘P’ represents the permeability coefficient. Permeability coefficient is the velocity of drug passage through the membrane in µg/cm2/h.
(7)
(7)
where D is the diffusion coefficient of the drug in the barrier, h shows the diffusional path length or the film thickness, K is the partition coefficient of a drug between the film and the receptor medium [Citation35].
2.11. Dissolution studies
The dissolution test on both the γ-irradiated and non-irradiated films was performed using USP Apparatus II (Galvano Scientific dissolution tester, China). Films exactly weighing 50 mg were suspended in a different buffer solution of pH 1.2, 4.5 and 6.8. To copy the pH changes besides the GI tract, three buffer mediums 1.2, 4.5 and 6.8 were consecutively applied, known to as sequential pH change method. Buffer medium of pH 1.2 (simulated gastric fluid) was obtained by adding 426 ml of HCl and 147 ml of KCl. Acetate buffer having pH 4.5 was prepared by mixing 2.99 g of sodium acetate with 14 ml of 2 N acetic acid solution. The volume of dissolution medium was set at 900 ml, and speed of paddle rotation was 50 rpm, the temperature was adjusted at 37 ± 0.5 °C and sink conditions were preserved throughout the experiment. During dissolution experiments, for the first 2 h, pH 1.2 medium was used. After its removal, the fresh medium of pH 4.5 was added for one an hour. After an hour, it was washed away, and replaced with the fresh phosphate buffer pH 6.8. Films were tied to paddle with the help of thread to prevent floating in each dissolution vessel. 5 ml of dissolution solutions were taken away at predetermined intervals (0, 0.5, 1, 1.5, 2, 2.5, 3, 4, 5, 6, 8, 10, 12 and 24 h) using a pipette and substituted with the equal volumes of fresh medium. The samples taken from the dissolution medium were analyzed at 288 nm in triplicate, by UV-visible spectrophotometer and percentage drug release was determined. The amount of release was estimated by the previously constructed calibration curve [Citation34].
2.11.1. Drug release kinetics
To estimate kinetics of drug release, data obtained from dissolution test was analyzed by using different model-dependent approaches like zero order, first order, Higuchi and Korsmeyer-Peppas as well as model-independent approach i.e. similarity factor (f2).
The zero-order model defines the system in which the drug release rate does not depend on the initial concentration of the drug.
Zero-order kinetics:
(8)
(8)
First-order kinetics:
(9)
(9)
Higuchi’s square root of time equation (diffusion model):
(10)
(10)
Korsmeyer–Peppas model:
(11)
(11)
where Wt is the amount of drug released at a time t, K is released constant, n is diffusional constant, C0 and Ct are the concentration of drug at an initial time and at time t, Mt/M∞ is the fractional drug release into dissolution medium. The drug release mechanism is characterized by the value of n [Citation11]. With n ≤ 0.5, (diffusion-controlled drug release) the diffusion of the drug from polymer matrix is Fickian diffusion- and quasi-Fickian diffusion transport respectively. n = 0.89 (swelling-controlled drug release) also called case-II transport [Citation11]. With 0.5<n < 1, the release behavior is expected to be a superposition of two mechanisms, that is called anomalous transport. Whereas n > 1 is called super-case II transport [Citation35].
The Similarity factor (f2) is defined as ‘logarithmic reciprocal square root transformation of one plus the mean squared (the average sum of squares) differences of drug percentage dissolved between the test and reference products [Citation36]. It is used to measure the proximity between the two formulations.
(12)
(12)
in which Rt and Tt are percentages of drug dissolved at every time point for reference and test products. An f2 value larger than 50 indicates significant similarity [Citation37].
2.12. In vitro antibacterial test
The antibacterial activity studies of non-irradiated and γ-irradiated films were detected by agar disc diffusion method. Three bacteria including Escherichia coli, Klebsiella pneumonia [Citation38] and Acetobacter aceti were exposed to these films. The bacteria were grown in sterile nutrient agar media (20 ml). Aliquot of 100 µl of every bacterial strain was mopped on Petri-plates having media. Sterile filter paper discs having impregnated with 5 μl of each nanocomposite film was placed on these plates. DMSO impregnated disc was used as negative control. Pure cefixime trihydrate served as positive control. These culture plates were then allowed to incubate at 37 °C for a time period of 24–48 h. The average diameter (mm) of the zone of inhibition of bacterial growth around the sample discs and control discs was measured and recorded. The zone of inhibition indicates the inhibitory activity of each sample and control compound on the growth of the Escherichia coli, Klebsiella pneumonia and Acetobacter aceti [Citation15].
3. Results and discussion
3.1. Characterization of formulation
In the present study non-irradiated and γ-irradiated nanocomposite films of cefixime trihydrate is prepared by a solvent casting method using chitosan and starch as polymer and sepiolite are used as nanofiller. To improve the properties of chitosan-starch films, glycerol is added as a plasticizer. The nanocomposite films have been found to have good film formation, smooth in appearance.
3.1.1. Fourier transform infrared (FTIR) spectroscopy
The Fourier transform infrared (FTIR) spectroscopy was studied to detect the possibility of intermolecular bonding or complexation between the mixing components. Samples used for FTIR study were F3, F8, F13, F18, F23, and F28. Each spectrum was scanned from 500–4000 cm−1 at the resolution of 6 cm−1 and an average of 132 scans was recorded. The typical spectra of the functional groups of chitosan, starch as well as cefixime were also seen in each spectrum of drug loaded films, , whereas the absorption peaks were given in . and show FTIR analysis of drug loaded and non-drug loaded formulations. The hydroxyl group appears in the range of 3267.5 cm−1 to 3285.32 cm−1. FTIR analysis explains the interaction between the polymers and the drug. The lower frequency shift of -OH group indicates strong hydrogen bonding and the compatibility of the mixing components. The maximum lower frequency shift was observed for F8 formulation whereas, maximum higher frequency shift was observed for F3 and F13 formulation, respectively. The stretching of carbonyl amide group of both the chitosan and cefixime is in range of 1630.66 to 1641.91 cm−1. Lower frequency shift was observed for F23 i.e. 1636.6 cm−1, which shows that 80 kGy irradiated films are more compatible as compared to others. It also shows that chitosan carbonyl amide is overlapped with carbonyl amide of cefixime. The drug-loaded formulations F3, F13 and F23 show a characteristic peak of N-O bond stretching of cefixime in range of 1536–1558 cm−1. The lower frequency shift of N-O bond for F13 as compared to F3 and F23 indicates the compatibility of 40 kGy irradiated films. The results show no interaction between polymers and drug [Citation22]. The sepiolite addition in the blend is indicated by Si-O-Si stretching frequency in the range of 980–1070 cm−1. The drug-loaded formulation overall shows more frequency shift towards lower values, that indicates the compatibility of mixing polymer and no interaction. Irradiated films are more stable as compared to non-irradiated films.
