?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Purpose of present study was to develop eight formulations of chlorpheniramine (CPM) niosomes according to 23 factorial design, characterise on the basis of various evaluation tests, i.e. in vitro drug release, SEM, FTIR, TGA and release kinetics, optimise the eight formulation on the basis in vitro drug release data, formulate gel of optimised dispersion, and to perform in vivo and histopathological study using gel of optimised dispersion on rabbits. Here, N3 having low level of cholesterol and span-80 but high level of span-60(0.1:0.2:0.05) was selected as optimised dispersion of niosomes that showed highest drug release i.e. 88.25% at pH 6 over 24 h of study and followed Korsmeyers-Peppas release kinetics with Fickian diffusion mechanism. After application of statistic by Analysis of variance (ANOVA) with 3D surface plots construction, gel of optimised dispersion of CPM niosomes was formulated, and evaluated by tests for i.e. viscosity, Spreadability, Extrudibility, drug content, drug entrapment, stability, SEM, FTIR, TGA, in vitro drug release, in vivo drug release following first order kinetics and histopathological study. Niosomal gel of CPM ensured successful development using suitable combination of non-ionic surfactants, and effective loading of drug for targeted delivery of drug.
1. Introduction
Transdermal delivery system (TDDS) ensures delivery of drug through transdermal route of application that presents a unique non-invasive route of application, avoid gastrointestinal tract passage, produce controlled effect maintaining sustained concentration of drug in plasma for longer period of time, and hence produce high patient compliance [Citation1,Citation2]. It has also been observed that transdermal route of application diminishes requirement for intravenous route of application, due to lower levels of Cmax (peak plasma concentration of drug) leading to lower dose associated side effects, i.e. both systemic hepatic first pass metabolism, and pre-systemic side effects [Citation3]. Other advantages of transdermal route are to deliver not only hydrophilic drugs, but also lipophilic drugs directly into blood circulation through skin, control amount of drug applied, control the area of application, release kinetics and extended time of application [Citation4]. Transdermal route of application also have some disadvantages regarding skin irritation [Citation5], lag time of drug absorption, onset of action of drug, metabolism of drug in skin, and limited application dose [Citation6]. Other disadvantage faced by transdermal route is low rate of turnover from transdermal products due to barrier skin layer of dermis, i.e. the stratum corneum [Citation7].
To avoid these disadvantages, to perform distinctive functions various dosage forms have been developed, and used but gel of niosomes (semisolid formulations) was selected as best dosage form, to encapsulate drug due to many reasons, i.e. gels melt at body temperature being dried right after application on skin and gels are formulations that are alcoholic based semisolid, viscous, non-greasy, easily speardable, easily extrudable, emollient, water soluble and highly compatible with excipients [Citation8]. Niosomes based gel entrap drug completely from surroundings and use for allergic diseases safely.
Basic component of gel is carbomer that is polymer of acrylic acid and forms hydrogel due to hydration of carboxylic group present in structure when come in contact with aqueous or alkaline solution [Citation9]. Carbomers have widely been used during numerous previous research works [Citation10] due to their unique characteristics, i.e. optimum viscosity even at low concentration, good stability at heating, no effect on ageing, non-irritating and non-supportive for growth of bacteria or fungi [Citation11]. One of the most common polymer used for topical drug delivery system is Carbopol that is synthetic,anionic polymer derivative of carbomer, and have ability to crosslink its chains to self-assemble into microgel structure with permeation enhancement along with organic compound in gel formula, i.e. polyethylene glycol (PEG) and propylene glycol (PG) [Citation8].
Hypersensitivity developed due to allergen contact can proceed to systemic symptoms, if not treated promptly. Inflammatory reaction initiated due to histamine in response to antigen–antibody reaction after interaction and attachment of foreign antigen with antibodies, produced from activated immune cells produced localised symptoms of itching, erythema, pain, inflammation and blisters at site of contact but if not treated lead to generalised symptoms of fever, chills, sleep disturbances, allergic rhinitis, disturbed gastrointestinal motility and sleep disturbances [Citation12].
Chlorpheniramine maleate (CPM) belonging to alkyl amine class has been widely used to develop various dosage forms of topical drug delivery system due to its pharmacokinetic, physicochemical and pharmacodynamics characteristics suitable for transdermal drug delivery [Citation13]. CPM has been used for various mild to moderate acute condition of inflammation and allergy, i.e. runny nose, sunburn, urticaria, pruritus, angioedema, cough and insect bites symptoms [Citation14]. Various dosage forms have been developed using CPM i.e. syrups, tablets, capsules but their oral formulation produced side effects, i.e. dizziness, muscular weakness, gastrointestinal disturbances and mild to moderate sedation to deep sleep. CPM-based organogels based on span60/tween20 were developed as an alternative dosage form avoiding oral route associated side effects where organogel having 21% span-60, 3% tween-20, 5% menthol, 2% CPM and sunflower oil (to make 100 g) showed best key results of physical properties along with good drug release and higher anti-inflammatory effects [Citation15]. Recently, topical skin therapy has obtained greater attention to treat dermatological diseases due to targeted delivery of drug, reduced loss by systemic uptake [Citation16] with high efficacy, safety than systemic delivery, enhanced bioavailability and transporting the drug to deeper and lower layers of skin, i.e. stratum corneum through various carriers [Citation17] where most feasible drug carriers were niosomes and liposomes [Citation18]. Niosmes were preferred due to increased stability, decreased cost, ability to encapsulate drugs with variety of physico-chemical properties [Citation19], enhanced drug permeation rate, release of drug in more sustained or controlled way, and ability to create drug depot. Niosomes can be modified by controlling, ratio of hydrophilic and/or the hydrophobic moiety and hydrated to form gel (hydrogel) using carbopol [Citation20]. Niosomal gels are more useful and advantageous than traditional semisolid dosage forms due to ability to have long residence time, maintain sustained, higher concentration in skin and retain its rheological behaviour [Citation19].
The present work was designed to develop and formulate CPM niosomes, optimise the prepared niosomes and convert the optimised niosomes into gel based on low and high levels of three variables, i.e. cholesterol, span 60 and span 80. Niosomal gel of CPM was formulated to estimate feasibility of niosomal gel for topical delivery of CPM in rabbit model.
2. Materials and methods
Chlorpheniramine maleate (CPM) 99.99% purity (gifted by Pfizer Pvt. Ltd, Multan), Cholesterol (Merck, Germany), Propylene glycol (PG), Polyethylene glycol (PEG-1000) (Fluka, Germany), Methanol (HPLC grade), Carbopol-940 (polymer, China), dichloromethane (Merck), PBS (pH 7.4), Double Distilled Water (Distillation Plant in Pharmacy Department, BahauddinZakariya University, Multan). All reagents and chemicals of analytical grade were purchased and used during this research work.
2.1. Formulation of chlorpheniramine maleate loaded gel of niosomes
Design of experiment for preparation of CPM niosomes was 23 factorial design (). A 23 full-factorial design was used to optimise the CPM noisomal gel by assessing the effect of independent variables (concentration of cholesterol, concentration of span-60 and span-80) at two levels of low and high on dependent variables (percentage yield, drug content, drug loading, entrapment efficiency, size of vesicles, thermal stability of gel, in vitro drug release and anti-inflammatory effect). The physical characteristics of the prepared CPM loaded niosomal gel formulations were carried out by determining percentage yield, drug content, drug loading, entrapment efficiency, size of vesicles, stability of gel, in vitro drug release, release kinetics and anti-inflammatory effect [Citation21].
Table 1. Experimental plan for niosomal dispersion formulation: (a) Three factorial design 23, and (b) Coded level translation in actual units.
Niosomal gel loaded with CPM was formulated using optimised dispersion of CPM-loaded niosomes.
Eight formulations of niosomes were prepared by modified method of ether injection introduced by Deamer and Bhangham in 1976.
Proper quantities (translated according to 23 three factorial design of experiment as in ) of cholesterol, span-60 and span-80 as mentioned in were dissolved in dichloromethane that resulted in organic phase.
Table 2. Composition of CPM loaded niosomes displaying amount (g) of all factors used in formula to make 100 g niosomal dispersion formulation.
This organic phase was injected slowly at rate of 0.25 ml/mint through 14-gauge needle into PBS having CPM swirling at 500 rpm with thermostat temperature 55–65 °C and pH 7.4 resulting in formation of niosomes loaded with CPM.
Niosomes were formulated due to difference in temperature between organic and phosphate buffer saline phase [Citation22].
After formulation of dispersion and optimisation of dispersion of niosomes, gel of optimised niosomal dispersion was prepared. First of all, 2% Carbopol-934 [Citation23] 2.5 g was dissolved in PG 25 g to form viscous solution and PEG 2 g was dissolved in methanol (15 ml almost) to make thin solution with addition of N3 (optimised niosomal dispersion of CPM) 50 g (give 10 mg/g of gel) in it. Thin solution was added to viscous solution at once with continuous stirring at 55 °C to form CPM loaded niosomal gel of pH 5.5–6 adjusted by dropwise Triethanolamine (TEA) under continuous stirring at temperature 55 °C to get homogenous gel and finally stored in collapsible tubes for further study [Citation24].
Concentration of drug CPM used in this niosomal gel formulation is 2 g (2000 mg) per 100 g of gel, larger as compared to total components in order to deliver 15–20 mg of CPM from single application of CPM loaded niosomal gel, i.e. 0.1 g delivers 20 mg of CPM that is standard dose of drug for therapeutic effect i.e. anti-inflammatory effect on allergic skin.
2.2. Physicochemical properties of CPM
The values of physicochemical properties of CPM are stated here: partition coefficient for CPM 7.1, Log P 0.85, pH 4–5 and pKa 9.2, while BCS class is Ι [Citation25].
