Abstract
Hepatocellular carcinoma (HCC) is associated with a high mortality rate. In this study, we aimed to investigate the therapeutic effect and safety of hepatic arterial administration of doxorubicin (Dox)-loaded hollow gold nanospheres (Dox@HAuNSs) combined with photothermal ablation (PTA) in a rabbit VX2 liver cancer model established by implanting VX2 tumour cells into rabbit livers. Rabbits were randomly divided into four treatment groups: physiological saline solution (control); lipiodol and doxorubicin (Dox + Lp); lipiodol and Dox@HAuNS (Dox@PEG-HAuNS + Lp); and lipiodol, Dox@PEG-HAuNS, and photothermal ablation (Dox@PEG-HAuNS + Lp + PTA). Dox release from Dox@PEG-HAuNS in the tumour was detected using fluorescence microscopy. Tumour size and liver-kidney function were assessed in each rabbit preoperatively and on postoperative days 3, 7, and 14. Necrosis, proliferation, and micro-vessel density of VX2 tumours were estimated in peripheral tumour tissues. Dox release from Dox@PEG-HAuNS was increased with a subsequent increase in tumour necrosis in liver tumours after PTA, whereas the tumour volume and proliferation decreased. However, the aspartate aminotransferase and alanine aminotransferase levels indicated transient liver damage. Thus, intra-arterial delivery of Dox@PEG-HAuNS combined with PTA can suppress VX2 liver cancer growth. Dox@PEG-HAuNS + Lp + PTA is also associated with transient liver damage in rabbits. The combination of Dox@PEG-HAuNS chemoembolisation and PTA could be a potential therapeutic approach for HCC and it has broad application prospects.
1. Introduction
Hepatocellular carcinoma (HCC) is a malignant tumour associated with high global morbidity and mortality rates. An estimated 826 000 liver cancer cases, were reported worldwide in 2018 with HCC constituting approximately 80% of all cases [Citation1]. The early diagnosis of liver cancer is low owing to the strong regenerative and compensatory capacity of the liver. At diagnosis, most patients are in advanced stages, where approximately 15% of the diagnosed patients need surgical resection of the liver [Citation2]. For unresectable HCC, local treatment with transcatheter arterial chemoembolisation (TACE) is the preferred method [Citation3, Citation4]. However, tumour ischaemia and hypoxia after embolisation can promote blood vessel regeneration and lead to incomplete management of HCC [Citation5]. Therefore, inhibition of tumour growth and attenuation of angiogenesis using TACE remain challenging in the treatment of HCC [Citation6]. Accordingly, a novel multimodal therapy is needed to improve tumour response and prolong the survival of patients with HCC.
With the development of nanomaterials and nanomedicine, hollow gold nanospheres (HAuNSs) have attracted research attention in the field of cancer therapy owing to their unique physicochemical properties [Citation7]. As an inorganic nanomaterial, HAuNSs have several advantages over organic and traditional drug-loaded materials as described as follows. Nano Au has been reported to inhibit angiogenesis [Citation8]. Additionally, the diameter of HAuNS is less than 100 nm which allows its entry to the tumour. The diameter of HAuNS coated with polyethylene glycol (PEG) and loaded doxorubicin (Dox) increases by approximately 10 nm [Citation9]; however, the diameter is still smaller than the gap of the tumour vascular endothelial cells. Therefore, Dox-loaded hollow Au nanospheres coated with PEG (Dox@PEG-HAuNS) have an enhanced permeability and retention (EPR) effect as they can effectively enter the tumour tissue and release Dox [Citation10]. In addition, TACE can increase the content of Dox@HAuNS in tumour tissue-selective drug delivery [Citation11]. HAuNS can bind to Dox through its inner and outer surfaces, thus increasing the drug loading efficiency by 3.5 times compared to that of solid Au nanoparticles (AuNPs) [Citation9]. Additionally, HAuNSs allow controllable drug release, as the AuNPs swell and accelerate movement under irradiation with near-infrared light (NIR), which can accelerate drug release [Citation12]. Another positive aspect of using HAuNSs is that the drug is not degraded during the delivery process in the body. The sulfhydryl bond of PEG improves the water solubility and stability of Dox@HAuNSs [Citation13]. Compared with traditional drug-loading materials, HAuNS has radiotherapy enhancement and X-ray imaging properties [Citation14]. Using surface plasmon resonance (SPR), HAuNSs can convert light energy into heat energy when exposed to NIR (700–850 nm). The temperature of the irradiated region is sufficiently high to achieve photothermal ablation (PTA) in this scenario [Citation15]. Therefore, Dox@HAuNSs combined with NIR provides an alternative treatment strategy for tumour lesions, while achieving controllable drug release and reducing adverse reactions caused by Dox. In this study, we aimed to investigate the efficacy and safety of trans-hepatic artery injection of Dox@HAuNSs combined with PTA for the treatment of rabbit VX2 liver cancer and to provide a reference for future clinical application of this combined approach.