Figure 1. FTIR analysis of various formulations: (A) F3, (B) F13, (C) F23, (D) F8, (E) F18, (F) F28.
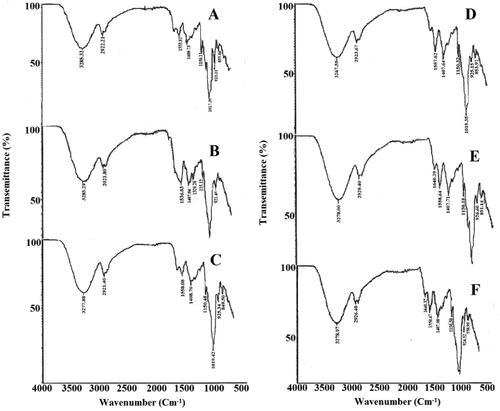
Table 2. FTIR absorption values of various formulations: (A) F3, (B) F13, (C) F23, (D) F8, (E) F18, (F) F28.
3.1.2. Thermogravimetric analysis (TGA)
The thermal stability of γ-irradiated and non-irradiated chitosan-starch polymeric bio-nanocomposite is shown in . Results showed that decomposition occurs in three steps.
Figure 2. TGA curves of non-drug and drug-loaded formulations of non-irradiated and γ-irradiated nanocomposite films.
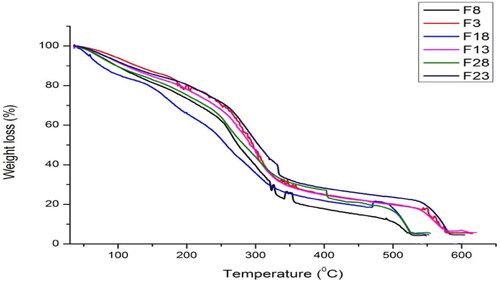
shows a weight loss of about 14–20% occurs at 150 °C which was the weight loss due to moisture evaporation. At the temperature of 250 °C, the second phase of weight loss is observed. The weight loss at this stage for F3 (non-irradiated) is more as compared to F23 which is a γ-irradiated film at a dose of 80 kGy. The weight of F8 non-drug samples is also more as compared to F28 (non-drug, γ-irradiated). At the temperature of 350 °C, the 80 kGy irradiated samples also showed maximum stability. The weight residue of γ-irradiated samples was higher which also showed maximum thermal stability for irradiated samples.
Table 3. Thermal studies of non-drug and drug-loaded formulations of non-irradiated and γ-irradiated bionanocomposite films at different temperatures.
Thermal stability of γ-irradiated and non-irradiated bionanocomposite showed that decomposition occurs in three steps. The first stage at 150 °C indicates the moisture contents. Sample F23 treated with 80 kGy radiation dose shows greater water with holding capacity and sample F18 shows least water with holding capacity at 150 °C. indicates that all drug loaded samples show maximum water with holding capacity as compared to non-drug loaded samples at 150 °C.
The second weight loss occurs at 250 °C. The maximum weight loss of 46.95% is observed for F18 formulation which is thermally least stable whereas, F23 treated with 80 kGy radiation dose shows maximum thermal stability at this stage as compared to all other formulations. The drug-loaded formulations indicate maximum thermal stability as compared to non-drug loaded samples at 250 °C. Cleavage of glycosidic bonds of chitosan and starch occurs at 350 °C. In this stage, the thermal stability of irradiated samples increases with increasing radiation dose. The maximum thermal stability was observed for F23 formulation. The increased thermal stability is attributed to the network formation at high radiation dose.
The weight residue is observed at 550 °C. The maximum weight residues are observed for irradiated drug loaded samples. TGA thermogram indicates that without drug films are thermally least stable as compared to drug-loaded formulations. It also indicates that the drug-loaded γ-irradiated films at 80 kGy are thermally more stable as compared to all other formulations as reported previously [Citation39].
3.1.3. Scanning electron microscopy (SEM)
The results of a scanning electron microscope of cefixime loaded 80 kGy gamma irradiated formulation F23 at 3 different magnification power as well as non-drug loaded F8 formulation at single magnification power respectively is shown in . From the scanning electron microscope results, no phase separation is found between starch and chitosan and it shows the compatibility of the mixing polymers. The films of homogenous and even surface indicate the structural compactness. Phase partition is shown evidently which shows that starch/chitosan and sepiolite (nanoclay) do not react with one another [Citation14]. In , the white spots indicate the sepiolite particles without drug film, and they are uniformly distributed throughout the polymeric matrix [Citation40].
Figure 3. SEM image of cefixime loaded formulation F23 irradiated with 80 kGy dose (a) at a magnification of 2500×, (b) at a magnification of 5000×, (c) at a magnification of 10,000×, (d) non-drug loaded (F8) at magnification of 10,000×.
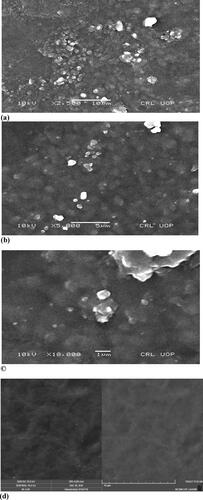
The γ-irradiated film is observed at three different magnification powers as shown in at ×500, ×5000 and ×10,000 magnifications, respectively. At ×5000, spherical agglomerates (large circles) indicate the presence of cefixime and also of some very small agglomerates of sepiolite. The high-resolution in confirms the particles size of sepiolite within the nano domain. Most of the particles are below 100 nm [Citation14]. It is also observed that radiation does not affect the homogeneity of the film and the inner structure of both drug loaded and non-drug-loaded formulations.