2.3. Physical examination
The optimised niosomalgel of CPM was examined physically to determine its physical state, uniformity, consistency, phase separation, colour, texture, presence of lumps and homogeneity by pressing small quantity of gel between index finger and thumb.
2.4. Homogeneity
Optimised niosomal gel was examined for homogeneity by placing in glass container to determine aggregate or lump [Citation25].
2.5. pH determination
Average pH (n = 3) of formulated dispersions of niosomes of CPM and niosomal gel of Optimised CPM niosomal Dispersion at room temperature 25 ± 0.5 °C by pH metre (Digital, WTW, pH 526 Germany) with probe calibration each time before use and pH adjustment up to 5.5–6 pH with Triethanolamine (TEA) [Citation26].
2.6. Viscosity
Viscosity of optimised niosomal gel of CPM was determined by rotational digital viscometer using N4 spindle at 12 rpm and room temperature [Citation26].
2.7. Spreadability
Spreadability explains the coming out behaviour of gel from collapsible tube. Spreadability of niosomal gel of CPM was determined using a wooden block apparatus (introduced by Multimer et al.) consisting of fixed glass slide of 7.5 cm length on one end and movable on other end that was tied to weight pan that was rolling on pulley set horizontally with fixed glass slide. To perform test about 1 g of niosomal gel was placed between glass slides, 1 kg weight was applied on slide for five minutes, then 60 g weight was added to pan and two glass slides were separated (time in seconds was noted). Spreadability was determined by formula:
where S=spreadability in g cm/s, m= weight tied upper slide, t= time in seconds l= length of glass slide (7.5 cm) and w= weight tied to upper slide was (60 g). The process was repeated three times to get average value of Spreadability (n = 3) [Citation27].
2.8. Extrudibility
Extrudibility test is simple test that determines flow ability and measures force required to extrude the gel from aluminium tube using hardness tester. Extrudibility test was performed by filling 5 g niosomal gel in lacquered aluminium collapsible tube, adjusting plunger to hold tube with application of pressure of 1 kg/cm2 for 30 s on tube that would extrude ribbon of study gel and calculating amount of extruded gel by calculating pressure in grams. Procedure was repeated three times to get average value with standard deviation (n = 3) [Citation26].
where Eb=extrudability, Wt = applied weight to extrude gel from tube (in g), D=area (in cm2).
Formula used to calculate extrudability was as stated below [Citation28]:
where Eb = extrudability, W= Weight applied on tube to extrude gel from tube and D= Area (cm2).
2.9. Partition coefficient (KO/PB system) studies
Pinch of CPM was added in 5 ml of n-octanol and phosphate buffer and mixedby vigorous shaking in separating funnel that was allowed to stand in vertical position in stand for 24 h after which solvent was centrifuged for half hour or less at 2000 rpm and analysed at 265 nm by UV spectrophotometer [Citation29]. Following formula was used (n = 3) to calculate KO/PB:
Partition coefficient of the drug (KP) =
3. Chemical examination
3.1. Determination of percentage yield
Percentage yield of all niosomal dispersions and optimised gel of CPM was calculated by dividing measured weight of formulations with formula weight and multiplying with 100 [Citation26] by following formula:
3.2. Determination of percentage drug content and percentage drug loading
100 mg or 0.1 g of CPM niosomes or optimised CPM niosomal gel was sealed in cellophane membrane soaked in conical flask of water for 24 h with subsequent withdrawal of 1 ml sample to check absorbance then concentration in µg/ml using UV spectrophotometer at 265 nm and formulas below [Citation26]:
Formula stated below determines capacity of niosomal gel/dispersion to load CPM [Citation30].
3.3. Drug content uniformity
Content uniformity was determined by repeating process three times to take three samples (n = 3) from top, middle and bottom points of glass container of niosomal gel [Citation31].
3.4. Drug entrapment efficiency of CPM niosomal gel
Free drug is always present in niosomal formulation of CPM as no dispersion/gel has entrapped total drug. To separate free drug 0.1 g of niosomal gel of CPM was hydrated with 10 ml PBS and sonicated in bath sonicator for 10 min. Dispersion of CPM niosomes was centrifuged at 10,000 rpm at 25 ± 0.1 °C for half hour and produced supernatant solution that was filtered to separate free drug. Supernatant solution was analysed at 265 nm by UV spectrophotometer [Citation21]. Results were used to estimate EE (%) by using formula:
where Ct = concentration of total drug and Cf = Concentration of free drug.
3.5. Fourier Transform infrared spectroscopy (FTIR)
FTIR spectrophotometer (Perkin Elmer-spectrum RX-I, Lamba USA), based on KBr disc method under hydraulic press by applying 600 kg/cm2 pressure was used to obtain spectra of formulated CPM niosomes, CPM niosomal gel and components of niosomes, i.e. cholesterol, span-60, span-80 and CPM individually. FTIR was used to investigate drug-ingredients interaction, compatibility and structural features of samples [Citation32].
3.6. Thermal gravimetric analysis
TGA was performed to estimate physical properties, compatibility between drug and ingredients of formula of optimised niosomal dispersion, and gel by placing sample of gel 6.285 mg in aluminium pan under pressure of Nitrogen gas at rate 20.0 ml/min with heating at 40–400 °C Perkin-Elmer thermal analyser. Heating under Nitrogen atmosphere produced sharp thermogram peaks due to initiation of reaction in pan [Citation26].
3.7. Scanning electron microscopy (SEM)
Surface morphology, size, texture of vesicles of optimised niosomal dispersion of CPM, and optimised niosomal gel of CPM were determined by Scanning electron microscopy (SEM Hitachi S-3600 N Japan), using platinum sputter technique. SEM photographs of niosomal dispersion (N3) were obtained [Citation21].
3.8. Stability studies and drug leakage study
Stability study was conducted to evaluate drug leakage, drug content, encapsulation efficiency and other physical behaviour parameters of optimised niosomal gel of CPM. After removal of free CPM niosomal dispersion was sealed in 10 ml sealed glass vial and niosomal gel was sealed in 10 collapsible aluminium tubes (n = 3), and kept at three different temperatures, i.e. refrigeration temperature (2–8 °C ± 2 °C), ambient temperature (20 °C ± 2 °C), elevated temperature (40 °C ± 2 °C) and relative humidity 75 ± 5% for period of 3 months in stability chamber. Sample of 0.5 g from each was taken at preset time interval, i.e. 1st day, 7th day, 2nd weeks, 1st month, 2nd month and 3rd month and analysed for free drug content. Drug leakage was determined by comparing drug content of niosomal formulations before and after storage. Procedure was followed by centrifuging sample at 20,000 rpm for half hour to separate leaked CPM and get supernatant (having free drug) evaluated spectrophotometrically at 265 nm by using formula:
where a = the amount of CPM measured in the supernatant (g); b = the initial amount of CPM entrapped in niosomes (g).
Samples of formulations were inspected visually to determine physical stability parameters and evaluated for amount of free and entrapped drug to determine chemical stability [Citation20].
3.9. In vitro drug release study of niosomal gel of CPM
Dissolution profile of all niosomal dispersions of CPM (N1-N8) and optimised niosomal gel of CPM was determined using USP apparatus Type II (paddle procedure) model no. D15/68 (Digital instrument) having PBS pH 6 at 37.0 ± 0.5 °C with speed 59 rpm [Citation33].
Drug release from CPM niosomal dispersion/optimised niosomal gel of CPM was determined by mounting Dialysis membrane (width 29.31 mm and average diameter 17.5 mm) in the USP apparatus type II (paddle). Dialysis membrane was soaked in freshly prepared pure boiling distilled water for 12–24 h. Temperature of dissolution medium outside glass dissolution bowl was maintained at 37 ± 1 °C and speed of paddle rotation was set at 100 rpm to rotate in dissolution medium of glass dissolution bowl (500 ml at pH 6). Niosomal dispersions and Optimised niosomal gel of CPM were sealed in dialysis membrane bag and attached to paddles. After starting apparatus 5 ml samples were taken at preset interval by replacement with 5 ml PBS (temperature maintained at 37 ± 1 °C) to maintain constant volume of dissolution medium and analysed after dilution at 265 nm by UV spectrophotometer by repeating process in triplicate n = 3 [Citation28]. Drug release (%) was calculated by using following calibration curve equation for CPM, i.e.
where y=absorbance, m=slope, x=concentration and b=intercept. Whereas, the value of R2 (Regression coefficient) was 0.9964 (values as per linearity curve of CPM).
3.10. Drug release kinetics study
Release kinetics were determined by using MS Excel 2010 with added DD Solver program and applying release models, i.e. First order, Zero order, Hixon-Crowell, Higuchi and Korsmeyer-Peppas models on drug release % data of CPM niosomal dispersions. All models were applied individually to determine best fit model followed by niosomes of CPM. Value of R2 (Regression analysis coefficient) of five models was used to determine accuracy of best fit model and its value should be near to 1.00 8]. Value of Akaike Information Criterion (A/C) of five models was used to determine validation of best fit model [Citation34]. Value of “n” was used to determine mechanism of drug release, i.e. if n = 0.45 mechanism is Fickian Diffusion, n lower than 0.45 but greater than 0.89 mechanism is Non-Fickian Diffusion: Anomalous, and if n greater than 0.89 mechanism is Erosion [Citation28].
3.11. Optimisation of formulation
Design expert 7.0.0 was used to apply factorial design on in vitro drug release data to optimise niosomal formulation of CPM that was further used for development and evaluation of optimised niosomal gel of CPM [Citation22].
4. In vivo study
4.1. Skin irritation study
Sample of about 1.00 g niosomal gel of CPM was applied in triplicate on back of hand and forearm skin (3in2) of five human volunteers and graded for any type of erythema and edoema for 3 h using Van-Abbe Dermal Scoring Criteria who stated that values between 0 and 9 indicate that formulation applied is not an irritant to skin [Citation26].
