?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Nanoparticles (NPs) find widespread applications in detectors, catalysis, optoelectronics, and medical devices, owing to their high surface-to-volume ratio and zero-dimensional confinement. However, addressing environmental concerns is crucial during the creation of novel nanostructured materials. Herein, ZnO NPs of different sizes were prepared via the pulsed laser ablation in liquid (PLAL) method at energies of 70, 90, and 130 mJ. The morphology and structural properties of the synthesized NPs were characterized by scanning electron microscopy, energy-dispersive X-ray spectrometry, and transmission electron microscopy. zeta-sizer and zeta-potential were used to ensure the physical stability of NPs. UV-Vis spectrophotometry measurement showed a blue shift in the band gaps with an increase in the pulsed laser energy leading to a decrease in the size of the NPs. Fourier-transform infrared spectroscopy technique confirmed the formation of ZnO NPs.
1. Introduction
The synthesis of metallic nanoparticles (NPs) has emerged as a promising area of study in recent years. The green creation of NPs has grown in significance over the past few years due to its many advantages, including ease of scaling up for large-scale synthesis, low cost, good stability of the nanoparticles produced, and non-toxic [Citation1]. The extraordinary properties of zero-dimensional materials such as NPs have led many researchers to explore various methods for their synthesis, behavior, and applications, owing to their higher surface-to-volume ratio and zero-dimensional confinement compared to other nanostructures [Citation2–7]. Therefore, NPs have been employed in many scientific applications. such as biomedical devices, optoelectronic technologies, detectors, nanofluids, and chemical catalyst nanostructures.
Recently, various methods have been developed to synthesize the chosen structure, size dispersal, and shape of NPs such as physical, chemical, and biological processes [Citation4, Citation8–12]; however, the ecological concern becomes a crucial account during the designing of novel materials. Pulsed Laser Ablation in Liquid (PLAL) is the most common, simple, straightforward, and environmentally friendly method with good reproducibility and high purity presenting a different way of thinking in chemistry intended to eliminate toxic waste, reduce energy consumption, and use ecological solvents [Citation6, Citation13]. It involves an intense laser beam projected onto a dense target, which can be a metal, semiconductor, polymer, metallic alloy, or complex multicomponent semiconductor, placed in a liquid environment to eliminate the nanoscale part of the target to form NPs [Citation14, Citation15].
The unique optical, electrochemical, piezoelectric, chemical, and optical properties of metallic oxides make them promising materials for various applications [Citation16–18]. In particular, ZnO has attracted attention because of its wide and direct bandgap (3.3 eV) and exciton binding energy (60 meV) at room temperature (RT), which are higher than those of other wide-bandgap semiconductors, as well as its chemical stability and environmental friendliness [Citation16, Citation19]. The polar surface of ZnO is very stable and has been used to induce the formation of many nanostructures using different techniques [Citation20–24]. Consequently, the development of new synthetic methods is significant for achieving high-performance nanoscale devices [Citation17], being applicable in field emitters [Citation25], solar cells [Citation26, Citation27], nanogenerators [Citation18], sensors [Citation28], bioimaging [Citation29], and drug delivery [Citation30]. Several studies explain the mechanism of NP preparation using PLAL. Patil P. et al. (1987) reported the foundational work on PLAL of solids in a confining liquid for material processing [Citation31]. Mitra et al. [Citation15] created highly stable and reproducible ZnO quantum dots by femtosecond laser ablation from zinc nitride targets (Zn3N2) in a liquid. that resisted degradation due to water or oxygen molecules. Liu Z. et al.[Citation32] utilized a high-power continuous wave (CW) laser to produce Ni-oxide and Ti-oxide NPs by ablation of solid targets in an aqueous environment, and studied the chemical composition, particle size, morphology, and distribution of the prepared NPs. Using an Nd-YAG laser with different wavelengths (1064 nm and 532 nm), Krstulovic et al. [Citation33] made Al-doped ZnO crystalline colloidal nanoparticles in Milli-Q water and analyzed the effect of varying the laser pulses and output energy on the NP size, composition (Al/Zn ratio), and physical characteristics (bandgap and crystallinity). A Zn plate placed in acetone was pulsed with a Q-switched Nd:YAG laser of 1064 wavelength at 7 ns pulse width by Dorranian D. et al. [Citation34]. They found that the laser fluence affected the NP characteristics. Al-Nassar SI et al. [Citation14] prepared ZnO nanoparticles from pure zinc metal inside a CTAP (10-3 M) in distilled water using a Ti:sapphire pulsed laser with a wavelength of 800 nm.