3.1.4. Energy dispersive x-ray (EDX) analysis
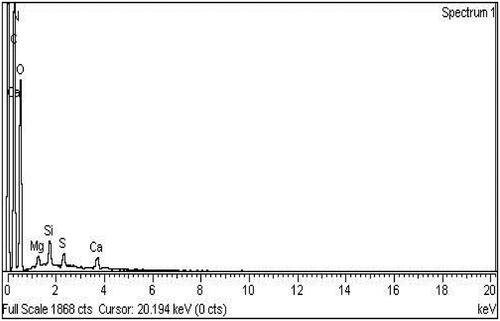
The elemental composition of 80 kGy γ-irradiated chitosan starch nanocomposite film F23 loaded with cefixime was studied by Energy Dispersive X-ray spectroscopy (EDX). shows the EDX analysis obtained from a selected region.
The EDX analysis shows the purity of mixing components. It shows that nanocomposite film consists of carbon 39.48%, nitrogen 10.84%, oxygen 46.93%, magnesium 0.47%, silica0.95%, sulphur 0.57% and calcium 0.75% by weight (). The result confirms that the γ-irradiation does not affect the elemental composition of bio-nanocomposite films and all elements remain intact as reported earlier [Citation41]. A low-intensity peak represents the elements present in low quantity. The intensity peaks corresponding to the concentration of elements within the sample. The elemental composition of cefixime loaded chitosan-starch bionanocomposite films is shown in .
Table 4. Elemental composition of 80 kGy γ-irradiated formulation F23.
3.2. Preliminary solubility studies of cefixime
Cefixime was less soluble in aqueous media whereas its solubility was observed high in organic solvents. Cefixime’s solubility depends upon pH that increased with an increase in pH. Cefixime showed the highest solubility (39 mg/ml) in methanol. The solubility of cefixime in different solvents is shown in .
Table 5. Solubility studies of cefixime in different solvent systems at 37 ± 2 °C for 24 h.
The solubility study of cefixime is performed in different solvent systems including organic solvents. Cefixime is less soluble or insoluble in aqueous media as it is a poorly water-soluble drug (hydrophobic drug) and it shows maximum solubility 39 mg/ml in methanol as reported formerly by [Citation42]. Its solubility is pH dependent, the solubility of cefixime increases with increase in pH. The solubility in acidic pH is higher than water which shows ionic potency dependent solubility. At pH 7.4, the solubility of cefixime is found to be 20 mg/ml whereas, at pH 7, the solubility is 14 mg/ml which shows that its solubility increases with increase in pH. Current results are in accordance as published previously [Citation43].
3.3. Preparation of calibration curve
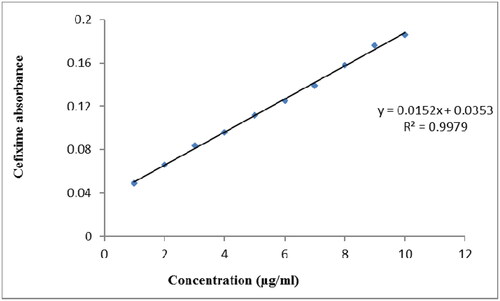
A standard calibration curve of cefixime in was made with a series of dilutions, ranging from 1 µg/ml to 10 µg/ml. The obtained Y-equation was 0.0152x + 0.0353 having a slope at 0.0152 and intercept at 0.0353 along with a coefficient of determination (R2) of 0.9979. The calibration equations and regression coefficients were shown using Microsoft Excel Software, in which x-and y-axis corresponds to the concentration (C) and optical absorbance (A) of cefixime, respectively.
3.4. Drug content uniformity/drug loading efficiency
The drug contents of all the formulations non-irradiated and γ-irradiated films were determined and found up to the standard range. The percentage drug content ± standard deviation of all the fifteen formulations are shown in . Drug content percentage yield is in the range of 90.76% to 107.36% that indicates the uniform and homogenous distribution of drug throughout the nanocomposite films.
Table 6. Drug content uniformity/drug loading efficiency of formulations.
3.5. Swelling studies of films
Water uptake studies of chitosan, starch, chitosan-starch blend films, and irradiated films loaded with a constant amount of drug in a different medium including acidic pH 1.2 and 4.5 as well as neutral pH 6.8, respectively were studied for 2.5 h. The results of the equilibrium swelling ratio versus time (min) are shown in . In order to understand drug release character of polymer-drug blend, the swelling studies are essential. It depends upon the porosity of polymer and degree of interaction between solvent molecules and polymer chains. It is seen that the highest swelling occurs at pH 1.2 than at pH 4.5 and the least water uptake is observed at pH 6.8.
Figure 6. The swelling ratio of chitosan-starch nanocomposite films in (A) HCl buffer pH 1.2, (B) Acetate buffer pH 4.5, and (C) Phosphate buffer pH 6.8.
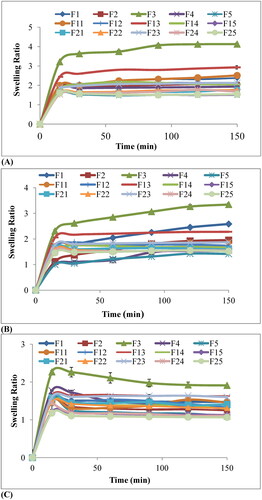
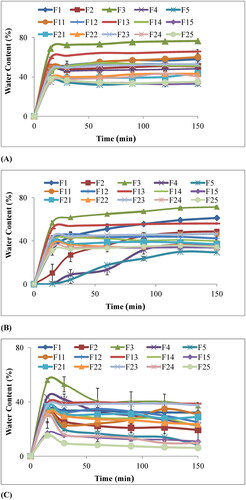
It is seen that swelling of chitosan film F1 is higher as compared to chitosan-starch blend films F2, F4. The equal ratio of two polymers chitosan: starch (i.e. 50:50) as in formulation F3 showed maximum water content and equilibrium swelling ratio. Percentage swelling or percentage of water content with time is shown in . The results point out that percentage of swelling has increased with time. Results depicted that the highest swelling occurs at 50% concentration of starch F3 and water content decreased linearly when starch concentration increased from 50% to 100% as in .