4.2. In vivo anti-inflammatory study
After approval from the Ethical Committee of Faculty of Pharmacy, B.Z. University Multan for using rabbits for ex vivo, in vivo and histopathological studies. Six healthy rabbits of 2.5 ± 0.19 kg weight were selected by assuring that selected rabbits have not been used for any laboratory experimental purpose for last 15 days, and not taken any medication for last six hours before starting study. Straight, broad and clearly seen skin area (10 cm2) of dorsal side of rabbit above belly was selected, shaved and rubbed gently with green chilli (Capsicum annum) for 1–2 min to induce inflammatory response. Optimised niosomal gel of CPM was applied with custom made applicator on shaved area for 2 h, i.e. 0.05 g of Niosomal gel (having CPM dose according to human dose, i.e. 10 mg) on 1 cm2 dorsal skin area. Inhibition of capsicum induced inflammatory response was used as measure of in-vivo anti-inflammatory activity of CPM in form of its niosomal gel.
4.3. Pharmacokinetic (bioavailability) study
In vivo studies of optimised niosomal gel for pharmacokinetic study were performed to determined drug uptake, clearance and release using rabbit model [Citation35]. Blood samples (5 cc) from marginal ear vein were withdrawn at preset time points, i.e. 0, 0.25, 0.5, 0.75, 1.0, 1.5, 2, 2.5, 3 and 4 h after application of gel on rabbit dorsal allergic skin (triplicate n = 3), and centrifuged for 25 min at 4000 rpm to obtain serum containing CPM (protein bound) which was stored at −20 °C until analysed. Serum was treated with de-proteinizer, i.e. methanol (volume equal to serum volume), centrifuged for 25 min at 6000 rpm to separate protein, and was analysed by UV-spectrophotometer at 265 nm to determine concentration of drug in serum at different time intervals [Citation21].
Pharmacokinetic model by PK solver (Version 2) was applied to determine bioavailability by calculating various pharmacokinetic parameters of niosomal gel of CPM using Microsoft Excel, Windows Professional XP Version 2013 along with calculation of mean, and standard deviation, i.e. time to reach peak serum concentration (tmax), Peak serum concentration (Cmax), half-life (tmax), Volume of distribution (Vd), Clearance(Cl) and Area under the plasma concentration time curve (AUC0-t and AUC0-α) [Citation36].
4.4. Histopathological study
Histopathological method using histological techniques and optical microscope was used to analyse numerous capsicum induced histopathological alterations in dorsal side pre, and post (treatment) skin samples of rabbit. Procedure was performed sequentially, i.e. rabbit skin tissues from normal, capsicum treated and gel treated areas were selected, excised and used to prepare slides stained with H&E dye (haematoxylin and Eosin) to locate, identify and demonstrate histopathological changes clearly. These slides were viewed under high powers of optical microscope to obtain photo-microscopic images of all slides to visualise whole skin tissue, i.e. Epidermis, Lamina propria, loose connective tissues, all type cells, all glands, secretory vesicles, vascular tissues, basal layers, and to confirm all histopathological alterations for detailed comparison of slides of various skin tissues. Procedure was found to be similar to procedure used by Bozzatto (2013), and also in accordance with histopathological method used by Javed (2018) to see histopathological alterations in goat nasal membrane [Citation26].
To perform histopathological studies 1 day after application of optimised CPM niosomal gel rabbits were biopsied, biopsies of normal rabbit skin tissue, capsicum treated rabbit skin tissue, and niosomal gel treated rabbit skin tissue were collected for histopathological studies [Citation37]. Images were taken to capture, demonstrate all incidents regarding capsicum induced inflammatory, vascular, and histological alterations from their first appearance to treatment of symptoms. Green chilli (Capsicum annum) was used as main allergen that damaged rabbit skin tissue mechanically with induction of inflammatory responses on site of application.
4.5. Statistical analysis
To develop, niosomes and niosomes based gel of CPM, Microsoft Excel 2013 was used to estimate values of mean and standard deviation with application of Analysis of variance (ANOVA) (p < 0.05, Regression analysis to evaluate difference, and impact of various levels of cholesterol, span-60 and span-80 (independent variables) on drug release (dependent variable) [Citation38].
5. Results and discussion
It was observed from current study that the independent variables (concentration of cholesterol, concentration of span-60 and span-80) greatly influenced the dependent variables (percentage yield, drug content, drug loading, entrapment efficiency, size of vesicles, thermal stability of gel, in vitro drug release and anti-inflammatory effect). A significant difference in all dependent variables was noticed using prepared CPM loaded niosomal gels, which in turn influenced the CPM release from gel.
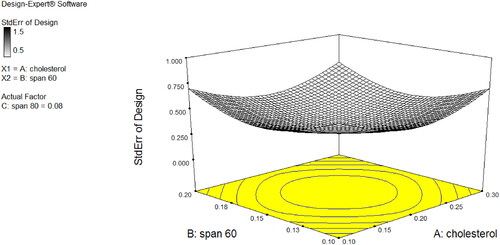
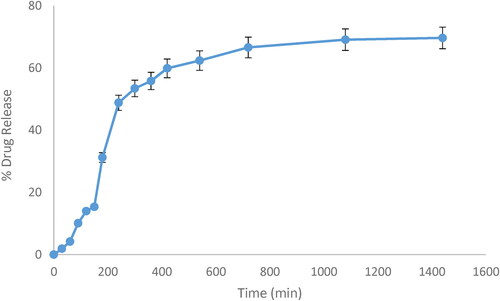
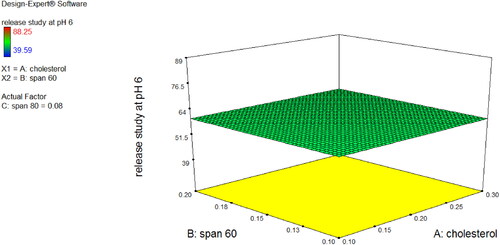
Niosomal dispersions of CPM were very light pale white (milky) in colour, clear with no particular odour, and uniform in appearance. The percentage yield of CPM niosomes was in a range of 95.04–98.51% while EE % results ranged between 87 and 96% (N1 = 94, N2 = 87, N3 = 96, N4 = 90, N5 = 95, N6 = 89, N7 = 96 and N8 = 91) that indicated its direct relation with amount of cholesterol and surfactant. In vitro drug release study of 24 h was performed for CPM Niosomal formulation (N1-8). All the formulations showed continuous and sustained release behaviour while selected formulation (N3) showed more controlled but slowly enhancing release of CPM ∼ 40–50% at 4th hour of study at pH 6 and at 24th hour study design with ∼12% amount of drug remaining in niosomes for further hours after 24 h of application. As shown in , it was clear from 3D surface graph that by decreasing amount of cholesterol drug release increased in all formulations of Niosomes and by increasing amount of cholesterol drug release was decreased. In case of span-60, by decreasing amount of span-60, drug release was decreased but when amount of span-60 was increased drug release was also increased. While in case of span-80, when amount of span-80 was decreased drug release was decreased, and when amount of span-80 was increased drug release was also increased.
Figure 1a. (a) Optimised Niosomes (N3) 3D Surface plot of % Drug release response at y = pH 6. (b) Optimised Niosomes N3 interaction diagram.
5.1. Physical examination of niosomal dispersion of CPM
The visual inspection revealed that CPM niosomal dispersions were clear, transparent, and showed good homogeneity with absence of lumps, aggregates or precipitates. The pH of CPM niosomes was in range of 5.1 ± 0.1 to 5.8 ± 0.1 that in range of skin pH probably producing, no skin irritation, and suitable for dermatological use. The particle size analysis ranged between 0.3 and 5.0 micrometer.
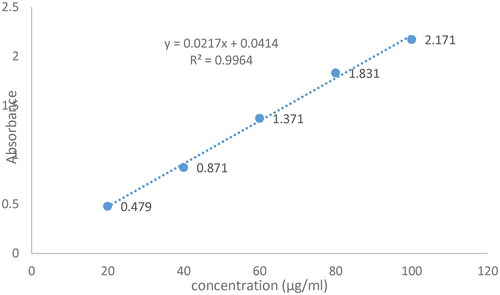
5.2. Calibration curve of CPM
CPM Linear regression curve displayed in was produced by plotting absorbance at 265 nm against concentrations (µg/ml). The acquired linear regression equation was y = 0.0217x + 0.0414, where R2 (regression coefficient) was 0.9964.
5.3. Solubility studies
The solubility of CPM was 681.51 ± 0.01 mg/ml in water, 731.51 ± 0.01 mg/ml in PBS and 542.15 ± 0.01 mg/ml in methanol, declaring that CPM was freely soluble in these solvents in an order of PBS > Water > Methanol. These values were in accordance with the previous study values [Citation29], who studied microsponge based CPM gel where CPM solubility was as given here: PBS (810.52 ± 3.8 mg/mL) > water (687.58 ± 2.9 mg/mL) > methanol (547.98 ± 2.3 mg/mL).
5.4. Partition coefficient (K O/PB) studies
The partition coefficient of CPM was 6.99 with logarithmic value (log P) equals 0.850 demonstrating good hydrophobicity of CPM, to formulate its topical dosage form. The values were in accordance with the previous where CPM partition coefficient was 0.851 (log p = 7.1) during the study of CPM transdermal patch [Citation39].