Controlling the crystallinity, doping, size, and concentration of NPs remains a significant requirement, and managing these factors by optimizing the growth conditions is crucial for improving the material’s performance [Citation10, Citation35]. In this work, highly crystalline, physically stable, and size-controlled ZnO NPs with a blue shift in band gap were produced via pulsed laser ablation of a ZnO target in distilled water, with a Nd:YAG laser of 1064 nm wavelength [Citation36–38]. We have studied the effect of varying the laser energy on the structural, morphological, and optical properties of the synthesized ZnO NPs.
2. Materials and methods
2.1. Synthesis of the ZnO NPs
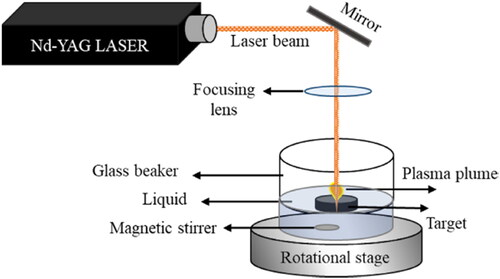
The ZnO NPs were synthesized by placing pure ZnO targets (99.99%) with dimensions of 2.5 cm and 6 mm thickness in a cylindrical glass beaker with 15 ml of distilled water at room temperature. Q-switched Nd:YAG lasers with a wavelength of 1064 nm, pulse duration of 10 ns, and pulse repetition rate of 10 Hz were used for the laser ablation.
Laser ablation pulses of energies 70, 90, and 130 mJ were employed to study the effect of pulse energy on the NPs. The laser beam was focused through a convex lens on a small spot of diameter ≅ 70 µm to have laser fluence of 18.2 J/mm2, 23.3 J/mm2, and 33.7 J/mm2 at pulsed laser energy 70, 90, 130 mJ, respectively, for 30 min on the target metal surface.
To avoid laser trapping inside the cavities caused by the target ablation, the glass beaker was placed on a rotating stage. An inner magnetic stirrer was used to ensure that the laser interacted with the metal target while the colloidal solution was circulating and homogenizing. The conventional setup for this process is shown in .
2.2. Characterization of the ZnO NPs
The morphologies and structural properties were characterized using SEM TESCAN VEGA scanning electron microscope (SEM) and a JEM 2100 F transmission electron microscope (TEM). An energy-dispersive X-ray spectroscope (EDX) was used to identify the chemical composition. The RT absorption measurements were carried out using a Shimadzu 1900 UV spectrophotometer to determine the absorption of UV or visible light. (MALVERN PANALYTICAL-ZEN3600) Zeta-sizer and the zeta-potential instrument were used to measure the average particle size and the effective electric charge on the NPs surface, respectively. Fourier transform infrared spectroscopy (FTIR) was performed using a SHIMADZU IR Affinity 1S to obtain the absorption, transmission, diffusion, and emission spectra of the synthesized ZnO NPs.
3. Results
The TEM images () were used to study the morphological features, elemental compositions, and structural properties of the as-prepared ZnO NPs using laser energies of 70, 90, and 130 mJ. The energy of the pulsed laser directly influences the size and concentration of ZnO NPs. This method tends to form NPs of uniform size and shape (). A mixture of spherical and hexagonal NPs were obtained. In addition, the size of the ZnO NPs decreased with increasing pulsed laser ablation energy, and a high concentration of ZnO NPs were obtained at higher energies.
Figure 2. HR-TEM images of the ZnO NPs produced at (a, d) 70 mJ, (b, e) 90 mJ, and (c, f) 130 mJ. Size distribution of the ZnO NPs obtained using the computer software ‘Image J’, based on several high magnification TEM images at (g) 70 mJ, (h) 90 mJ, and (i) 130 mJ.