Figure 7. Percentage water content of chitosan-starch nanocomposite films in (A) HCl buffer pH 1.2, (B) Acetate buffer pH 4.5, and (C) Phosphate buffer pH 6.8.
3.5.1. Effect of γ-radiations on percentage swelling of bio-nanocomposite films
The water uptake capacity of γ-irradiated films (40kGy and 80kGy) has been determined by immersing them into buffer media of pH 1.2, 4.5 and 6.8. Cross-linking by γ-irradiation is very helpful for controlling percentage swelling in blended films. The effect of different doses of γ-rays on equilibrium swelling ratio and percentage swelling or water content of chitosan-starch nanocomposite films are shown in and , respectively. From the figures, it has been concluded that equilibrium swelling ratio and water content has decreased by increasing dose of γ-rays. The results showed that water content steadily decreased from 78%, 65% and 58% of the non-irradiated films to 60%, 55% and 40%of the radiated films at 40 kGy radiation dose and 45%, 43% and 35% of the radiated films at 80 kGy dose at pH 1.2, 4.5 and 6.8, respectively.
3.5.2. Effect of different pH media on swelling of bio-nanocomposite films
It has seen that for both the γ-irradiated and non-irradiated films the highest swelling occurs at pH 1.2 then at pH 4.5 while the least water uptake was observed at pH 6.8 as shown in and . At the start, swelling occurred at an elevated rate which slowly decreased until the films attained equilibrium. It is notable that all the films remain intact in the neutral medium i.e. at pH 6.8 while the films in acidic medium become disposed-off during the experiment. The film containing (50:50) chitosan starch ratio F3 showed maximum swelling at all pH i.e. 1.2, 4.5 and 6.8. At pH 1.2 and 4.5, a film containing chitosan only F1 show the highest swelling after F3 because chitosan is acidic in nature and shows maximum swelling at acidic pH. The percentage swelling occurred in order of F3 > F13 > F11 > F1 > F23 > F12 > F14 > F2 > F4 > F5 > F21 > F22 > F24 > F15 > F25.
In case of swelling, the polymeric blend with chitosan-starch 50:50 ratio shows maximum swelling ( and ). When the concentration of chitosan is further increased beyond 50% as in F2 and F4 the percentage of water uptake decreases as compared to F3 and F1. This may be because of the lesser amount of starch in these films that has slowed down the dispersion of solvent into these films. This is due to the surface containing more starch that became coarse possibly owing to starch agglomeration and phase partition. This decrease in water content due to the increase in starch concentration is due to a well-arranged crystalline arrangement of starch which inhibits the diffusion of media into matrix [Citation44]. In case of gamma-irradiated films at a dose of 40 kGy and 80 kGy, more swelling is observed at lower dose because high doses of gamma rays may deactivate the polymers and cause strong cross-linking between the polymer chains that restrict the penetration of solvent molecules and decreases the water content as reported previously by [Citation45].
The highest swelling of films in acidic media (pH 1.2) is because of the protonation of amino groups of chitosan (NH2 becomes NH3+) that may cause repulsions among polymer chains leading to an increase in swelling. At the same time at pH 6.8, deprotonation of amine group occurs that reduces the repulsive forces between the polymer chains and in turn decreases the swelling [Citation15].
3.6. Erosion studies of films
The percentage of erosion studies of both the γ-irradiated and non-irradiated films were conducted by gravimetric method and results are shown in . The matrix erosion of these formulations is directly related to water uptake by the bionanocomposite films. The films showing greater water uptake have also shown greater erosion. F3 film has greater water uptake so its percentage erosion is 40.5%. The same pattern was exhibited by the remaining films i.e. their erosion decreases with the increase in the concentration of starch above 50%. The erosion percentage was high at acidic pH 1.2 as compared to pH 4.5 and 6.8. As the films have more percentage of swelling at acidic pH, their rate of erosion has also elevated. Irradiated films, especially with dose of 80 kGy, were relatively more stable as compared to 40 kGy because irradiation causes strong-cross linking and water molecules are trapped inside.
Table 7. Percentage matrix erosion of non-irradiated and γ-irradiated bionanocomposite films at pH 1.2; 4.5 and 6.8.
3.7. Ex vivo skin permeation studies
Ex vivo skin permeation studies of both the γ-irradiated and non-irradiated cefixime loaded chitosan-starch nanobiocomposite films were carried out with the help of Franz diffusion cell by using rat skin.
3.7.1. Effect of polymer concentration on cefixime permeation through rat skin
Skin permeation profile revealed that the release behavior of non-irradiated and γ-irradiated films depends on the concentration of polymer. Amount of drug permeated changes with the change in the concentration of polymer. As the concentration of chitosan increases, the amount of permeation decreases, . The cumulative amount permeated was found to be 1959.24 μg/cm2, 1440.812 μg/cm2, 1112.684 μg/cm2, 779.7575 μg/cm2 and 689.5453 μg/cm2 from the formulation F5, F4, F3, F2 and F1 respectively. As the concentration of chitosan increases from F5to F1, the amount of drug permeated begins to decrease. The F5 (0% chitosan) formulation showed the highest cumulative amount of permeated drug after 24 h, while F1 (100% chitosan) showed the lowest amount of permeated drug. The irradiated films also follow the same pattern that by increasing concentration of chitosan their cumulative amount of drug permeated decreases after 40 h, . F15 and F25 (0% chitosan) films which are γ-irradiated at a dose of 40 kGy and 80 kGy, respectively have shown the highest release as compared to other formulations. The release patterns of films were controlled and extended over a prolonged period of time. The values of flux, kp, k, D for both irradiated and non-irradiated films are given in . The value of flux for F5 is 5.0257 μg/cm2/min which is also greater as compared to other formulations, which shows that the amount of drug passing through the specific area per unit time is also greater.
Figure 8. (A) In vitro permeation profile of non-irradiated cefixime nanobiocomposite films; (B) 40kGy γ-irradiated cefixime nanobiocomposite films; (C) 80 kGy γ-irradiated cefixime nanocomposite films.