5.5. Fourier transform infrared spectroscopy (FTIR)
FTIR spectra of niosomal gel of CPM and its main components (cholesterol, span-60, span-80 and carbopol-934) were recorded and shown in . CPM spectra showed a characteristic peak at 1431.09 and 1089.55/cm due to C–H group and phenyl group for maleate salts, respectively. Cholesterol spectra showed characteristics and broad peaks at 2901.78, 2961.51, 3299.92, 2846.23 and 1790.24/cm due to strong aromatic stretching of CH = CH, acetyl groups, hydroxyl group, symmetric –CH3 and vinyl group, respectively. In addition, span-60 spectra showed sharp, and broad peaks at 2916.22, 1735.58 and 2849.13/cm due to strong aromatic –CH3 group, strong C = O ester bond and hydroxyl OH group, respectively and small peaks in range of 1000–1100/cm were due to aliphatic structure. Moreover, span-80 spectra showed characteristic peaks at 2922.48, 1738.97 and 1457.64/cm were due to –OH group, 5 membered ring and –CH3, respectively [Citation40]. Furthermore, carbopol-934 spectra showed broad peaks at 2916.47 (in range of 3000–3800/cm) due to amine group.
Figure 3. FTIR peaks of a = drug (CPM), b = cholesterol, c = span-60, d = span-80, e = PEG-1000, f = PG, g = carbopol-940, and h = the optimised niosomal gel of CPM (N3).
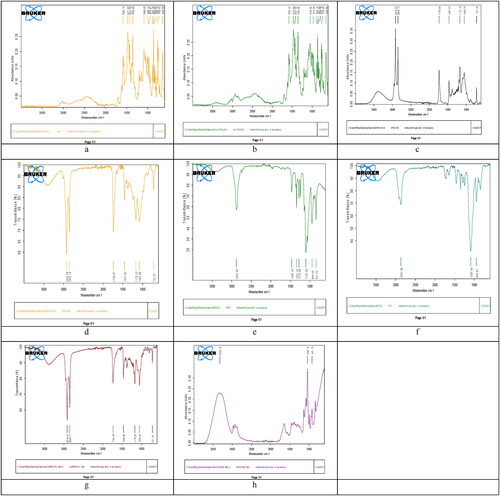
FTIR of CPM niosomal gel showed sharp peaks at 1040.18/cm due to phenyl group that were sharper in FTIR of niosomal gel of CPM than in FTIR of Carbopol 934 alone due to coordination of linkages in niosomal gel, while the broad peaks at 921.73/cm were due to –OH group, and were more sharp in spectrum of niosomal gel of CPM. Furthermore, FTIR of niosomal gel of CPM showed very slight shifting of peaks and smoothening of peaks indicating strong physical interaction between CPM, and its components responsible for formation of niosomal gel. No new peaks were obtained that indicated no interaction between CPM and other components. No major shifting of peak was seen that ensured no chemical interaction and good compatibility between formula components leading to conclusion that CPM niosomes, can be successfully incorporated into gel having span-60, span-80, cholesterol, PG, PEG and Carbopol-934. Hence FTIR study has shown that pure CPM, cholesterol, PEG, PG, Carbopol 934, Span-60, and span-80 showed no significant difference among peaks alone or in combination ensuring their good activity in final formulation without any chemical interaction [Citation41].
5.6. Analysis of 23 factorial design
Analysis of 23 Factorial Design Based on 23 full-factorial design studies, it was observed that the concentration of cholesterol, san-60 and span-80 have significant effect on percentage yield, drug content, drug loading, entrapment efficiency, size of vesicles, thermal stability of gel, in vitro drug release, and anti-inflammatory effect. These results support the selection of independent variables, in the current CPM loaded niosomal gel study, and explained under concerned variable.
5.7. Effect of independent variables on percentage yield
The percentage yield of the optimised CPM niosomal gel was 98.88 ± 0.01 (n = 3) as found by using formula (N3 at A −, B + and C − levels), indicating that percentage yield of niosomal gel was high, when low concentration of Cholesterol was used with high concentration of non-ionic surfactant span-60, and low concentration of span-80 keeping concentration of Carbopol-934 constant.
5.8. Effect of independent variables on percentage drug content and percentage drug loading
CPM content ranged from 96.88 ± 0.02 to 98.78 ± 0.02 after 24 h and 94.96 ± 0.02 to 96.77 ± 0.02 after 90 days (within required range for CPM, i.e. 93–107%), with percentage loading of CPM niosomes was 0.94 ± 0.01 to 0.97 ± 0.01 after 24 h, and 0.969 ± 0.01 to 0.987 ± 0.01 after 90 days. These values demonstrated high drug content and loading values of CPM niosomal gel were due to, optimum formula having high amount of span-60, and low amount of cholesterol and remained stable at 2–8 °C, and ambient temperature up to 90 days showing little changes at high temperature. These readings were found almost similar to that of study of [Citation21], i.e. 97% of acyclovir content was remained at end of 3 months. According to previous reports, it was stated that nature of drug entrapped in niosomal vesicles affect its percentage drug loading and tendency of drug to interact with lipid bilayer by various forces for example, non-polar, polar and/or electrostatic forces decide its loading in aqueous, or lipid bilayer portion of niosomes [Citation42]. During this study work it was suggested that CPM was incorporated into aqueous compartment of niosomes, and showed maximum entrapment with span-60 (96.55%±0.02) [Citation26].
5.9. Effect of independent variables on drug entrapment efficiency of niosomal gel of CPM
The entrapment efficiency % of optimised niosomal gel was 97.62 ± 0.37%, and the reason for this high value of entrapment efficiency was optimum concentration of cholesterol: span-60: span-80 (in ratios 0.1:0.2:0.05), because various studies reported direct relationship of Concentration of cholesterol, and non-ionic surfactant with entrapment efficiency of niosomal vesicles. During this study, it was observed that on increase in concentration of cholesterol from 0.5 to 1% entrapment efficiency was increased, but by increasing concentration from 1% to 1.5 or 2% entrapment, efficiency was decreased due to reason that increase in cholesterol increased hydrophobicity, rigidity, and stability of lipid bilayer of vesicles membrane, leading to low permeability, and entrapment that were similar previous findings [Citation43]. The entrapment efficiency of CPM niosomes of gel was also found in accordance with that of aceclofenac niosomes by Mishra in 2014, who reported that when concentration of cholesterol was low (upto optimum level) with constant level of Non-ionic surfactant, the entrapment efficiency was high. Reason of this high entrapment efficiency with low amount of cholesterol could be the low characteristic effects produced by cholesterol, i.e. less rigidity, less cementing effect, decreased micro viscosity of lipid bilayer leading to high leaking spaces in lipid bilayer of niosomes, and increased chances of uptake of CPM in vesicles of niosomes of gel. This phenomenon was observed at low concentration of cholesterol upto particular level beyond which, membrane loses its integrity leading to disruption, and breakage of vesicles. Another study, confirming values of this study was, reports from Korchowiec (2006) stating that optimum concentration of cholesterol provided sufficient rigidity to stabilise niosomes with reduced leaky effect, leading to reduced efflux with increased entrapment effect [Citation44]. One other reason for high concentration of cholesterol leading to low entrapment efficiency might be due to, high concentration of cholesterol formed cluster affecting integrity of vesicles, causing non uniform distribution of drug along lipid bilayer being reported by Finean [Citation45]. Various contrasting conclusion studies have been reported to study effect of cholesterol concentration on entrapment efficiency, i.e. some studies showed that cholesterol has no effect on entrapment efficiency [Citation46], some studies showed that inverse relation was present between cholesterol concentration and entrapment efficiency [Citation47], and some studies reported that direct proportional relationship was present between cholesterol concentration, and entrapment efficiency [Citation48].
Entrapment efficiency was also found to be affected by amount of non-ionic surfactants (span-60 and span-80), i.e. in this case Entrapment efficiency of Optimised niosomal gel was due to, high level of Span-60 might be attributed to high gel to liquid phase transition temperature of Span-60, and longer saturated alkyl chain length of Span-60 leading to stable niosomes of its gel and these findings were found to be in accordance with that of nimesulide-span-60 niosomes [Citation49]. Other reason for high entrapment, when concentration of non-ionic surfactant was increased, was due to reason of decreased drug leakage from vesicles [Citation50]. Optimised formula of niosomal gel of CPM of present study (cholesterol:span-60, 1:2) was also in accordance with formula optimised by Jigar (2011) during preparation of Erythromycin niosomal gel where concentration ratio of cholesterol:Noionic surfactant was 1:2 High, amount of span-60 than span-80 produced, high entrapment than with high amount of span-60 with high amount of span-80 also because span-80 alone produces high entrapment, but with span-60 its entrapment efficiency decreases, and same effect was observed ring this study of Erythromycin niosmal gel where span-80 alone produced high entrapment efficiency (82.26%), while when combine with span-20 (49.51%) or 2pan-60 (72.62%) hence, confirming optimised formula of this study [Citation47].
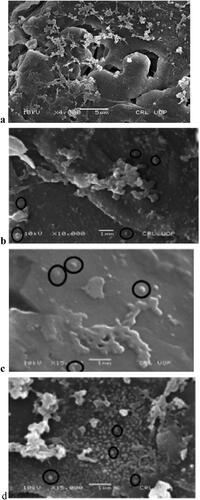
5.10. Effect of independent variables on vesicle size (scanning electron microscopy SEM)
Study of SEM of optimised niosomal gel of CPM showed (SEM images) that niosomal gel sample produced spherical shape and morphology of vesicles of size range 0.1–5µm on different magnification power of 4000, 10,000 and 15,000 as shown in . Optimum size of niosomal vesicles of its gel was produced, due to reason of having optimum concentration of span-60, and cholesterol with span-80 in formula. Critical packing parameter (CPP) of Span-60 was 0.5–1 that was considered to be responsible for formation of niosomal vesicles in its gel formulation [Citation51]. Span-60 and span-80 produced proper shaped and spherical vesicles than tween in formula because Span are water insoluble, and have high lipophilic nature leading to proper visualisation in aqueous medium, while tween is water soluble and high hydrophilic property no vesicles are formed in aqueous medium [Citation47]. High amount of Span-60 used in optimised formula have low free surface energy that reduce vesicle size, because of high hydrophobicity and low HLB 4.3 of span-60 [Citation27]. Vesicle size of niosomal vesicles of gel was in accordance with Diacerein niosomal vesicle size produced by Khan, i.e. 1 μm, Diclofenac niosomes by Jaiswal (2016), i.e. 1–6 μm, aceclofenac niosomes by Mishra (1997), i.e. 4.2–4.8 μm, and spherical shape and morphology of vesicles was similar to shape of naltrexone niosomal vesicles produced by Khan [Citation41].