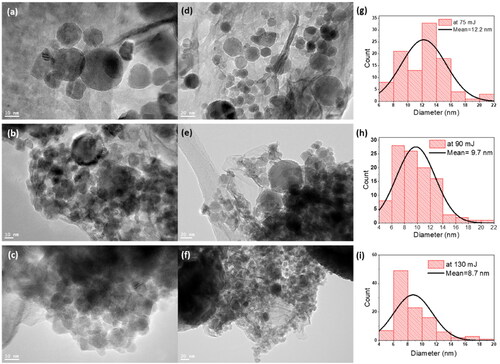
shows the size distribution of the ZnO NPs produced with a pulsed laser ablation energy of 70 mJ; an average NP size of 12.2 nm was obtained. In contrast, the ZnO NPs created with A laser ablation energy of 90 mJ had an average size of 9.7 nm (). In contrast, shows the ZnO NPs at 130 mJ with an average size of 8.7 nm. The inverse relationship between the pulsed laser ablation energy and the size of the ZnO NPs may be due to the increased ionic kinetic energy and plasma temperature [Citation39]. In addition, two mechanisms can account for this inverse relationship considering the interaction between produced NPs and laser beam [Citation40]: firstly, the Coulomb explosion that occurs when highly charged NPs absorb light resulting in the ejection of electrons into the liquid (photochemical bleaching) [Citation41], and secondly when the laser pulses raise the temperature of the NPs above the point of melting or vaporization [Citation42].
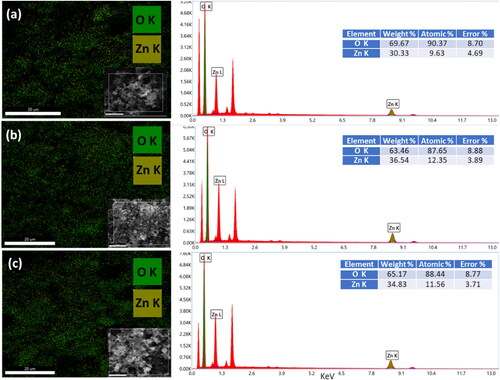
The SEM-EDX elemental maps of ZnO NPs are extracted from X-ray intensity measurements by background subtraction. These elemental maps exhibit a uniform spatial distribution of zinc and oxygen elements. They properly show that Zn and O were the main components of the NPs. The quantitative information on the ZnO NPs by analyzing the SEM EDX spectrum is presented in . It shows that the sample includes only the Zn and O X-ray peaks. To determine the quantitative composition of each element we used ZAF method as implemented in the EDX genesis software. The proportion of each element is given in the table in the inset of each figure.
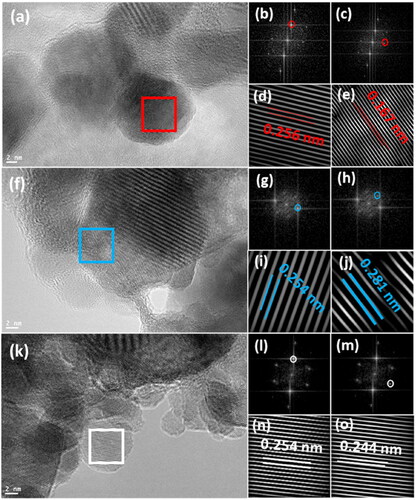
shows the TEM images of the ZnO NPs and the corresponding fast Fourier transform (FFT) and inverted FFT patterns. To calculate the d-spacing values, an inverted FFT process was utilized for the FFT images after selecting specific diffraction spots. The d-spacing for the ZnO NPs prepared at pulse energies of 70, 90, and 130 mJ were 0.256, 0.254, and 0.254 nm, respectively (), which are directly associated with the (002) plane in the hexagonal crystalline system of ZnO. The c lattice parameter is computed (1/d2=4/3(h2+hk + k2/a2)+l2/c2) to be 5.12 Å for the ZnO NPs prepared at 70 mJ, and 5.08 Å at 90 and 130 mJ. The lattice parameter c of the ZnO NPs slightly decreases with decreasing average particle size [Citation43, Citation44]. In addition, the interplanar lengths in correspond to the (110), (100), and (101) planes, respectively, which were indexed based on the hexagonal structure (P63/m), JCPDS card number 36-1451.
Figure 4. HR-TEM images of ZnO NPs (a) at 70 mJ, (b)(c) FFT images of the red square after selecting diffraction spots, (d)(e) the IFFT for selected diffraction spots in b, c, (f) at 90 mJ, (g)(h) FFT images of the blue square after selecting diffraction spots, (i)(j) the IFFT for selected diffraction spots in g, h, (k) at 130 mJ, (l)(m) FFT images of the white square after selecting diffraction spots, (n)(o) the IFFT for selected diffraction spots in (l), (m).