2. Materials and methods
2.1. Preparation of Dox@PEG-HAuNS (Dox@HAuNS)
Dox@HAuNSs were synthesised according to a previously reported method [Citation9]. Briefly, the following procedure was followed. The first step of preparing HAuNS was to synthesise cobalt NPs with deoxygenating deionised water containing 4.5 mL of 1 mol/L sodium borohydride, 2.8 mL of 0.1 mol/L sodium citrate, and 1.0 mL of 0.4 mol/L cobalt chloride. Au ions were reduced to the surface of cobalt nanoparticles and cobalt was oxidised to cobalt oxide when chloroauric acid was added to the solution containing cobalt nanoparticles. All remaining cobalt cores were oxidised by air and the final product was HAuNSs. HAuNS (5.0 × 1012 particles/mL) particles were added to an argon-purged aqueous solution containing various concentrations of PEG-SH to form the PEG-HAuNS. The reaction was allowed to proceed overnight. Later, the reaction mixture was centrifuged (14 000 rpm for 20 min) for further purification and the obtained pellets were resuspended using deionised water. This process was repeated twice to ensure that unreacted PEG molecules were completely removed. Aliquots of free Dox in water (1.0 mM Dox at a volume of 0.02–0.3 mL) were added to an aqueous solution of HAuNSs or PEG-HAuNSs (1.0 × 1011 particles/0.1 mL) and the mixtures were stirred at 25 °C for 24 h to prepare Dox@PEG-HAuNS. After centrifugation (14 000 rpm for 20 min), the precipitate was washed with phosphate-buffered saline (PBS) and centrifuged at the same speed. The washing cycle was repeated until the supernatant turned colourless.
2.2. DOX release from dox@PEG-HAuNS (dox@HAuNS) in vitro
Dox@PEG-HAuNS was placed in 2.0 mL PBS at pH of 7.4 in a 5 mL test tube at room temperature. A laser detector was placed on the side 5.0 cm away from the centre of the test tube. The samples were irradiated by NIR (Changchun New Industry Optoelectronic Technology Co., Ltd., China) with the output power of 2 W/cm2 at 808 nm for 5 min. Before and after near-infrared laser irradiation, the nanoparticle solution was centrifuged (14 000 rpm for 20 min) and the supernatant was extracted for Dox analysis by spectrophotometry. Percentage release was calculated as (cumulative amount of Dox released each time)/(total amount of Dox carried by Dox@PEG-HAuNS) * 100 [Citation16].
2.3. Establishment of the rabbit VX2 liver cancer model
New Zealand rabbits were provided by the Animal Experimental Centre and the study design was approved by the Ethics Committee of General Hospital of the Chinese People’s Liberation Army. The rabbits were maintained in cages and fed standard food and water. The Ethical Committee of Animal Research approved the study. Twenty-four adult male rabbits (2.5–3.0 kg) were used in the in vivo experiment. The rabbits were randomly divided into four groups with six rabbits per group: (A) control, (B) Dox + Lp, (C) Dox@PEG-HAuNS + Lp, and (D) Dox@PEG-HAuNS + Lp + PTA. The rabbit VX2 liver cancer model was established by extracting a VX2 tumour mass and preserving a cell suspension of the mass in liquid nitrogen. For use, tumour suspensions were placed in a 37 °C water bath for resuscitation, and the supernatant was discarded after centrifugation (1 000 rpm for 3 min). PBS was added to prepare the tumour mass suspension. VX2 tumours, with a volume of 0.5 mL, were implanted subcutaneously in the femoribus internus of the rabbits for proliferation. After 1.5–2 weeks, the tumour texture was hard, and the solid VX2 tumour mass was removed, divided into 1 × 1 × 1 mm3 pieces, and placed in saline. To establish the rabbit VX2 liver cancer model, the rabbits were anaesthetised using thiazide hydrochloride and ketamine hydrochloride (0.3 mg/kg) with a ratio of 1:1, and their skin was disinfected and towelled. An abdominal median incision was made and three VX2 tissue pieces were microinjected into the left side of the hepatic central lobe. The puncture was blocked with a gelatine sponge to avoid liver haemorrhage; then, the incision was sutured. Penicillin (300 000 units) was injected intramuscularly to prevent infection after the implantation of VX2 tumours.