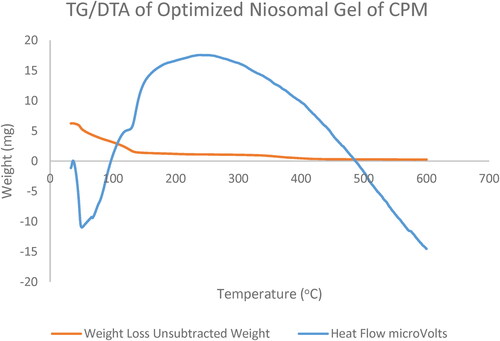
5.11. Effect of independent variables on thermal stability of CPM loaded niosomal gel (thermal gravimetric analysis)
Thermal analysis of optimised CPM niosomes (N3) shows stability of niosomal gel of CPM on different temperatures and melting point of niosomal gel (). There is a sharp single exothermic peak between 120 and 350 °C with loading temperature of 30 °C that went to 50 °C, and loading weight of niosomal gel of CPM was 6.285 mg that decreased to 0.2 mg at 410–600 °C crossing melting point of CPM (CAMEO chemicals reports). Hence it declared the niosomal gel of CPM to be more thermostable than dispersion at molecular level in accordance with reported melting point [Citation52].
6. Physical examination
6.1. Homogeneity
On physical examination it was found that CPM niosomal gel was translucent milky white to off-white in colour, without any colour intensity difference, clear without any aggregate or phase separation, equally opaque with good tendency, odourless, without any grittiness, and homogenous in appearance being similar to niosomal gel of Goyal [Citation31].
6.2. pH determination
pH of Optimised CPM niosomal gel was almost 6.06 ± 0.15 (n = 3) lying in range of pH making it suitable for topical delivery of CPM, without causing any skin irritation, and readings of pH were consistent with that of niosomal gel of Benzoyl peroxide by Goyal (2015) where pH of niosomal gel, was 6.2 ± 0.162, Meloxicam niosomal gel by Usama (2016) where pH was 6.2 ± 0.4 and readings of Kamboj (2013), where pH of mefenamic acid niosmal gel was 6.4–6.7 ± 0.12 [Citation53].
6.3. Viscosity
Viscosity of optimised niosomal gel of CPM calculated by Brookfield viscometer (Model RVTDV 11, Brookfield Engineering Laboratories, lnc, Stoughton, MA using spindle 06, and 2.5 rpm at 25 ± 1 °C was 3768 ± 0.57*102cP (n = 3) was determined. Viscosity of formulated niosomal gel of CPM was lying in desirable range providing sufficient consistency, Spreadability, and Extrudability of gel leading to its easy application in thin film with low resistance to flow (Non-Newtonian flow system) by applying small stress force, easy removal with good feeling sensation, and enhanced permeability across skin [Citation54]
6.4. Spreadability
Spreadability and Extrudability of Mefenemic acid niosomal gel by Kamboj [Citation55] also showed, good outcomes, due to optimum concentration of formula producing viscosity smaller than CPM niosomal gel of this study, hence, higher spread ability values were obtained by Kamboj and workers (2013). Values of Spread ability of optimised CPM niosomal gel obtained in triplicate was 3.91 ± 0.1 g cm/s, and showed inverse relation with viscosity as confirmed by that of Goyal [Citation31]. Optimum Spreadability with small force application was considered to be because of optimum concentration of various formula major components, and major reason was considered to be loose gel matrix of gel, due to presence of vesicles of niosomes [Citation38]. Readings were found to be similar to that of Etodolac niosomal gel [Citation50]. In fact spread ability value of niosomal gel of CPM was affected by various factors, i.e. amount of cholesterol, span-60, PEG-1000, and span-80 and showed that gel was easily spread with small shear force due to PEG-1000 in formula being in concordance with those reported by Boushra and Gosyal [Citation56]. It was already reported by Garg (2002) that PEG-1000 and PG (permeation enhancers) in formula of niosomal gel enhanced spreadability, and consistency [Citation57].
6.5. Extrudibility
Niosomal gel of CPM had optimum viscosity that allowed it to flow out from plastic collapsible tubes easily, quickly and with application of small force on tube maintaining its good consistency hence showed good Extrudibility determined as average of three values (n = 3), i.e. 0.950.03 g/cm. Values were found in accordance with values of mefenemic acid niosomal gel [Citation55].
All physical examination tests suggested good homogeneity, optimum viscosity, desirable pH, sufficient Spreadability [Citation58], and Extrudibility of formulated optimised CPM niosomal gel being safe for topical delivery of CPM
6.6. Stability studies and drug leakage study
Stability studies of optimised CPM niosomal gel performedfor three months byusing Stability chamber set at refrigerated temperature (2–8 °C), ambient temperature (20 °C), and elevated temperature (40 °C) showed no particular change in physical appearance, i.e. colour, consistency, viscosity, homogeneity, phase, pH, drug entrapment, and drug content (drug leakage) at refrigerated temperature (2–8 °C), very slight changes at ambient temperature (20 °C), and slight change at elevated temperature (40 °C).
The findings of stability study of CPM niosomal gel were summarised that suggested that gel was more stable at refrigerated conditions, less stable at ambient temperature after 2nd month, while unstable at elevated temperature after 3rd month, because its CPM content and CPM entrapment were non-significantly (p > 0.05) decreased to 95.01 and 96.56, respectively.
Increased stability of niosomal gel of CPM at refrigerated and room temperature than elevated temperature might be due to elevated temperature induced fluidity of niosomal gel lipid bilayer, leading to higher CPM leakage at elevated temperature, and stability were found parallel to the stability measured by Bhaskaran and Jousma [Citation59], during their research, where room or higher temperature enhanced drug leakage. Stability of CPM niosomal gel was higher that stability of niosomes might be, because of prevention of fusion of niosomal vesicles after incorporation of them into Carbopol 934 P based gel, and this value was found to be in accordance with values of [Citation47].
Values of this stability study were almost similar to one month stability study of Diclofenac niosomal gel by Jaiswal (2016), where initial entrapment efficiency was 89.55 ± 3.90 at 2–8 °C, 89.47 ± 3.59 at 20 °C and 89.38 ± 2.89 at 40 °C, and after 1 month entrapment efficiency was 89.38 ± 0.34 at 2–8 °C, 89.23 ± 1.04 at 20 °C and 88.98 ± 0.88 at 40 °C. These observations were in concordance with three month stability study of niosomal gel of Erythromycin by Jigar et al. [Citation47], where amount of drug retained on skin and drug release was reduced at end of 12th week at higher temperatures than at refrigerated temperature.
Stability study of optimised CPM niosomal gel (at 2–8 °C, 75 ± 5% RH) showed no change in physical appearance (milky white), pH (6.2), viscosity (3768 cP*102), Spreadability (3.81–3.91 g cm/min), homogeneity (clear), drug entrapment efficiency (97.22–97.62%), and drug content (98.12–98.78) during three months of stability study. Stability study of optimised CPM niosomal gel (at 20 °C, 75 ± 5% RH) showed mild changes in parameters, i.e. same physical appearance (milky white), mild decrease in pH (6.2–5.1), decrease in viscosity (3768 to 3710 cP*102), increase in spreadability (up to 3.99 g cm/min), homogeneity (clear till end of 1st month, and lumps during 2nd and 3rd month), decrease in drug entrapment efficiency to 97.11%, and decrease in drug content to 97.09 with the passage of time. But the Stability study of optimised CPM niosomal gel (at 40 °C, 75 ± 5% RH), showed prominent changes in parameters, i.e. light yellow appearance, decrease in pH to 4.1, decrease in viscosity to 3508 cP*102, increase in spreadability to 4.87 g cm/min, larger yellow lumps, decrease in drug entrapment efficiency to 97.11%, and decrease in drug content to 97.09 on 3rd month of stability study [Citation58].
According to this CPM niosomal gel was stable at 2–8 °C showing little less stability at 40 °C stating that best storage temperature for niosomal gel of CPM should be refrigerated temperature for maintained efficacy with reduced leakage, and decreased stability problems being in concordance with that of niosomal gel of erythromycin and diclofenac [Citation27].
In one study conducted by Nagalakshmi(2016), and his workers in 2016 to formulate herbal niosomal gel stability study showed drug content was lower, i.e. 66.52% at high temperature (25 °C ± 2 °C), than 82.28% at 4 °C ± 2 °C confirming findings of this study. The main reason for lower retention of drug (drug content) on skin at higher temperature, was because high temperature cause phase transition of surfactant leading to degeneration of polymer, lipid vesicles leakage, and poor drug retention [Citation59].
6.7. Effect of independent variables on in vitro drug release study of niosomal gel of CPM
CPM release from Optimised niosomal gel studied for 24 h, using linear regression equation Y = mx + b, and R2 (regression coefficient) at pH 6, i.e. values for CPM Y = 0.0217x + 0.0414 and R2 = 0.9964 showed that maximum drug release was, 48.81% at 4th hour, 66.69% at 12th hour, 69.08% at 18th hour, and 69.64% at 24th hour of study as shown in . Study demonstrated that drug release was rapid during first 12 h, and slow during last 24 h indicating sustained release of CPM from niosomal gel. Effect of this slow and excellent CPM release study were in concordance with effects of Herbal niosomal gel formulated, and evaluated by Nagalakshmi (2016) and his workers in 2016 who stated that release of drug from niosomal gel was 52% at 12th hours, and 85% at 24th hour of study (24 h study) [Citation59].