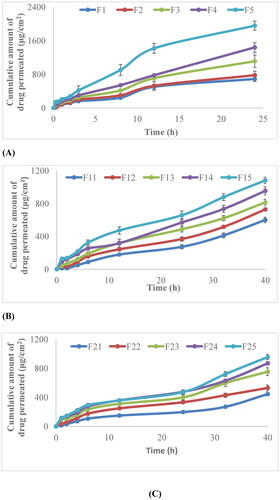
Table 8. Ex vivo skin permeation kinetic data of cefixime from both γ-irradiated and non-irradiated films.
3.7.2. Effect of radiation dose on cefixime permeation through rat skin
In the case of γ-irradiated films at different doses i.e. at 40 kGy and 80 kGy, the amount of drug permeated decreases with increasing the radiation dose. It is seen from that the cumulative amount of drug permeated from γ-irradiated nanocomposite films at a dose of 40 kGy i.e. F11, F12, F13, F14 and F15 is 600.7842 μg/cm2, 728.0258 μg/cm2, 814.5201 μg/cm2, 954.4866 μg/cm2 and 1082.287 μg/cm2, respectively. Whereas for formulations irradiated at dose of 80kGy i.e. F21, F22, F23, F24 and F25, , the cumulative amount of drug permeated was 446.03 μg/cm2, 530.26 μg/cm2, 753.528 μg/cm2, 868.80 μg/cm2 and 955.51 μg/cm2, respectively. The value of flux for F15 (irradiated at dose 40kGy) is 2.746 μg/cm2/min which is higher than that of F25 (irradiated at a dose of 80 kGy) i.e. 2.216 μg/cm2/min. It is because radiation has deactivated the cefixime and causes the cumulative amount permeated to decrease as compared to non-irradiated nanocomposite films.
The permeation profile of cefixime loaded non-irradiated and γ-irradiated nanocomposite films show that the amount of drug permeated decreases with increase in chitosan concentration, , as reported previously [Citation46]. The formulation F5 (0% chitosan) shows the maximum amount of drug permeated in 24 h and its flux is also the highest. This is because as chitosan is viscous in nature when its concentration is increased it may contribute to enhance the viscosity of the solution and in turn molecules of cefixime diffuse out from the polymeric chain networks slowly, which is according to work reported previously [Citation37]. Cefixime is a hydrophobic and pH-sensitive drug and it is acidic in nature, and also chitosan exhibits pH-sensitive behavior and dissolves rapidly at low pH. The permeation studies are conducted at neutral pH [Citation47]. Permeation occurs in biphasic mode, initial abrupt release, followed by the controlled release of the drug. The formulations F5, F15, and F25 have the highest flux values because the concentration of chitosan is minimum in these non-irradiated and γ-irradiated films.
The permeation profile of γ-irradiated films shows that the amount of drug permeated is lower in the case of films irradiated at a dose of 80 kGy. When radiation dose is increased, because of immense crosslinking density and complexation, the free volume room accessible in the polymeric template is reduced as the film become rigid. That’s why γ-irradiated films are less permeable.
3.8. Dissolution studies
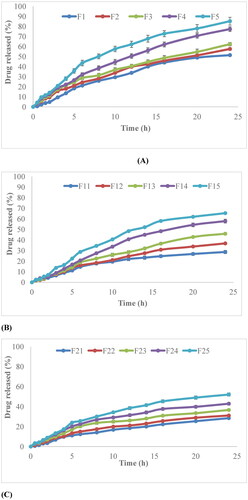
The dissolution studies of both non-irradiated and γ-irradiated nanobiocomposite films were performed by at three different pH; 1.2, 4.5 and 6.8 by sequential change method. The results obtained are shown in . The rate of drug release decreased with increase in the concentration of chitosan. It is evident from results that formulation F5 (0% chitosan concentration) show maximum drug release i.e. 85%. In this case, almost 50% of the drug was released during the first 3 h. The drug release rate was in order of F5 > F4 > F3 > F2 > F1. The same pattern was observed in the case of γ-irradiated films, their release also decreases also with the increase of chitosan concentration.
Figure 9. (A) Release behavior of non-irradiated nanocomposite films, (B) γ-irradiated films at a dose of 40 kGy, (C) γ-irradiated films at a dose of 80 kGy.
3.8.1. Effect of different pH on the release of cefixime
The dissolution profile of cefixime reveals that the release of cefixime from films was markedly affected by the pH of the medium. When pH was higher, the rate of drug release was slower, and when pH was decreased, the release was accelerated. The total release amount during pH 1.2 was 50% in the case of F5. Whereas, at pH 6.8 the release was controlled and become constant after 12 h. Γ-Irradiated films also show the same behavior.
3.8.2. Effect of γ-irradiation on cefixime release
The release rate of γ-irradiated films is shown in . It is seen that with the increase of γ radiation dose, the release of cefixime was affected. The 80 kGy irradiated films show lesser release as compared to 40 kGy nanocomposite films. F15 film shows maximum release (65.35%) and F25 shows (52%) release. This was due to the cross-linking of drug molecules within the polymeric matrix.
3.8.3. Kinetic analysis
All the dissolution profiles were plotted against time, and curves conclude about the mode of drug release from films. The model independent and model dependent approaches have been used for the comparison and selection of one formulation on the basis of release pattern. The best formulation was selected on the basis of maximum drug release in both γ-irradiated and non-irradiated films. The kinetic analysis shows that for non-irradiated and γ-irradiated films the First order model (concentration-dependent) is best fit to data of all formulations, except F23, F24, and F25. In case of these three formulations best-fit the model to data is Higuchi (diffusion controlled), as confirmed by the high values of determination coefficient (R2) in case of all formulations as shown in . It also indicates the drug release is diffusion controlled. The Korsmeyer-Peppas is used to calculate the value of ‘n’, the value of ‘n’ for all the formulations indicates non-Fickian diffusion mechanism. The drug release profile of various formulations was compared with reference product F1 and also formulations irradiated at 40 kGy were compared with 80 kGy formulations, to calculate the effect of different variables by using similarity factor, f2. The results showed in show the f2 value of different formulations. The values of less than 50 show the considerable effect of cross-linking and polymer concentration. The reference formulation F1 was compared with the cross-linked bionanocomposite films at a dose of 40 kGy and 80 kGy, and also it was compared with other non-irradiated films. The γ-irradiated films at a dose of 40 kGy were also compared with the 80 kGy films. It is evident that irradiated films were similar to one another. The F1 formulation having only pure chitosan was not similar to the F5 formulation having only starch. The F1 formulation was also not similar to irradiated formulations having chitosan only i.e. F11 and F21.