2.4. TACE procedure
The rabbits were anaesthetised after fasting for 12 h. The skin was disinfected and towelled at the femoral artery in the right groin area. For the hepatic artery cannula, the femoral artery was bluntly separated by making a 2-cm incision along the right groin area. A 2.2 F microcatheter (Japan ASAHI INTECC, STD130-22S) was then inserted along the femoral artery and the hepatic artery supplying blood to the tumour for chemoembolization. Treatments were injected into the feeding artery of the rabbits based on their allocation to the four groups. The doses of Dox and iodised oil were determined according to a previously reported method [Citation17]. The control group was treated with 2 mL of 0.9% physiological saline solution. Dox + Lp treatment consisted of a combination of 2 mL of lipiodol and 0.5 mg of doxorubicin. Dox@PEG-HAuNS + Lp and Dox@PEG-HAuNS + Lp + PTA treatments consisted of a combination of 1 mL of lipiodol, and 1 mL of Dox@PEG-HAuNS (Dox content 0.5 mg/mL, PEG-HAuNS content 1.0 mg/mL). After TACE, ligation of the femoral artery in addition to stitching and sterilization of the incisions were completed. Penicillin (300 000 units) was then intramuscularly injected into the treated rabbits for 3 days.
2.5. Photothermal ablation
Six hours after TACE, the VX2 liver cancer of the rabbits in the D group was exposed along the abdominal median incision and irradiated (808 nm; 4 W/cm2; 4 min) using NIR (Changchun New Industry Optoelectronic Technology Co., Ltd., China).
2.6. Fluorescent detection of Dox
Four hours after PTA, two rabbits from the C group and two rabbits from the D group were sacrificed using an overdose of pentobarbital (100 mg/kg). VX2 tumours were temporarily removed and stored in the dark. Tumours were sectioned on a − 20 °C cryostat (Germany, FRICELL CM1950) with a thickness of 5 mm. Dox release in the tumour area was visualised using immunofluorescence microscopy (Japan, OLYMPUS IX73) at excitation and emission wavelengths of 488 and 565 nm, respectively.
2.7. Measurement of tumour size
We used 16-slice spiral computed tomography (CT) (Siemens, SOMATOM EMOTION, Germany) for VX2 liver cancer imaging (scanning parameters: 130 kV, 130 mA, 3.0 mm layer thickness). The mixture of saline and iodixanol in a ratio of (1:1) was used as a contrast agent. CT scanning was used to measure the arterial phase (7 s) and the venous phase (23 s). Tumour size (V) was calculated using the formula V = A × B2/2 [Citation18]. The maximum diameter (A) and transverse diameter (B) of the tumour were measured preoperatively and on postoperative days 3, 7, and 14.
2.8. Detection of alanine transaminase (ALT), aspartate transaminase (AST), creatinine (CREA), and urea levels
Blood from the auricular vein of each rabbit was obtained preoperatively and on days 3, 7, and 14 after TACE. The levels of ALT, AST, CREA, and urea were measured using a Cobas 8000 modular analyser (Roche, Germany).
2.9. Histopathological evaluation
The rabbits in the four groups were sacrificed and anatomised on postoperative day 14. Peripheral tumour tissues were obtained and embedded in 10% formalin. Subsequently, the tissues were sliced (3 μm) and stained with haematoxylin and eosin (H&E) to evaluate tumour necrosis. TdT-mediated dUTP nick-end labelling (TUNEL), tumour proliferation marker (Ki67 antigen), cluster of differentiation (CD)31, and hypoxia-inducible factor 1a (HIF-1a) assays were performed to evaluate tumour cell apoptosis, proliferation, micro-vessel density (MVD), and HIF-1a expression, respectively.