Drug release from Optimised niosomal gel of CPM N3 was high (i.e. 69.64%) at ratios of cholesterol:span-60:span-80 as 0.1:0.2:0.05, i.e. lower concentration of cholesterol with high amount of span-60 and low amount of span-80 because as amount of span-80 was also increased (from 0.05 to 0.1%) with increased amount of span-60 (0.2%) produced a huge amount of surfactant in aggregate (0.3%) that might act as depot and prevent drug leakage hence drug release from niosomal vesicles of gel leading to low drug release hence authenticating the optimised formula that was further validated by Salih et al. (2013) [Citation60] formulated etodolac niosomal gel drug release. In case of etodolac niosomal gel higher, i.e. 94.91% drug release was obtained at end of 1 day at 1:1 ratio of cholesterol and surfactant than drug release at 1:1.5 ratio of cholesterol and surfactant [Citation50].
Values of drug release from CPM niosomal gel (69.64%) at 24th hour of study was lower as compared to CPM simple organogel (80.3%) at even 8th hour of study [Citation15], because network of niosomal gel vesicles entrapped CPM making unavailable to surrounding gel, and CPM entrapped in niosomal vesicles leaked out from vesicles into surrounding gel slowly, and gradually than plain or organo gel of CPM being in concordance with values of Korchowiec [Citation44]. Another reason for this slow release from niosomes, was presence of cholesterol in niosomal gel formula, because cholesterol present in niosomal vesicle lipid layer limit drug mobility cause spontaneous mixing between two membrane lipids, increase stability of lipid layer, and perform membrane stabilising role [Citation53].
6.8. Drug release kinetics study
Values of five models of release kinetics applied on in vitro drug release data from niosomal gel of CPM using DD solver and M S Excel calculated, on the basis of coefficient of regression (R2) [Citation59], and Akaike Information Criterion (A/C) [Citation26] showed that R2 Value of First order kinetics was highest than that of others, i.e. 0.85 near to one suggesting the First order kinetics was the best fit release model followed by niosomal gel (in vitro), whose goodness was further validate by its low A/C 110.2 than that of others [Citation34]. Values of all release kinetics parameters, i.e. value of K (rate constants), R2, nd A/C of five release models were displayed in . The outcome of this study confirmed that optimised niosomal gel of CPM followed, the First order release kinetic model being similar to CPM microsponge based gel [Citation29].
Table 3. Drug release kinetics of optimised CPM niosomal gel (N3) at pH 6.
6.9. In vivo study
In vivo studies of skin irritation study, anti-inflammatory study, pharmacokinetic, bioavailability study, and histopathological study of CPM loaded optimised niosomal gel were narrated individually. Only optimised gel is selected for study rather than eight niosomal gels of niosomal formulations (N1–8), as previous sections of this study published earlier, as niosomal dispersion of CPM stated that N5 produced better result than all others. Hence, to avoid extra length, and irrelevant features of study only optimised gel was used. Although, the in vivo findings are not in agreement with that of in vitro results, it could be due to narrow pore size of dialysis membrane. Furthermore, it was also observed from results of current study that niosomal gel of CPM is far better than emulgel, or simple gel because it has enhanced targeting, enhanced loading due to its lipid structure similar to skin, longer residence time sustained effect from lipid-based vesicles serving as best drug depot system, and finally automatic absorption of lipid-based drug delivery system due to its biodegradable nature.
6.10. Skin irritation study
Skin irritation study of formulated niosomal gel of CPM showed, mean skin irritation score of 0.02 ± 0.01 confirming non irritancy, and safety of gel for topical use on human skin without producing any sign of irritation, i.e. redness, lesion, abrasion or erythema [Citation61]. The effects were in accordance with those of Benzoyl peroxide niosomal gel skin irritation test having score of 1.13 ± 0.21 being safe for skin use [Citation31].
6.11. Effect of independent variables on anti-inflammatory studies
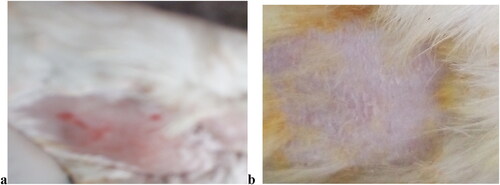
In vivo studies of CPM niosomal gel was conducted by applying gel sample on dorsal side skin area (10 cm2) of six rabbits (2.5 ± 0.19 kg) (n = 6) to observe inflammatory effect of capsicum, and anti-inflammatory effect of CPM. After washing, drying, shaving and cleaning rabbit skin area was labelled as Normal skin area, and capsicum treated skin area where normal skin area was area not having capsicum induced allergy or niosomal gel treatment but, used to study histology of normal rabbit skin and to compare it with inflamed skin area for observation of histopathological changes, and Capsicum treated area was the area treated with allergen (green chilli: Capsicum annum) to study, histopathological changes induced by capsicum treated allergy. Procedure was followed by rubbing half cut portion of fresh green chilli (Capsicum annum), gently on selected area for 2 min that induced topical allergic or inflammatory reaction by producing red small round spots or patches along with itching, shivering, rashes, redness, and inflammation etc. After induction of symptoms half of capsicum treated area, was kept untreated with gel, and used as control to observe capsicum induced histopathological changes while remaining half capsicum treated area was treated with 0.05 g of niosomal gel of CPM (10 mg dose of drug CPM) to observe effect of treatment rapidly after 61 min of application of niosomal gel of CPM as shown in .
6.12. Pharmacokinetic (bioavailability) study
The pharmacokinetic parameters of CPM niosomal gel by using rabbit as experimental model are shown in .
Table 4. Pharmacokinetic parameters of CPM (10.00 mg) (niosomal gel) applied topically on allergenic rabbits.
Values were in concordance with pharmacokinetic of CPM muco-adhesive buccal patch Sekhar (2008), i.e. for oral and buccal routes Cmax (ng/ml) 5.73 ± 1.08, and 6.16 ± 0.99, Tmax (h) 2.17 ± 0.41, and 3.33 ± 0.82 and AUC0 − n (ng h/ml) 57.85 ± 15.50 and 84.99 ± 17.96, respectively demonstrating that Cmax of niosomal gel was higher with lower Tmax. These readings suggested that niosomal gel was more fast and efficient drug delivery system than buccal or oral patch of CPM, and topical route of CPM niosomal gel application was best route for enhanced absorption, and bioavailability due to high bioavailability of CPM niosomal gel than both buccal, and oral CPM patch where low bioavailability might be due to route of application and complications associated with patch dosage form (buccal/oral). Another reason for high pharmacokinetic parameters of CPM niosomal gel, might be suggested that CPM enclosed in lipid directly was directly applied to skin, where these vesicles having lipid structure, and composition similar to that of human bi-layered lipid membrane diffuse and permeate easily through membrane without any hindrance hence showing enhanced bioavailability than buccal CPM patch, and oral CPM tablets where CPM was embedded in matrix polymer causing delayed CPM release, and low bioavailability and CPM was granulated in oral tablet facing first pass effects of oral route, and reducing its bioavailability to only 25–45%, respectively [Citation36].
In vivo CPM release study showed that cumulative drug released during 1st hour of application being highest at 0.75th hour (43.76 ± 0.21), and decreased slowly and continuously during late hours showing sustained drug release pattern. During in vivo study of four hours, duration it was noticed that maximum drug was released during first hour, and decreased during later being in concordance with previous study reports of CPM sustained release tablet by Masumoto and his colleagues (1974) where, maximum drug amount released was, i.e. Cmax (ng/ml) 10.71 ± 0.89. It was also noticed that mean peak plasma concentration from in vivo CPM niosomal gel was higher than its in vitro values, and mean time to reach peak plasma concentration of in vivo was lower than in vitro being in agreement with great difference lying in mean peak plasma concentration, and time obtained from in vitro than from in vivo with area under curve (AUC) value being same ensuring no decrease in bioavailability from sustained release dosage forms [Citation62].
6.13. Histopathological studies
Histological examination of capsicum treated skin tissue revealed major changes, and phenomenon of histological examination of capsicum treated rabbit skin tissue demonstrated that green chilli induced inflammatory process causing, histopathological alterations in skin hence, acted as a strong irritant due to its major chemical ingredient, i.e. capsaicin that caused dose dependent hypersensitivity in rabbit skin, because higher the amount of capsaicin greater the histopathological, and morphophysiological damage produced in skin. These changes were found in accordance with those of Bozzatto [Citation63]. Capsicum treated skin also showed some vascular changes along with stated histological changes, i.e. some blood vessel haemorrhages in few portion of skin tissue. These all changes were also observed by Germuth (1953), during study of hypersensitivity induced responses resulting in epitheloid, and giant cell reactions in skin tissue [Citation64]. Observations of histological examination of CPM niosomal gel treated area of rabbit skin tissue demonstrated, reduced inflammatory process along with reduced necrosis, reduced tissue disorganisation, reduced haemorrhages, and increased cell infiltration, improved epithelialization, and better tissue organisation, and enhanced tissue strength being similar to observations of Oryan and Zakar [Citation37].
6.14. Histopathological study of normal rabbit skin
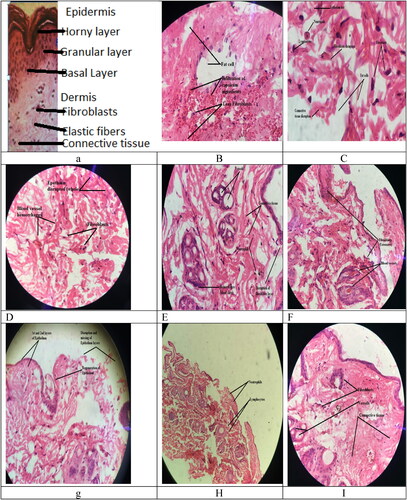
Normal rabbit skin after selection of appropriate portion, staining with haematoxylin, and eosin (H&E), and visualising under low, and high magnification powers (i.e. 10× and 40×) of optical microscope displayed prominent histopathological alterations () showing regular and symmetrical arrangement of all layers and cells. Histopathological study of normal skin showed, symmetrical and smooth surfaced lamina propria with symmetrical collagen fibres, and glands along with their normal secretions of eosinophilic leukocytes, lymphocytes, macrophages, mast cells, plasma cells, and fibroblasts. Fibroblasts and collagen fibres were found to make a regular network of loose connective tissue covering lamina propria, and performing function by mechanism of diffusion through capillaries to supply nutrients, and oxygen to the cells and to remove carbon dioxide, and water from cell being in accordance with findings of normal skin histology by Javed [Citation26].