Table 9. Kinetic profile of γ-irradiated and non-irradiated bionanocomposite films.
Table 10. Comparison of similarity factor (f2) of different formulations.
The F5 formulation containing zero percent chitosan shows maximum release. The γ-irradiated films also follow the same sequence, . It has been suggested that drug release from polymeric matrices frequently implies penetration of water molecules, hydration, swelling, dispersion of dissolved drug, and finally erosion of matrix [Citation21].
The release of cefixime from bionanocomposite films is markedly affected by pH value. The release is greater at acidic pH 1.2 and it is lower at pH 6.8. This is because most of the hydrogen bonds are formed among hydroxyl groups of chitosan and starch, hydrogen bonds are affected by low and high pH, which are the solitary support of nanocomposite film as reported previously [Citation48]. At low pH, the destabilized hydrogen bonds obtained by the hydrogen ions interference may enhance the degradation time of the film, thus causing immense drug release. In case of high (pH 6.8), the hydrogen bonds of the film are fairly stable and amino groups of chitosan cannot be protonated, due to which release of drug through the film becomes quite persistent [Citation49].
The release of cefixime from γ-irradiated films is greatly influenced by radiation dose. The release is slower at 80 KGy as compared to 40 KGy because high dose may deactivate the polymeric matrix and cross-linking also hinders the movement of polymer chains, increases the polymeric group strength and cross-linking compactness. Therefore, it leads to the reduced swelling capacity which in turn decreases the drug release as mentioned above drug release also depends on swelling, which is in accordance with the previous results [Citation50]. The kinetic model proved that the best fit model was First order and Higuchi also since there R2 values were not significantly different from one another. High values of the regression coefficient for the Higuchi model indicate diffusion controlled drug release. The value of ‘n’ indicates non-Fickian diffusion showing gradient dependent release. This anomalous mode of drug release was due to the high concentration of polymer and also due to cross-linking. The high t25% value of irradiated samples indicates that with the increase of cross-linking the formulation becomes a controlled release. In 80 kGy irradiated samples, the highest value of t25% indicates strong cross-linking.
3.9. In vitro antibacterial test
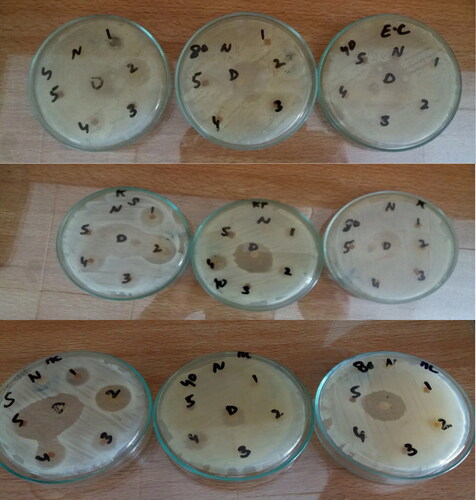
The in vitro antibacterial studies of starch, chitosan, chitosan-starch blends of non-irradiated and irradiated films were performed against three Gram-negative bacteria i.e. Escherichia coli, Klebsiella pneumonia, and Acetobacter aceti. It is evident from that the blends of chitosan and starch have the greatest zone of inhibition as the blend enhances antibacterial activity.
Table 11. Antibacterial activities of different bionanocomposite films (γ-irradiated and non-irradiated) against different bacteria.
3.9.1. Antimicrobial activity against Acetobacter aceti
In case of Acetobacter aceti, the polymeric blend of chitosan and starch F2 and F3 in ratio of 75:25 and 50:50, show the greatest zone of inhibition 29 mm and 21 mm, respectively as shown in as compared to zone of inhibition of standard drug which is 25 mm. It reveals the better antimicrobial activity of the prepared formulation.
3.9.2. Antimicrobial activity against Escherichia coli
In the case of Escherichia coli, the antimicrobial activity depends on the drug release pattern. The films having better drug release showed more antibacterial activity as F5, F4, F3, and F2 have a zone of inhibition as 20 mm, 13 mm, 12 mm, 9 mm, respectively as shown in . Whereas, the antibacterial activity of irradiated films depends on chitosan concentration. The γ-irradiated films having pure chitosan and 50:50 polymeric blends showed antibacterial activity.
3.9.3. Antimicrobial activity against Klebsiella pneumonia
In the case of Klebsiella pneumonia, the antibacterial activity also relates to drug release. F5, F4, F3, F2 and F1 in which F5 have maximum drug release, whereas F1 have a minimum release, also had a zone of inhibition in order of 25 mm, 20 mm, 17 mm, 15 mm and 10 mm, respectively as shown in . While 40kGy irradiated films show the highest activity of blend of 75:25 (chitosan: starch). The only one 80kGy irradiated film F25 show activity which has the highest drug release. showed a zone of inhibition of different bacterial strains by chitosan-starch nanocomposites films.
The antibacterial activity of nanocomposite films against Escherichia coli, Klebsiella pneumonia as reported previously [Citation38] and Acetobacter aceti. Starch shows the additive effect in addition to chitosan on enhancing the antimicrobial activity as reported previously [Citation51]. The irradiated films have lesser or no activity as compared to non-irradiated due to degradation consequence of γ-radiation on chitosan, and . It causes reduction of molecular weight of chitosan, which in turn decreases its antimicrobial activity [Citation52]. Strong cross-linking and binding of a drug within the polymeric matrix may also slow-down or completely restrict the drug release.