2. 10. Statistical analysis
SPSS version 26.0 (IBM Corporation, Armonk, NY, USA) was used for all statistical analyses. Data are presented as mean ± standard deviation, and comparisons among groups were performed using the one-way analysis of variance (ANOVA) followed by Duncan test and Least-Significant Difference (LSD) post-hoc tests for analysis. Student’s t-test was used to compare the differences between groups. Statistical significance was set at p < 0.05.
3. Results
3.1. Baseline characteristics in VX2 liver cancer rabbits
A total of 24 rabbits were included in the subsequent in vivo experiments. The baseline characteristics of the 24 rabbits before TACE are presented in .
Table 1. Baseline characteristics (n = 24).
3.2. NIR laser irradiation can trigger dox release from Dox@PEG-HAuNSs
In the Dox release in vitro experiment, the cumulative release of Dox was approximately 42.5% after 30 h of NIR irradiation and approximately 15.2% without NIR irradiation. These data showed that NIR could effectively promote the Dox release from Dox@PEG-HAuNSs in vitro ().
Figure 1. (A) Dox Release from Dox@PEG-HAuNS in vitro with or without NIR Irradiation. Irradiation with NIR laser effectively promote Dox release from Dox@PEG-HAuNSs in vitro. (B) VX2 liver cancer from rabbits that received intra-hepatic arterial injections of doxorubicin-loaded hollow Au nanospheres coated with PEG (Dox@PEG-HAuNS) and lipiodol with or without photothermal ablation (PTA). In tumour not treated with PTA, Dox was mainly combined with PEG-HAuNS and was agglomerated. After PTA, most of Dox was released from the Dox@PEG-HAuNS. It is dotted in the tumour tissue.
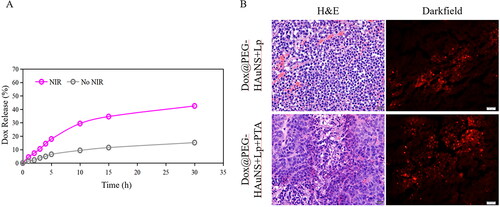
In addition, the Dox release in the lesion was found to be minimal in group B in the in vivo experiments. Dox was mainly released in the PEG-HAuNS-treated rabbits, 6 h after hepatic artery perfusion of group C. However, free Dox release in the tumour area increased after 4 h of NIR irradiation in rabbits treated with Dox@PEG-HAuNS + Lp + PTA (p < 0.05) ().
3.3. Dox@PEG-HAuNS + Lp + PTA treatment can effectively inhibit VX2 tumour growth
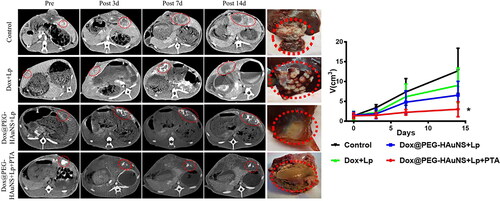
The preoperative tumour volumes were 1.30 ± 0.50 cm3 (control; group A), 1.28 ± 0.86 cm3 (Dox + Lp; group B), 1.44 ± 0.75 cm3 (Dox@PEG-HAuNS + Lp; group C), and 1.31 ± 0.61 cm3 (Dox@PEG-HAuNS + Lp + PTA; group D). While the tumour volumes on day 3 after TACE were 3.40 ± 0.76 cm3 (group A), 2.20 ± 0.86 cm3 (group B), 1.86 ± 1.21 cm3 (group C), and 1.49 ± 0.69 cm3 (group D). On postoperative day 7, the tumour volumes were 7.55 ± 3.47 cm3 (group A), 6.35 ± 3.21 cm3 (group B), 4.79 ± 2.87 cm3 (group C), and 2.24 ± 0.72 cm3 (group D). On postoperative day 14, the tumour volumes were 12.70 ± 5.73 cm3 (group A), 9.19 ± 4.25 cm3 (group B), 6.56 ± 3.49 cm3 (group C), and 3.18 ± 1.75 cm3 (group D). Thus the growth of VX2 liver cancer in groups B and C was suppressed following the TACE treatments on the three postoperative days 3, 7, and 14 compared with that of the control group A. However, the tumour growth was significantly inhibited on days 3, 7, and 14 in group D only compared with that of the control group A ().
Figure 2. Tumour size changes after treatment. Enhanced computed tomography showed changes in tumour size after treatment of rabbit VX2 liver cancer. Red circles represent tumour lesions in the arterial phase. During the 15-day observation period, intra-hepatic arterial injections of doxorubicin-loaded hollow Au nanospheres coated with PEG combined with photothermal ablation significantly inhibited tumour growth.