6.15. Histopathological study of treated rabbit skin tissue
Two portions of excised rabbit skin, mainly capsicum treated skin tissue and CPM niosomal gel treated skin, were selected, stained with H&E dye, and visualised using high magnification power (40×) of optical microscope.
Photo-microscopy of capsicum treated skin after induction of allergy and initiation of inflammatory reaction showed, major histopathological changes as displayed in –e, i.e. lamina propria showed clear surface changes, cells, and glands became asymmetrically arranged, blood vessels became wide spaced, fibroblasts increased to huge number, inflammatory cells increased in numbers, and made clusters called Inflamosomes, collagen fibres became roughly connected to Inflamosomes, and loosely packed connective tissue un evenly covered Inflamosomes. Photo-microscopic images also showed, various other major visible changes beside these alterations, i.e. disruption and dissolution of whole or part of epithelium called de-epithelialization, lamina propria composition modulation, appearance of hypersensitivity cells mainly the neutrophils and lymphocytes in lamina propria, rupturing of blood capillaries leading to accumulation of blood, and clear haemorrhages, and rupturing, dissolution and accumulation of fibroblasts called disruption of fibroblasts. These major alterations produced after induction of allergy with capsicum confirmed the capsicum (mainly its capsaicin) to be very strong irritant, and lethal allergen because it dissolved epithelium, ruptured fibroblast, and even blood vessels.
Photo-microscopy of CPM niosomal gel treated skin slides after application of gel, and initiation of anti-inflammatory reaction showed, major histological alterations as displayed in i, i.e. regeneration of epithelium where only 1-2 layers were regenerated while, remaining layers were missing, somewhere total disruption of epithelium, and lamina propria with reduced number of lymphocytes in lamina propria, and moderate fibroblasts indicating treatment of allergenic symptoms by applying anti-allergic gel application. Vascular changes were much reduced, i.e. no haemorrhages were seen that showed good response of anti-allergy treatment. Hence application of niosomal gel of CPM reduced allergic reaction and declared CPM niosomal gel, as therapeutically active anti-histaminic treatment. displayed gel induced alterations in skin, i.e. lamina propria disappearance or asymmetry, clustering of inflammatory cells, extensive fibroblasts, and prominent blood vessels while, showed fibroblasts, vessels, and connective tissue while showed lymphocytes, and neutrophils, and showed, disruption of epithelium with 1–2 layers along with regeneration of epithelium clearly. Niosomal vesicles being lipophilic were used to entrap CPM due to hydrophilic nature CPM needed to be loaded in some lipophilic carriers to cross skin epithelium more efficiently by trans-cellular passive diffusion mechanism. Gel of these niosomal vesicles was used to obtain enhanced, facilitated permeation, and absorption of CPM through epidermis by concentration, dependent mechanism due to, presence of additional permeation enhancers like Propylene glycol and Polyethylene glycol in gel [Citation65].
After observing all slides form –i, it was clear that niosomal gel proved to be a good medicine, because initiated healing response by activating defense mechanism, attaching many inflammatory cells towards induced allergic site on skin, and production of various Inflamosomes, i.e. lymphocytes, neutrophils, and fibroblast more than in normal skin. Enlarged view at 40× produced enlargement of histopathological changes for prominent view. Underlying mechanism of action of CPM in its niosomal gel was, its anti-histaminic activity (compete with Histamine for binding to H1 receptors) because, various inflammatory agents mediate inflammatory response by involving Histamine H1 receptors [Citation66].
In short, it was suggested that good alteration in lamina propria caused enhanced CPM permeability, increased penetration of drug across skin for longer time, i.e. 1 day, increased production of collagen by chronic fibroblasts, increased healing of wounds, increased mucin the a-glycoprotein secretion overlapping the cell leading to, increased anti-inflammatory reaction, and enhanced drug transport. Underlying mechanism for mucin-action was described in few steps, i.e. increased mucin secretions, increased overlapping of all cells, increased tight attachment of niosomal lipophilic vesicles of CPM to hydrophilic end of mucin, increased production of wide spaces in loose network of connective tissue that allowed increased drug passage in membrane easily, hence, facilitated transport of drug inside membrane [Citation67].
During this study, capsicum was used as strong inflammatory agent to study induced inflammatory response by infiltration of inflammatory cells, i.e. lymphocytes and neutrophil into capsicum treated rabbit’s dorsal skin site. The major reason for selection of capsicum induced inflammation, as a model inflammation induction method, was the smaller number of reports or less available data regarding this method than frequently used histamine induction method used to study rat paw edoema by many reporter [Citation68].
Anti-histaminic and anti-inflammatory activity of CPM observed during this study, was in concordance with effects of previous studies reports that measured anti-inflammatory activity of CPM (administered by various routes) in terms of percent inhibition of paw edoema, i.e. CPM administration by intravenous injection in rat’s paws, inhibited histamine-induced paw edoema [Citation69], CPM administered by subcutaneous injection in rat’s paw inhibited Substance p-induced (s/c) paw edoema, CPM given by oral route in rat inhibited histamine induced paw edoema, and CPM administered by intradermal injection in rat’s paw also inhibited histamine induced edoema [Citation66].
Hence, topical application of niosomal gel of CPM proved, to be successful, and stable dosage form providing sufficiently facilitated, and potentiated anti-inflammatory effect of CPM by avoiding oral routes side effects like, poor bioavailability due to first pass metabolism causing only 25–45% of CPM reaching blood circulation [Citation70], by the presence of permeation enhancers like Propylene glycol, Polyethylene glycol etc., and by the presence of penetration enhancers like Span-60 and Span-80 in formulation that enhanced partitioning of CPM into skin, being in parallel to phenomenon of span-60 based topical CPM organogel formulated by Balata and co-workers (2017)[15s].
7. Conclusion
It can be concluded that CPM-based niosomal gel was successfully developed by using suitable combination of non-ionic surfactants for effective drug loading and good bioavailability. This gel could be an efficient carrier for sustained drug release across the skin and an improved anti-allergic effect.
Data availability statement
All the data has been incorporated into this article.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Gyati SA, Abhay A, Davinder S, et al. Etodolac containing topical niosomal gel: Formulation development and evaluation. J Drug Deliv. 2016;2016:9324567.
- Nicoli S, Colombo P, Santi P. Release and permeation kinetics of caffeine from bioadhesive transdermal films. AAPS J. 2005;7(1):E218–223.
- Guy RH. Current status and future prospects of transdermal drug delivery. Pharm Res. 1996;13(12):1765–1769.
- Tymes NW, Shah VP, Skelly JP. In-vitro profile of oestradiol transdermal therapeutic systems. J Pharm Sci. 1990;79(7):601–602.
- Zhai H, Maibach H. Occlusion versus skin barrier function. Skin Res Technol. 2002;8(1):1–6.
- Misra AN. Controlled and novel drug delivery. In: Jain NK, editors. Transdermal drug delivery. New Delhi: CBS Publishers; 1997. 8(1)100–101.
- Heather AE. Transdermal drug delivery: Penetration enhancement techniques. Curr Drug Deliv. 2005;2(1):23–33.
- Ayoub RK, Murtaza G, Imran M, et al. Formulation and permeation kinetic studies of flurbiprofen gel. Trop J Pharm Res. 2015;14(2):195–203.
- Ozeki T, Yuasa H, Kanaya Y. Application of carbopol to controlled release preperations I. Carbopol as a novel coating material. Int J Pharm. 2000;199(1):77–83.
- Yahagi R, Machida Y, Onishi H, et al. Mucoadhesive suppositories of ramasetron hydrochloride utilising carbopol. Int J Pharm. 2000;193(2):205–212.
- Macedo T, Block LH, Shukla AJ. Release of tolmetin from carbomer gel systems. Drug Dev Ind Pharm. 1993;19(8):887–902.
- Parson ME, Ganellin CR. Histamine and its receptors. Brit J Pharmacol. 2006;147(S1):S127–S135.
- Barry BW. Novel mechanisms and devices to enable successful transdermal drug delivery. Eur J Pharm Sci. 2001;14(2):101–114.
- Ceschel GC, Maffei P, Gentile M. Design and evaluation of a new transdermal formulation containing chlorpheniramine maleate. Drug Dev Ind Pharm. 1999;25(9):1035–1039.
- Balata GF, Shamardl AM. Formulation of chlorpheniramine maleate in span 60/tween20 based organogels for transdermal delivery. JIPBS. 2017;4(1):49–57.
- Fireman S, Toledano O, Neimann K, et al. A look at emerging delivery systems for topical drug products. Dermatol Ther. 2011;24(5):477–488.
- Korting HC, Schäfer-Korting M. Carriers in the topical treatment of skin disease. HandbExpPharmacol. 2010;197(1):435–468.
- Sinico C, Fadda AM. Vesicular carriers for dermal drug delivery. Expert Opin Drug Deliv. 2009;6(8):813–825.
- Bayindir ZS, Yuksel N. Provesicles as novel drug delivery systems. Curr Pharm Biotechnol. 2015;16(4):344–364.