In the case of Acetobacter aceti, the polymeric blends which contain a higher concentration of chitosan show a greater zone of inhibition. This behavior depends on chitosan concentration since chitosan itself possess antimicrobial activity [Citation53]. Similarly, the irradiated films F12 and F22 show greater activity. In the case of Escherichia coli, the antibacterial activity relies on drug release [Citation51]. The film having greater drug release shows more antibacterial activity as in the case of F5. Whereas, the antibacterial activity of irradiated films depends on chitosan concentration. Only F11, F12, F21, and F22 which are pure chitosan films and blends of chitosan and starch show activity.
In the case of Klebsiella pneumonia, antibacterial activity is also related to drug release. F5 has a maximum zone of inhibition while 40 kGy irradiated films show the highest activity of polymeric blend of chitosan: starch (75:25). Only one 80 kGy (F25) irradiated film shows the activity which also has the highest drug release.
4. Conclusion
The film composition had a particular impact on drug release properties. It is possible to alter swelling of films, drug release, and permeation rate by changing the composition of chitosan-starch in nanocomposite films. The γ-irradiated films could provide the delivery of drug at a sustained rate through the skin. Due to the γ-irradiation the release of the drug became sustained as evident from values of t25%. It indicates that due to an increase in cross-linking dose, the drug release became controlled. The polymeric blends also showed greater antibacterial activity against three selected strains. Moreover, the antibacterial activity depends on drug release, the films which showed greater drug release, also showed higher antibacterial activity.
Acknowledgment
This work was supported by Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2022R73), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Data availability
All the data have been added to this article.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Yuan C, Jiang B, Xu X, et al. Anti-human ovarian cancer and cytotoxicity effects of nickel nanoparticles green-synthesized by Alhagi maurorum leaf aqueous extract. J Exp Nanosci. 2022;17(1):113–125.
- Li X, Feng H, Mei Y, et al. Describing a modern therapeutic drug prepared by in situ decorated gold nanoparticles on starch-modified magnetic nanoparticles to treat the cutaneous wound: a preclinical trial study. J Exp Nanosci. 2022;17(1):150–162.
- Mahendran D, Kavi Kishor PB, Geetha N, et al. Efficient antibacterial/biofilm, anti-cancer and photocatalytic potential of titanium dioxide nanocatalysts green synthesised using Gloriosa superba rhizome extract. J Exp Nanosci. 2021;16(1):11–30.
- Hao Q, Cheng L, Dong Z. Two Zn(II)-organic frameworks: catalytic Knoevenagel condensation and treatment activity on spine surgery incision infection via inhibiting Staphylococcus aureus biofilms formation. J Exp Nanosci. 2021;16(1):31–42.
- Dufresne A. Nanocellulose: from nature to high performance tailored materials. Walter de Gruyter; 2013.
- Huang J, Chang PR, Lin N, et al. Polysaccharide-based nanocrystals: chemistry and applications. Hoboken, New Jersey: John Wiley & Sons; 2015.
- Bilia AR, Bergonzi MC, Boulos JC, et al. Nanocarriers to enhance solubility, bioavailability, and efficacy of artemisinins. World J Tradit Chin Med. 2020;6(1):26–38.
- Kittur FS, Kumar ABV, Tharanathan RN. Low molecular weight chitosans—preparation by depolymerization with Aspergillus niger pectinase, and characterization. Carbohydr Res. 2003;338(12):1283–1290.
- Xu LL, Zhang Y, Chai Y, et al. Differentiation of belamcandae rhizoma and iridis tectori rhizoma by thin-layer chromatography and high-performance liquid chromatography. World J Tradit Chin Med. 2021;7(1):63–70.
- Berger J, Reist M, Mayer JM, et al. Structure and interactions in chitosan hydrogels formed by complexation or aggregation for biomedical applications. Eur J Pharm Biopharm. 2004;57(1):35–52.
- Anirudhan T, Parvathy J, Nair AS. A novel composite matrix based on polymeric micelle and hydrogel as a drug carrier for the controlled release of dual drugs. Carbohydr Polym. 2016;136:1118–1127.
- Yuen CWM, Yip J, Liu L, et al. Chitosan microcapsules loaded with either miconazole nitrate or clotrimazole, prepared via emulsion technique. Carbohydr Polym. 2012;89(3):795–801.
- Ma J, Bilotti E, Peijs T, et al. Preparation of polypropylene/sepiolite nanocomposites using supercritical CO2 assisted mixing. Eur Polym J. 2007;43(12):4931–4939.
- Mathew AP, Dufresne A. Plasticized waxy maize starch: effect of polyols and relative humidity on material properties. Biomacromolecules. 2002;3(5):1101–1108.
- Qian YX, Xie HM, Zuo TT, et al. Ultra-high performance liquid chromatography/ion mobility-quadrupole time-of-flight massspectrometry and database-driven automatic peak annotation for the rapid profiling and characterization of the multicomponents from Stephaniae Tetrandrae radix (Fang-Ji). World J Tradit Chin Med. 2021;7(1):120–129.
- Liu H, Adhikari R, Guo Q, et al. Preparation and characterization of glycerol plasticized (high-amylose) starch–chitosan films. J Food Eng. 2013;116(2):588–597.
- Paul Y, Kumar M, Singh B. Formulation and in vitro evaluation of gastroretentive drug delivery system of Cefixime trihydrate. Int. j. drug Dev. res. 2011;3:170–173.
- Shafiq M, Yasin T. Effect of gamma irradiation on linear low density polyethylene/magnesium hydroxide/sepiolite composite. Radiat Phys Chem. 2012;81(1):52–56.
- Khan RA, Beck S, Dussault D, et al. Mechanical and barrier properties of nanocrystalline cellulose reinforced poly (caprolactone) composites: effect of gamma radiation. J Appl Polym Sci. 2013;129(5):3038–3046.
- Nohales A, Solar L, Porcar I, et al. Morphology, flexural, and thermal properties of sepiolite modified epoxy resins with different curing agents. Eur Polym J. 2006;42(11):3093–3101.
- Brinckmann JA, Cunningham AB, V. Harter DE. Reviewing threats to wild Rhodiola sachalinensis, a medicinally valuable yet vulnerable species. World J Tradit Chin Med. 2021;7(3):299–306.