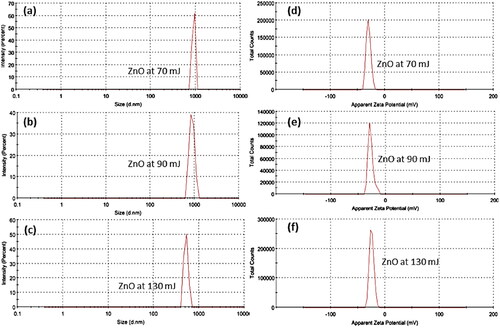
A Zeta-sizer instrument measures the average particle size of ZnO NPs by using dynamic light scattering (DLS). Static Light Scattering (SLS) and Electrophoretic Light Scattering (ELS) are also used to analyze particle mobility and charge (Zeta-potential) using the Zeta-Sizer technique. shows the mean particle size of ZnO NPs of each sample to be 905, 876, and 519 nm at 70, 90, and 130 mJ, respectively. TEM and Zeta-sizer measurements display different particle sizes since TEM estimates particle size from drier samples, while Zeta-sizer measures particle size in the dispersion. The Zeta-sizer uses DLS for an intensity-based particle size measurement, whereas TEM is a number-based measurement. The comparison of results from a number-based approach with those from an intensity-based approach is always a trap. A reasonable compromise is a decrease in NPs size with an increase in laser energy. Hence, it is always a trap to compare the results of an approach based on numbers with a method based on intensity [Citation45]. Therefore, a reasonable compromise is a decrease in NPs size with an increase in laser energy.
Zeta-potential has been used to evaluate the effective electric charge on the surface and the charges the stability of ZnO NPs (). It presents the zeta-potentials are −29.9, −27.4, and −24.7 mV at 70, 90, and 130 mJ, respectively. It is known that positively charged particles will bind to negatively charged surfaces, and vice versa. In addition, Zeta-potential values over 60 mV display excellent stability, over 30 mV show physical stability, below 20 mV have limited stability, and below 5 mV are indicative of agglomeration [Citation46]. Therefore, the zeta-potential value of synthesized NPs is almost physically stable.
To measure the effect of laser ablation on the ZnO NPs, optical absorption measurements were performed. shows the UV-vis absorbance spectra of the ZnO NPs in the range of 300–600 nm. The energy bandgaps can be calculated using Tauc’s equation, as follows:
(1)
(1)
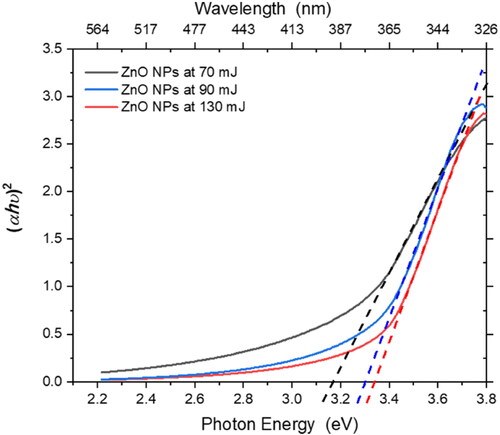
where α is the absorption coefficient, h is the Planck’s constant, υ is the frequency of the incident photon, K is a proportionality constant, and Eg is the bandgap energy. In electronic transitions, the critical term is the exponent n, which denotes the type of transition. If n = 2, the transition is direct, and if n = 1/2, the transition is indirect. The n values of 2/3 and 1/3 represent an indirect forbidden transition and a direct forbidden transition, respectively. Thus, a plot of (αhν)n against (hν) can provide the Eg from the intersection of the dotted line () with the x-axis. The bandgap energies of ZnO NPs increased with decreasing size: 3,12, 3.26, and 3.3 eV at 70, 90, and 130 mJ, respectively (), owing to the confinement effect and minimization of defects in the structure of the NPs [Citation47, Citation48].