Note: * indicates P < 0.05.
3.4. Dox@PEG-HAuNS + Lp + PTA treatment can increase VX2 tumour cell apoptosis and suppress VX2 tumour proliferation
According to the TUNEL assay, the apoptosis rates of peripheral tumour tissues were 5.03% ± 4.48% (group A), 18.04% ± 7.09% (group B), 20.87% ± 8.19% (group C), and 34.47% ± 13.14% (group D) 14 days after TACE (). This suggests that the apoptosis of tumour cells in group D was significantly higher than that in the other groups (PA-D < 0.001, PB-D = 0.004 < 0.01, PC-D = 0.015 < 0.05).
Figure 3. Histopathologic examination of tumour tissues in the four study groups. Tissue sections were stained with haematoxylin and eosin (H&E), TdT-mediated dUTP nick-end labelling (TUNEL), tumour proliferation marker (Ki67 antigen), cluster of differentiation (CD)31, and hypoxia-inducible factor 1a (HIF-1a). Intra-hepatic arterial injections of doxorubicin-loaded hollow Au nanospheres coated with PEG combined with photothermal ablation significantly inhibited tumour growth.
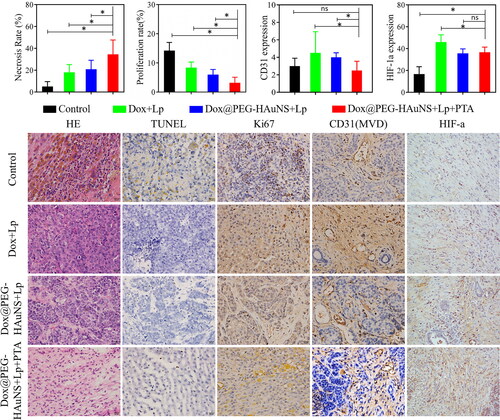
The proliferation rates of tumours measured using the Ki67 assay were 14.23 ± 2.77% in group A, 8.39 ± 1.91% in group B, 5.97 ± 1.79% in group C, and 3.2 ± 1.89% in group D on day 14. This suggested that the proliferation of tumour cells under Dox@PEG-HAuNS + Lp + PTA treatment was significantly decreased compared with that in the other three groups (PA-D < 0.001, PB-D < 0.001, PC-D = 0.036 < 0.05).
3.5. Dox@PEG-HAuNS + Lp + PTA treatment can attenuate VX2 tumour angiogenesis and decrease HIF-1a expression
The MVD in peripheral tumour tissues was 3.00±0.89 for group A, 4.5±2.43 for group B, 4.00 ± 1.26 for group C, and 2.50 ± 1.05 for group D on day 14 (). This suggests that the tumour MVD for Dox@PEG-HAuNS + Lp + PTA was significantly decreased compared with that for Dox + Lp (PB-D = 0.035 < 0.05).
The HIF-1a assay suggested that the number of positive cells in groups A–D was 16.67 ± 6.74, 46.00 ± 6.57, 35.67 ± 4.03, and 36.67 ± 4.72, respectively, on day 14. This suggested that, HAuNS may significantly inhibit HIF-1a expression compared with group B (PB-C = 0.005 < 0.01, PB-D = 0.01 < 0.05).
3.6. Dox@PEG-HAuNS + Lp + PTA can induce transient liver damage
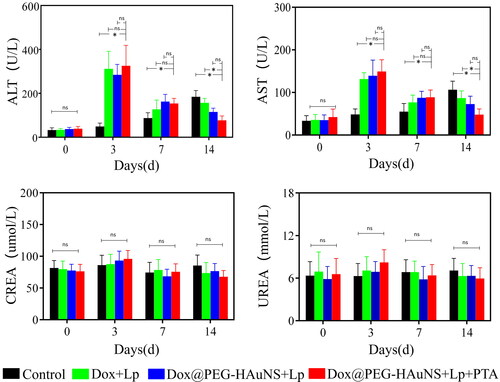
Postoperative liver and kidney functions, as assessed by measuring AST, ALT, CREA, and urea levels in the treatment groups, are illustrated in . On day 3, the levels of CREA and urea slightly increased in groups B, C, and D compared with those in group A (PCREA = 0.63, PUrea = 0.31) and returned to normal levels 7 days after surgery. This suggests that Dox@PEG-HAuNS + Lp + PTA treatment did not increase nephrotoxicity compared with traditional TACE. However, consistent with the findings for traditional TACE, the AST and ALT levels after Dox@PEG-HAuNS + Lp + PTA treatment were significantly increased compared with those of the control on day 3 after TACE (PAST < 0.001, PALT < 0.001) and returned to normal levels 7 days after surgery.