- Hussein AA, HSh M. Preparation and evaluation of cefixime nanocrystals. Iraqi J Pharm Sci. 2014;23:1–12.
- Maheshwari R, Rajagopalan R. Formulation and evaluation of paracetamol syrup made by mixed-solvency concept. Pharm Lett. 2012;4:170–174.
- Dol H, Gandhi S, Pardhi D, et al. Formulation and evaluation of in situ ophthalmic gel of moxifloxacin hydrochloride. J Pharm Innov. 2014;3:60–66.
- Shivashankar M, Mandal BK. Design and evaluation of chitosan-based novel pH sensitive drug carrier for sustained release of cefixime. Trop J Pharm Res. 2013;12:155–161.
- Khan S, Ranjha NM. Effect of degree of cross-linking on swelling and on drug release of low viscous chitosan/poly (vinyl alcohol) hydrogels. Polym Bull. 2014;71(8):2133–2158.
- Ige P, Swami B, Patil T, et al. Design and development of sustained release swelling matrix tablets of glipizide for type II diabetes mellitus. Farmacia. 2013;61:883–901.
- Lamoudi L, Chaumeil JC, Daoud K. Swelling, erosion and drug release characteristics of Sodium Diclofenac from heterogeneous matrix tablets. J Drug Deliv Sci Technol. 2016;31:93–100.
- Namazi H, Rakhshaei R, Hamishehkar H, et al. Antibiotic loaded carboxymethylcellulose/MCM-41 nanocomposite hydrogel films as potential wound dressing. Int J Biol Macromol. 2016;85:327–334.
- Ahad A, Aqil M, Ali A. Investigation of antihypertensive activity of carbopol valsartan transdermal gel containing 1, 8-cineole. Int J Biol Macromolec. 2014;64:44–149.
- National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the care and use of laboratory animals. National Academies; 1985.
- Khan IN, Khan MI, Mazumder K, et al. Characterization and ex-vivo skin permeation study of domperidone maleate transdermal patch. Bull Pharma Res. 2012;2:15–21.
- Zhang J, Li J, Huang JQ. Network Meta-analysis of four Chinese patent medicines combined with angiotensin converting enzyme inhibitors or angiotensin receptor blockers in early diabetic nephropathy treatment. World J Tradit Chin Med. 2020;6(1):51–60.
- Kouchak M, Handali S, Boroujeni BN. Evaluation of the mechanical properties and drug permeability of chitosan/eudragit RL composite film. Osong Public Health Res Perspect. 2015;6(1):14–19.
- Shahzad Y, Afreen U, Nisar Hussain Shah S, et al. Applying response surface methodology to optimize nimesulide permeation from topical formulation. Pharm Dev Technol. 2013;18(6):1391–1398.
- Cheng G, An F, Zou M-J, et al. Time- and pH-dependent colon-specific drug delivery for orally administered diclofenac sodium and 5-aminosalicylic acid. World J Gastroenterol. 2004;10(12):1769–1774.
- Murtaza G, Ahmad M, Khan SA, et al. Evaluation of Cefixime-loaded chitosan microspheres: analysis of dissolution data using DDSolver. Dissolut Technol. 2012;19(2):13–19.
- Zaid AN, Qaddomi A, Ghanem M, et al. Development of a dissolution method to compare tablet formulations containing Valsartan/Amlodipine. Dissolut Technol. 2015;22(3):32–38.
- Anacona JR, Estacio J. Synthesis and antibacterial activity of cefixime metal complexes. Transition Met Chem. 2006;31(2):227–231.
- Tuhin MO, Rahman N, Haque M, et al. Modification of mechanical and thermal property of chitosan–starch blend films. Radiat Phys Chem. 2012;81(10):1659–1668.
- Olivato J, Marini J, Pollet E, et al. Elaboration, morphology and properties of starch/polyester nano-biocomposites based on sepiolite clay. Carbohydr Polym. 2015;118:250–256.
- Tripathi S, Mehrotra G, Dutta P. Chitosan–silver oxide nanocomposite film: preparation and antimicrobial activity. Bull Mater Sci. 2011;34(1):29–35.
- Prasanna S, Koppula SB. Enhancement of dissolution rate of cefixime trihydrate by using various solid dispersion techniques. Int J Biopharma. 2013;2:104–107.
- Kumar N, Tripathi R, Nath G, et al. Xanthan gum based once a day matrix tablet of cefixime trihydrate: development and evaluation. IJPSR. 2013;4:3390.
- Singh V, Kumari K. Synthesis and characterization of chitosan–starch crosslinked film for controlled drug release. Int J Mater Biomater Appl. 2014;4:7–13.
- Rimdusit S, Somsaeng K, Kewsuwan P, et al. Comparison of gamma radiation crosslinking and chemical crosslinking on properties of methylcellulose hydrogel. EJ. 2012;16(4):15–28.
- Guo YF, Xu KX, Hong JW, et al. Analysis of phytochemical constituents of zuogui wan in rat serum and its effects on early embryonic development of mice. World J Tradit Chin Med. 2020;6(3):324–330.
- Natarajan R, Nathum R. Cefixime trihydrate loaded chitosan-alginate transdermal patches. World J Pharm Sci. 2014;2:997–1008.
- Belbekhouche S, Bras J, Siqueira G, et al. Water sorption behavior and gas barrier properties of cellulose whiskers and microfibrils films. Carbohydr Polym. 2011;83(4):1740–1748.
- Thakur A, Monga S, Wanchoo R. Sorption and drug release studies from semi-interpenetrating polymer networks of chitosan and xanthan gum. Chem Biochem Eng Q. 2014;28:105–115.
- Huo W, Xie G, Zhang W, et al. Preparation of a novel chitosan-microcapsules/starch blend film and the study of its drug-release mechanism. Int J Biol Macromol. 2016;87:114–122.
- Pasanphan W, Rimdusit P, Choofong S, et al. Systematic fabrication of chitosan nanoparticle by gamma irradiation. Radiat Phys Chem. 2010;79(10):1095–1102.
- Khalil S, Hassan M, Ali N. Characterization and antimicrobial properties of gamma irradiated starch/chitosan/Ag nanocomposites. Arab J Nucl Sci Appl. 2016;94:1–1.