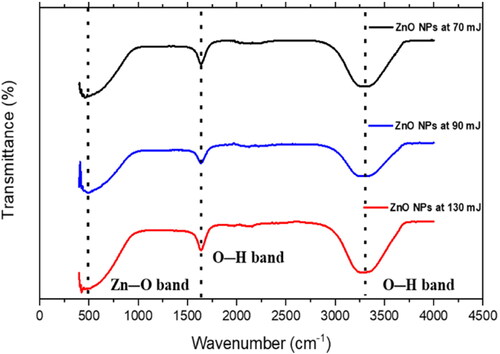
The FTIR spectra of the as-prepared ZnO NPs were recorded in the range of 400-4000 cm−1 using a KBr reference (). FTIR is an effective technique for measuring the absorption, transmission, diffusion, and emission spectra of materials in the infrared region. When a sample is exposed to infrared light, inelastic collisions between the molecules and infrared photons produce vibrations with a characteristic vibrational frequency and of varying modes depending on the nature of the bond [Citation49].
The absorption bands and values indicate the formation of zinc oxide nanoparticles, O-H stretching vibration from ZnOH species, and O-H stretching vibration as shown in . The reaction of zinc oxide nanoparticles and hydroxyl group results in the free O-H stretching bond, which revealed from 3408 cm−1 absorption peak value, these results are consistent with those reported in the literature. The characteristic peak between 450 cm−1 and 500 cm−1 appears due to the ZnO stretching mode (), and indicating the presence of ZnO NPs. The broad peaks at 3300 cm−1 (stretching) and 1630 cm−1 (bending) indicate the formation of a hydroxyl residue, which is caused by atmospheric moisture [Citation14, Citation50, Citation51].
4. Conclusions
Synthesis of ZnO NPs using different laser ablation energy was investigated. The energy of pulsed laser ablation affects the size and concentration of the prepared ZnO NPs; at higher laser ablation energies, the particle size is reduced and the concentration increases. An average NP size of 12.2, 9.7, and 8.7 nm was obtained at pulsed laser energy 70, 90, and 130 mJ, respectively. The c lattice parameter is calculated to be 5.12 Å for the ZnO NPs prepared at 70 mJ, 5.08 Å at 90, and 5.08 Å 130 mJ. The lattice parameter c of the ZnO NPs slightly decreases with decreasing average particle size. The morphologies and structural properties were characterized using SEM and TEM. The UV-visible absorption spectra of ZnO NPs indicate a blue shift in the bandgap at higher laser energy owing to the confinement effect and reduction of defects in the structure of the NPs. The zeta-potential value of synthesized NPs indicated almost physically stable. The formation of ZnO NPs is confirmed by the ZnO stretching peak at 450-500 cm−1 in the FTIR spectra.
Author contributions
Tahani Flemban wrote the main manuscript, prepared the NPs, performed UV-Vis spectrophotometry and FTIR measurements, and analyzed all experimental results.
Acknowledgments
The author thanks Prof. Khalid Elsayed for providing access to the Nd-YAG laser facility in his lab at the College of Engineering, Imam Abdulrahman Bin Faisal University, and for his timely help. In addition, I would like to express my gratitude to the King Abdullah Institute for Nanotechnology for providing the TEM images. A preprint of this paper has previously been published [Citation45].
Disclosure statement
No potential conflict of interest was reported by the author(s).
Availability of data and materials
All materials are owned by the author and/or no permissions are required.
References
- Malhotra SPK, Alghuthaymi MA. Biomolecule-assisted biogenic synthesis of metallic nanoparticles. Agri-Waste and Microbes for Prod Sustainable Nanomater. Elsevier: Amsterdam, The Netherlands, 2022:139–163.
- Reguera J, Langer J, de Aberasturi DJ, et al. Anisotropic metal nanoparticles for surface enhanced Raman scattering. Chem Soc Rev. 2017;46(13):3866–3885.
- Liu LQ, Zhang XN, Yang LF, et al. Metal nanoparticles induced photocatalysis. Nat Sci Rev. 2017;4:761–780.
- Slavin YN, Asnis J, Hafeli UO, et al. Metal nanoparticles: understanding the mechanisms behind antibacterial activity. J Nanobiotechnol. 2017;15:1–20.
- Hanske C, Sanz-Ortiz MN, Liz-Marzan LM. Silica-coated plasmonic metal nanoparticles in action. Adv Mater. 2018;30:1706558.
- Zeng HB, Du XW, Singh SC, et al. Nanomaterials via laser ablation/irradiation in liquid: a review. Adv Funct Mater. 2012;22:1333–1353.