Figure 4. Preoperative and postoperative Changes in liver and kidney function-related indexes. As with traditional transcatheter arterial chemoembolisation, liver function-related indicators increased within 3 days after surgery and decreased to preoperative levels within 7 days postoperatively in the groups treated with intra-hepatic arterial injections of doxorubicin-loaded hollow Au nanospheres coated with PEG (Dox@PEG-HAuNS) and lipiodol with or without photothermal ablation (PTA). These findings are different from the control group, where liver function-related indicators gradually increased. Intra-hepatic arterial injections of Dox@PEG-HAuNS and lipiodol with PTA were associated with a gradual return of the liver function to normal levels after transient liver damage.
4. Discussion
TACE can be administered locally; however, a lower drug concentration limits its effectiveness in the treatment of HCC. Therefore, increasing the concentration of chemotherapeutic drugs in the tumour area is a possible strategy to improve the efficiency of TACE. The high specific surface area of HAuNS makes its potential use for drug loading greater than that of other nanomaterials. However, considering the diameter of Dox@PEG-HAuNS (∼50 nm), it could pass through the tumour vascular endothelium and enter the tumour tissue to release the drug. In addition, the tumour microenvironment is acidic owing to its high metabolic rate [Citation19]. You et al. [Citation9, Citation20] have reported that an acidic environment can enhance Dox@PEG-HAuNS-mediated release of Dox. Moreover, NIR irradiation can further accelerate Dox release. The current study demonstrated significant Dox release within the tumour tissue after exposure to 808 nm NIR. Hence, active and passive targeted drug release can facilitate accumulation inside the tumour area and improve the effectiveness of interventional treatments for HCC.
The effectiveness of the single-treatment strategy is limited. Recently, there has been considerable progress in the therapeutic strategy of TACE combined with thermal ablation in the treatment of liver cancer [Citation21, Citation22]. Using NIR, HAuNSs can convert light into heat energy. Li et al. [Citation23] showed that the temperature of HCC could reach 60 °C within 2 min after exposure to NIR irradiation at 808 nm (5 W/cm2) after intra-arterial delivery of Dox-loaded HAuNSs. An increase in temperature causes protein denaturation and necrosis. Abo-Elfadl et al. [Citation24] found that PEG-coated Au nano-semi-cubes combined with 700 nm (50 mW/cm2) NIR irradiation had a positive clinical effect on the treatment of Sk-Mel-28 melanoma in mice. In the current study, we combined NIR irradiation with Dox@PEG-HAuNS treatment and effectively inhibited the growth of VX2 liver tumour cells in rabbits. The TUNEL and Ki67 detection results showed that the apoptosis of tumour cells in the Dox@PEG-HAuNS + Lp + PTA treatment group was significantly increased and the proliferation was significantly reduced compared with that in the other treatment groups. This could be attributed to the fact that DNA and RNA in cells undergo denaturation or damage at high temperatures [Citation25]. In this study, laser was effectively converted into heat by Dox@PEG-HAuNS, thus increasing the necrosis of tumour. The release of Dox in NIR stimuli increased the free Dox concentration. There was a synergistic effect between the photothermal effect and Dox cytotoxicity. Therefore, administering Dox@PEG-HAuNS intra-arterially followed by NIR irradiation can not only increase the release of Dox and improve the concentration of Dox in the tumour area but also offer a combined PTA treatment strategy for HCC.
The tumour microenvironment is highly adaptable. After embolisation of blood vessels as a part of TACE, the internal changes associated with ischaemia and hypoxia can promote the secretion of certain cytokines such as HIF-1a, VEGF, and CXCL12, which have been shown to promote the development and recurrence of tumours [Citation26]. In the current study, MVD significantly increased after traditional TACE in rabbit VX2 liver cancer. Angiogenesis is strongly associated with cancer progression. Therefore, inhibition of adaptive changes in the tumour microenvironment is a key factor in the treatment of tumours. Li et al. [Citation27] reported that HAuNSs can normalise the tumour vasculature and inhibit neovascularisation of HCC lesions. In the current study, compared with that under traditional TACE (Dox + Lp), the level of HIF-1a, a cytokine that promotes angiogenesis [Citation28], was significantly suppressed in VX2 tumour cells after injection of HAuNSs into the hepatic artery. Thus, MVD was decreased in the tumour.