- El-Badry M, Fetih G, Fathalla D, et al. Transdermal delivery of meloxicam using niosomal hydrogels: in vitro and pharmacodynamic evaluation. Pharm Dev Technol. 2015;20(7):820–826.
- Jacob S, Nair AB, Al-Dhubiab BE. Preparation and evaluation of niosome gel containing acyclovir for enhanced dermal deposition. J Liposome Res. 2017;27(4):283–292.
- Varghese V, Vitta P, Bakshi V, et al. Niosomes of primaquine: effect of sorbitan esters (spans) on the vesicular physical characteristics. Indian Drugs. 2004;41(2):101–103.
- VardeNeha M, ThakorNamita M, SiniSrendran C. Shah viral H. Formulation optimisation and evaluation of liposomal gel of prednisolone by applying statistical des. IJRPB. 2013;1(2):180–187.
- Ravalika V, Sailaja AK. Formulation and evaluation of etoricoxib noisome by thin film hydration technique and ether injection method. Nano Biomed Eng. 2017;9(3):242–248.
- Zhang F. Physicochemical properties and mechanisms of drug release from melt-extruded granules consisting of chlorpheniramine maleate and eudragit FS. Drug Dev Ind Pharm. 2016;42(4):563–571.
- Javed H, Shah SNH, Iqbal MF. Formulation development and evaluation of diphenhydramine nasal nano-emulgel. AAPSPharmSci’Tech. 2018;19(4):1730–1743.
- Jaiswal PH, Dr. Gujarathi NA, Dr.Rane BR, Dr., et al. Formulation of niosomal gel of diclofenac sodium and its in-VitroCharacterization. IJPPR Human. 2016;6(4):585–600.
- Bhattacharya SA, Prajapati BG. Formulation, design and development of niosome based topical gel for skin cancer. Med Clin Res. 2017;2(2):1–23.
- Riaz R, Shah SNH, Javed H, et al. Development and evaluation of microsponge based chlorpheniramine maleate gel formulation. Lat Am J Pharm. 2017;36(10):2028–2036.
- Ruan G, Feng SS. Preparation and characterisation of poly (lactic acid) poly (ethylene glycol)–poly (lactic acid) (PLA–PEG–PLA) microspheres for controlled release of paclitaxel. Biomaterials. 2003;24(27):5037–5044.
- Goyal G, Garg T, Malik B, et al. Development and characterisation of niosomal gel for topical delivery of benzoyl peroxide. Drug Deliv. 2015;22(8):1027–1042.
- Fathalla D, Abdel-Mageed A, Abdel-Hamid A, et al. In-vitro and in-vivo evaluation of niosomal gel containing aceclofenac for sustained drug delivery. IJPSR. 2014;1(1):105.
- Mandal S, Mandal SS, Sawant KK. Design and development of microemulsion drug delivery system of atorvastatin and study its intestinal permeability in rats. Int J Drug Deliv. 2010;2(1):69–75.
- Obata Y, Ashitaka Y, Kikuchi S, et al. Statistical approach to the development of a transdermal delivery system for ondansetron. Int J Pharm. 2010;399(1–2):87–93.
- Nair A, Jacob S, Al-Dhubiab B, et al. Basic considerations in the dermatokinetics of topical formulations. Braz J Pharm Sci. 2013;49(3):423–434.
- Sekhar KC, Naidu KV, Vishnu YN, et al. Transbuccal delivery of chlorpheniramine maleate from mucoadhesive buccal patches. Drug Deliv. 2008;15(3):185–191.
- Oryan A, Zaker SR. Effects if topical application of honey on cutaneous wound healing in rabbit. Zentralbl Veterinarmed A. 1998;45(3):181–188.
- Shinde UA, Kanojiya SS. 2014 SerratiopeptidaseNiosomal gel with potential in topical delivery. J Pharm. 2014;2014(1):1–9.
- Iman IS, Nadia AS, AbdouEbtsam M. Formulation and stability study of chlorpheniramine maleate transdermal patch. AJP. 2010;4(1):17–23. S
- Shan X, Chen L, Yuan Y, et al. Quantitative analysis of haemoglobin content in polymeric nanoparticles as blood substitutes using Fourier transform infra-red spectroscopy. J Mater Sci Mater Med. 2010;21(1):241–249.
- Khan MI, Madni A, Ahmad S, et al. Formulation design and characterisation of a non-ionic surfactant based vesicular system for the sustained delivery of a new chondroprotective agent. Brazilian JPS. 2015;51(3):608–615.
- Kulkarni SB, Betageri GV, Singh M. Factors affecting microencapsulation of drugs in liposomes. J Microencapsul. 1995;12(3):229–246.
- Balakrishnan P, Shanmugam S, Lee WS, et al. Formulation and in vitro assessment of minoxidilniosomes for enhanced skin delivery. Int J Pharm. 2009;377(1-2):1–8.
- Korchowiec B, Paluch M, Corvis Y, et al. Langmuir film approach to elucidating interactions in lipid membranes: 1, 2-dipalmitoyl glycero3phosphoethanolamine cholesterol metal cation systems. Chem Phys Lipids. 2006;144(2):127–136.
- Finean JB. Interaction between cholesterol and phospholipid in hydrated bilayers. Chem Phys Lip. 1990;54(3–4):147–156.
- Uchegbu IF, Double JA, Turton JA, et al. Distribution, metabolism and tumoricidal activity of doxorubicin administered in sorbitanmonostearate (span 60) niosomes in the mouse. Pharm Res. 1995;12(7):1019–1024.
- Jigar V, Puja V, Krutika S. Formulation and evaluation of topical niosomal gel of erythromycin. Int J Pharm Pharm Sci. 2011;3(1):123–126.
- Yoshioka T, Sternberg B, Florence AT. Preparation and properties of vesicles (niosomes) of sorbitan monoesters (span 20, 40, 60 and 80) and a sorbitantriester (span 85). Int J Pharm. 1994;105(1):1–6.
- Singh CH, Jain CP, Kumar BN. Formulation, characterisation, stability and invitro evaluation of nimesulideniosomes. Pharmacophore. 2011;2(3):168–185.
- Asthana GS, Asthana A, Singh D, et al. Etodolac containing topical niosomal gel: Formulation development and evaluation. JDD. 2016;16(1):8.
- Uchegbu IF, Vyas SP. Non-ionic surfactant based vesicles (niosomes) in drug delivery. Int J Pharm. 1998;172(1–2):33–70.
- Siddiqui N, Garg G, Sharma P. Fast dissolving tablets: preparation, characterisation and evaluation. Int J Pharm Sci. 2010;4(2):87–96.
- Usama A, Fetih G, El-Faham T. Performance of meloxicam niosomal gel formulations for transdermal drug delivery. BJPR. 2016;12(2):1–14.
- Swami H, Bilandi A, Kataria MK, et al. Formulation and Evaluation of Liposomal Gel of Lornoxicam. WJPR. 2015;4(9):2312–2338.
- Kamboj S, Saini V, Bala S, et al. Formulation and characterization of drug loaded niosomal gel for anti-Inflammatory activity. IJMH. 2013;12(7):1–5.
- Boushra M, El-Houssieny H, Hamouda M. Formulation and evaluation of clotrimazole from pluronic F127 gels. DDT. 2010;4(1):33–43.
- Garg A, Aggarwal D, Garg S. Spreading of semisolid formulation. Pharm Tech. 2002;9(1):89–105.
- Bhaskaran S, Panigrah L. Formulation and evaluation of niosomes using different non-ionic surfactants. Ind. J. Pharm. Sci. 2002;1(1):63–65.
- Nagalakshmi S, Krishnaraj K, Arul Jothy M, et al. Fabrication and characterization of herbal drug –loaded nonionic surfactant based niosomal topical gel. J Pharm Sci Res. 2016;8(11):1271–1278.
- Salih OS, Samein LH, Ali WK. Formulation and in vitro evaluation of rosuvastatin calcium niosomes. IJPPS. 2013;5(4):525–535.
- Van-Abbe NJ, Nicholas P, Boon E. Exaggerated exposure in topical irritancy and sensitisation testing. J SocCosmet Chem. 1975;26(1):173–187.
- Masumoto K, Matsumoto K, Yoshida A, et al. In vitro dissolution profile and in vivo absorption study of sustained release tablet containing chlorpheniramine maleate with water insoluble glucan. Chem Pharm Bull. 1984;32(9):3720–3723.
- Bozzatto V, de Oliveira PR, Camargo-Mathias MI. Histopathology of the tegument of rabbits infested by rhipicephalussanguineus (acari: Ixodidae) ticks and exposed to selamectin (active principle of acaricide revolution, pfizer). Parasitol Res. 2013;112(7):2551–2560.
- Germuth FG. A comparative histological and immological study in rabbits of induced hypersensitivity of the serum sickness type. J Exp Med. 1953;97(2):257–282.
- Baroody FM. Nasal and paranasal sinus anatomy and physiology. Clin Allergy Immunol. 2007;19(1):1–21.
- Arya S, Kumar VL. Anti-inflammatory efficacy of extracts of latex of calotropisprocera against different mediators of inflammation. Mediat Inflamm. 2005;2005(4):228–232.
- Gartner LP, Hiatt JL. Colour textbook of histology e-book. Philadelphia: Elsevier Health Sciences. 2006. 2006(1).
- Farshid AA, Tamaddonfard E, Morvaridi A. Effects of histidine and dexamethasone on the local inflammation induced by histamine in rats. Vet Res Forum. 2011;2(1):31–36.
- Inoue H, Nagata N, Koshihara Y. Involvement of tachykinin receptors in oedema formation and plasma extravasation induced by substance P, neurokinin A, and neurokinin B in mouse ear. Inflamm Res. 1996;45(7):316–323.
- Tas C, Özkan Y, Savaser A, et al. In vitro release studies of chlorpheniramine maleate from gels prepared by different cellulose derivatives. Farmaco. 2003;58(8):605–611.