- Tahani Flemban Ridha Hamdi, Alkhabbaz H, Alheshibri M, et al. Physicochemical properties of nanofluids produced from oxidized nanoparticles synthesized in a liquid by pulsed laser ablation. Lasers in Manufact Mater Process. 2022;9:18–36.
- Gomez JL, Tigli O. Zinc oxide nanostructures: from growth to application. J Mater Sci. 2013;48:612–624.
- Loza K, Heggen M, Epple M. Synthesis, structure, properties, and applications of bimetallic nanoparticles of noble metals. Adv Funct Mater. 2020;30:1909260.
- Abdul Subhan A-H, Al-Douri Y. Influence of laser process parameters, liquid medium, and external field on the synthesis of colloidal metal nanoparticles using pulsed laser ablation in liquid: a review. Nanomaterials. 2022;12:2144–2177.
- Gherab YK, Al-Douri Y, Hashim U, Ameri M, et al. Fabrication and characterizations of Al nanoparticles doped ZnO nanostructures-based integrated electrochemical biosensor. J Mater Res Technol. 2020;9:857–867.
- Gherab YK, Voon CH, Hashim U, et al. Aluminium nanoparticles size effect on the optical and structural properties of ZnO nanostructures synthesized by spin-coating technique. Results in Phys. 2017;7:1190–1197.
- Liang W, Yuhas BD, Yang P. Magnetotransport in Co-Doped ZnO nanowires. Nano Lett. 2009;9(2):892–896.
- Al-Nassar SI, Hussein FI, Adel KM. The effect of laser pulse energy on ZnO nanoparticles formation by liquid phase pulsed laser ablation. J Mater Res Technol-JMR&T. 2019;8:4026–4031.
- Mitra S, Aravindh A, Das G, et al. High-performance solar-blind flexible deep-UV photodetectors based on quantum dots synthesized by femtosecond-laser ablation. Nano Energy. 2018;48:551–559.
- Ozgur U, Alivov YI, Liu C, et al. A comprehensive review of ZnO materials and devices. J. Appl Phys. 2005;98:041301.
- Willander M, Nur O, Zhao QX, et al. Zinc oxide nanorod based photonic devices: recent progress in growth, light emitting diodes and lasers. Nanotechnology. 2009;20(33):332001.
- Wang ZL, Song JH. Piezoelectric nanogenerators based on zinc oxide nanowire arrays. Science. 2006;312(5771):242–246.
- Nagaraju G, Ko YH, Yu JS. Effect of diameter and height of electrochemically-deposited ZnO nanorod arrays on the performance of piezoelectric nanogenerators. Mater Chem Phys. 2015;149:393–399.
- Yang PD, Yan HQ, Mao S, et al. Controlled growth of ZnO nanowires and their optical properties. Adv Funct Mater. 2002;12:323–331.
- Zhao QX, Willander M, Morjan RE, et al. Optical recombination of ZnO nanowires grown on sapphire and Si substrates. Appl Phys Lett. 2003;83:165–167.
- Wang XD, Summers CJ, Wang ZL. Large-scale hexagonal-patterned growth of aligned ZnO nanorods for nano-optoelectronics and nanosensor arrays. Nano Lett. 2004;4(3):423–426.
- Gao PX, Ding Y, Wang IL. Crystallographic orientation-aligned ZnO nanorods grown by a tin catalyst. Nano Letters. 2003;3:1315–1320.
- Kong XY, Wang ZL. Spontaneous polarization-induced nanohelixes, nanosprings, and nanorings of piezoelectric nanobelts. Nano Letters. 2003;3:1625–1631.
- Wang XD, Zhou J, Lao CS, et al. In situ field emission of density-controlled ZnO nanowire arrays. Adv Mater. 2007;19:1627.
- Gonzalez-Valls I, Lira-Cantu M. Vertically-aligned nanostructures of ZnO for excitonic solar cells: a review. Energy & Environ Sci. 2009;2:19–34.
- Gonzalez-Valls I, Lira-Cantu M. Dye sensitized solar cells based on vertically-aligned ZnO nanorods: effect of UV light on power conversion efficiency and lifetime. Energy & Environ Sci. 2010;3:789–795.
- Wan Q, Li QH, Chen YJ, et al. Fabrication and ethanol sensing characteristics of ZnO nanowire gas sensors. Appl Phys Lett. 2004;84:3654–3656.