In our study, PTA did not damage the normal liver parenchyma around the tumour, but liver function was transiently damaged after the intra-arterial injection of Dox@PEG-HAuNSs followed by PTA. The liver function returned to normal levels by postoperative day 7. This change was consistent with that observed after TACE. However, in the current study, we demonstrated that the biochemical parameters associated with liver function were slightly increased compared with those observed with traditional TACE. This can be attributed to the following reasons: the toxicity of HAuNSs and adverse reactions of PTA. In previous studies, AuNSs showed promising biocompatibility and safety [Citation29]. However, recent studies have shown that the toxicity of AuNS may be related to its shape, dose, surface charge, and modification [Citation30]. Additionally, to avoid leaving residual tumour cells, PTA must cover the tumour and normal liver parenchyma around the tumour, which will inevitably cause damage to normal liver tissue.
This study had some limitations. Firstly, the efficacy of free Dox and local PTA released on the elimination of residual tumour cells and the prevention of tumour regeneration lack validation of long-term study. Secondly, in the treatment of HCC, it is equally important to control the damage to normal liver parenchyma and enhance the anti-tumour efficacy. Further studies are needed to assess the potential hepatotoxicity of HAuNSs and PTA. In addition, the number of research animals should be appropriately increased to reduce potential errors and improve statistical efficiency.
In conclusion, the current study demonstrated that intra-arterial injection of Dox@PEG-HAuNSs combined with PTA via NIR irradiation significantly inhibited tumour growth and attenuated tumour angiogenesis in a rabbit model of VX2 liver cancer, with a significant short-term therapeutic effect. Transient liver damage was observed, in terms of safety. Dox@PEG-HAuNSs, a combination therapy of chemoembolisation and PTA, is a novel nano-therapeutic platform for liver cancer and has many clinical applications and transformation potential.
Data availability statement
The data and materials that support the results or analyses presented in my paper are freely available upon request.
Disclosure statement
No potential conflict of interest was reported by the authors
Additional information
Funding
References
- Rumgay H, Ferlay J, de Martel C, et al. Global, regional and national burden of primary liver cancer by subtype. Eur J Cancer. 2022;161:108–118.
- Ghavimi S, Apfel T, Azimi H, et al. Management and treatment of hepatocellular carcinoma with immunotherapy: a review of current and future options. J Clin Transl Hepatol. 2020;8(2):168–176.
- Nishikawa H, Kita R, Kimura T, et al. Transcatheter arterial embolic therapies for hepatocellular carcinoma: a literature review. Anticancer Res. 2014;34(12):6877–6886.
- Arizumi T, Ueshima K, Iwanishi M, et al. The overall survival of patients with hepatocellular carcinoma correlates with the newly defined time to progression after transarterial chemoembolization. Liver Cancer. 2017;6(3):227–235.
- Ben-Yosef Y, Miller A, Shapiro S, et al. Hypoxia of endothelial cells leads to MMP-2-dependent survival and death. Am J Physiol Cell Physiol. 2005;289(5):C1321–C1331.
- Gbolahan OB, Schacht MA, Beckley EW, et al. Locoregional and systemic therapy for hepatocellular carcinoma. J Gastrointest Oncol. 2017;8(2):215–228.
- Muddineti OS, Ghosh B, Biswas S. Current trends in using polymer coated gold nanoparticles for cancer therapy. Int J Pharm. 2015;484(1-2):252–267.
- Pan Y, Wu Q, Qin L, et al. Gold nanoparticles inhibit VEGF165-induced migration and tube formation of endothelial cells via the akt pathway. Biomed Res Int. 2014;2014:418624.
- You J, Zhang G, Li C. Exceptionally high payload of doxorubicin in hollow gold nanospheres for near-infrared light-triggered drug release. ACS Nano. 2010;4(2):1033–1041.
- Wu J. The enhanced permeability and retention (EPR) effect: the significance of the concept and methods to enhance its application. J Pers Med. 2021;11(8):771
- Singhana B, Slattery P, Chen A, et al. Light-activatable gold nanoshells for drug delivery applications. AAPS PharmSciTech. 2014;15(3):741–752.