- Salah N, Al-Shawafi WM, Alshahrie A, et al. Size controlled, antimicrobial ZnO nanostructures produced by the microwave assisted route. Mater Sci Eng C Mater Biol Appl. 2019;99:1164–1173.
- Zamiri R, Zakaria A, Ahangar HA, et al. Aqueous starch as a stabilizer in zinc oxide nanoparticle synthesis via laser ablation. J Alloys Compounds. 2012;516:41–48.
- Patil PP, Phase DM, Kulkarni SA, et al. Pulsed-laser induced reactive quenching at a liquid-solid interface - aqueous oxidation of iron. Phys Rev Lett. 1987;58(3):238–241.
- Liu Z, Yuan Y, Khan S, et al. Generation of metal-oxide nanoparticles using continuous-wave fibre laser ablation in liquid. J Micromech Microengin. 2009;19:054008.
- Krstulovic N, Salamon K, Budimlija O, et al. Parameters optimization for synthesis of Al-doped ZnO nanoparticles by laser ablation in water. Appl Surface Sci. 2018;440:916–925.
- Dorranian D, Eskandari AF. Effect of laser fluence on the characteristics of ZnO nanoparticles produced by laser ablation in acetone. Mol Crystals and Liquid Crystals. 2015;607:1–12.
- Al-Douri Y, Abdulateef SA, AbuOdeh A, et al. Nanosecond pulsed laser ablation to synthesize Gao colloidal nanoparticles: optical and structural properties. Optik. 2019;178:337–342.
- Alhujaily M, Albukhaty S, Yusuf M, et al. Recent advances in plant-mediated zinc oxide nanoparticles with their significant biomedical properties. Bioengineering 2022;9:541.
- Alyamani AA, Albukhaty S, Aloufi S, et al. Green fabrication of zinc oxide nanoparticles using phlomis leaf extract: characterization and in vitro evaluation of cytotoxicity and antibacterial properties. Molecules. 2021;26:6140.
- Khashan KS, Sulaiman GM, Hussain SA. Synthesis and characterization of aluminum doped zinc oxide nanostructures by Nd: YAG laser in liquid. Iraqi J Sci. 2020;61:2590–2598.
- Kim M, Osone S, Kim T, et al. Synthesis of nanoparticles by laser ablation: a review. Kona Powder and Particle J. 2017;34:80–90.
- Semaltianos NG. Nanoparticles by laser ablation. Crit Rev Solid State Mater Sci. 2010;35:105–124.
- Kamat PV, Flumiani M, Hartland GV. Picosecond dynamics of silver nanoclusters. Photoejection of electrons and fragmentation. J Phys Chem B. 1998;102:3123–3128.
- Takami A, Kurita H, Koda S. Laser-induced size reduction of noble metal particles. J Phys Chem B. 1999;103:1226–1232.
- Qi WH, Wang MP. Size and shape dependent lattice parameters of metallic nanoparticles. J Nanoparticle Res. 2005;7:51–57.
- Qi WH, Wang MP, Su YC. Size effect on the lattice parameters of nanoparticles. J Mater Sci Lett. 2002;21:877–878.
- Flemban TH. Synthesis of ZnO nanoparticles using varying pulsed laser ablation energies in liquid. PREPRINT (Version 1). Available at Research Square; 2022.
- Müller RH. Zetapotential und partikelladung in der laborpraxis. 1st ed. Stuttgart: Wissenschaftliche Verlagsgesellschaft; 1996.
- Ton-That C, Phillips MR, Nguyen TP. Blue shift in the luminescence spectra of MEH-PPV films containing ZnO nanoparticles. J Luminescence. 2008;128:2031–2034.
- Jiang DX, Cao L, Liu W, et al. Synthesis and luminescence properties of core/shell ZnS: mn/ZnO nanoparticles. Nanoscale Res Lett. 2009;4(1):78–83.
- Peter J, Griffiths R. Fourier transform infrared spectrometry. 2nd ed. Hoboken, New Jersey: John Wiley & Sons, INC; 2007.
- Hower PL, Gupta TK. Barrier model for zno varistors. J Appl Phys. 1979;50:4847–4855.
- Wahab R, Ansari SG, Kim YS, et al. Low temperature solution synthesis and characterization of ZnO nano-flowers. Mater Res Bull. 2007;42:1640–1648.