- Han J, Li J, Jia W, et al. Photothermal therapy of cancer cells using novel hollow gold nanoflowers. Int J Nanomed. 2014;9:517–526.
- Tao W, Ji X, Xu X, et al. Antimonene quantum dots: Synthesis and application as near-infrared photothermal agents for effective cancer therapy. Angew Chem Int Ed Engl. 2017;56(39):11896–11900.
- Hainfeld JF, Smilowitz HM, O'Connor MJ, et al. Gold nanoparticle imaging and radiotherapy of brain tumors in mice. Nanomedicine (Lond). 2013;8(10):1601–1609.
- Gupta S, Stafford RJ, Javadi S, et al. Effects of near-infrared laser irradiation of biodegradable microspheres containing hollow gold nanospheres and paclitaxel administered intraarterially in a rabbit liver tumor model. J Vasc Interv Radiol. 2012;23(4):553–561.
- Yuan H, Li X, Tang J, et al. Local application of doxorubicin-loaded iron oxid nanoparticles and the vascular disrupting agent via the hepatic artery: chemoembolization-photothermal ablation treatment of hepatocellular carcinoma in rats. Cancer Imaging. 2019;19(1):71.
- van Breugel JMM, Geschwind J-F, Mirpour S, et al. Theranostic application of lipiodol for transarterial chemoembolization in a VX2 rabbit liver tumor model. Theranostics. 2019;9(13):3674–3686.
- Zhang LJ, Wu S, Wang M, et al. Quantitative dual energy CT measurements in rabbit VX2 liver tumors: Comparison to perfusion CT measurements and histopathological findings. Eur J Radiol. 2012;81(8):1766–1775.
- Boedtkjer E, Pedersen SF. The acidic tumor microenvironment as a driver of cancer. Annu Rev Physiol. 2020;82:103–126.
- You J, Zhang R, Zhang G, et al. Photothermal-chemotherapy with doxorubicin-loaded hollow gold nanospheres: a platform for near-infrared light-trigged drug release. J Control Release. 2012;158(2):319–328.
- Kim W, Cho SK, Shin SW, et al. Combination therapy of transarterial chemoembolization (TACE) and radiofrequency ablation (RFA) for small hepatocellular carcinoma: comparison with TACE or RFA monotherapy. Abdom Radiol (NY). 2019;44(6):2283–2292.
- El-Agawy W, El-Ganainy SA, Gad MAA, et al. Combined transarterial chemoembolization with microwave ablation versus microwave alone for treatment of medium sized hepatocellular carcinoma. Curr Cancer Drug Targets. 2022;22(1):77–85.
- Li J, Zhou M, Liu F, et al. Hepatocellular carcinoma: Intra-arterial delivery of doxorubicin-loaded hollow gold nanospheres for photothermal ablation-chemoembolization therapy in rats. Radiology. 2016;281(2):427–435.
- Abo-Elfadl MT, Gamal-Eldeen AM, Elshafey MM, et al. Photothermal therapeutic effect of PEGylated gold nano-semicubes in chemically-induced skin cancer in mice. J Photochem Photobiol B. 2016;164:21–29.
- Nault JC, Sutter O, Nahon P, et al. Percutaneous treatment of hepatocellular carcinoma: State of the art and innovations. J Hepatol. 2018;68(4):783–797.
- Barker HE, Paget JTE, Khan AA, et al. The tumour microenvironment after radiotherapy: mechanisms of resistance and recurrence. Nat Rev Cancer. 2015;15(7):409–425.
- Li W, Li X, Liu S, et al. Gold nanoparticles attenuate metastasis by tumor vasculature normalization and epithelial-mesenchymal transition inhibition. Int J Nanomed. 2017;12:3509–3520.
- Darweesh RS, Ayoub NM, Nazzal S. Gold nanoparticles and angiogenesis: molecular mechanisms and biomedical applications. Int J Nanomed. 2019;14:7643–7663.
- Rambanapasi C, Zeevaart JR, Buntting H, et al. Bioaccumulation and subchronic toxicity of 14 nm gold nanoparticles in rats. Molecules. 2016;21(6):763.
- Sukhanova A, Bozrova S, Sokolov P, et al. Dependence of nanoparticle toxicity on their physical and chemical properties. Nanoscale Res Lett. 2018;13(1):44.
