Abstract
As fossil fuel is depleted day by day, there is a vast gap in the market and supply of fuel to the different industries that consume oil. In order to improve fuel properties, performance, combustion, and emissions from diesel engines, microalgae oil biodiesels are used as fuel with 20% of diesel on a mass basis by volume and 50 and 100 ppm of CeO2 nanoparticles as additives in combinations of biodiesel/diesel blends. The engine configuration consists of a single-cylinder, four-stroke, 3.5 kW, 1500 rpm diesel engine. Experiments were performed at constant speed on experimental test fuels for various load conditions. The addition of cerium oxide (CeO2) in the added biodiesel diesel blends improved the brake thermal performance (45%) compared to engine output. With CeO2 in the B20 blends, better combustion properties were observed, such as increased length of combustion (36 CA°), ignition delay (16 CA°), and heat release rate (55 J/deg) in the cylinder. The enhanced and improved fuel characteristics and combustion characteristics of CeO2 in the added biodiesel diesel blends minimise HC (0.54 g/kWh) and CO (4.6 g/kWh) emissions and smoke (0.61%) while increasing NOx (10.2 g/kWh) emissions from the engine cylinder.
1. Introduction
The world mainly relays on Petroleum products to meet its energy needs for all its development as well as transportation purposes, which is much greater in developing countries. Having a huge population right next to China, India faces a mammoth energy demand to meet its per capita energy requirement [Citation1–3]. The transport sector is one of the major sectors that require the highest demand for liquid fuel. The diesel engine is widely used due to its higher torque capacity and higher thermal efficiency values. Apart from automobiles, they are also used as a major power source in agricultural equipment, locomotives, generators, Marine engines, etc. The harmful tailpipe emission especially carbon dioxide has made an adverse effect on the global temperature [Citation2, Citation4]. Further, the other emissions from the vehicles such as HC, CO, oxides of nitrogen, and smoke opacity have a serious impact on air pollution. It should be noted that the major metropolitan cities in India suffer from a critical level of particulate emission gas pollution from the usage of fossil fuels necessitates the hunt for green energy supplies for which biofuels are a valuable substitute [Citation5–7]. Biodiesel processing methods are currently being researched; however, the techniques are not yet successful. The advantages and drawbacks of various forms of catalysers are studied and debated in this article focusing on enzymatic catalysers that could be used in commercial trans-esterification for biodiesel production in the future [Citation8,Citation9]. Recent events have also helped raise the consciousness of the impact of human activities on global warming. Algae oil, made from Algae biomass, is viscous, heavy oil widely available in India. Algae seeds from India contain 60% oil, of which 60% can be extracted. Ricinoleic acid which has a higher affinity for alcohol is found in nearly 89.5% of algae oil [Citation10]. It is possible to make a homogeneous mixture of algae oil and alcohol in any proportion without phase separation. Algae oil has a 100-fold higher viscosity than diesel. As a result, algae oil’s viscosity is a major stumbling block to using it as a CI engine fuel. Algae biodiesel has a viscosity of about five times that of diesel even after conversion. Botryococcus braunii produces a high amount of polysaccharides and extracellular hydrocarbons. This makes it an ideal organism for the production of biofuels because these polysaccharides and hydrocarbons can be converted into liquid or gaseous fuel. Botryococcus braunii is also able to tolerate extreme environmental conditions, making it easy to cultivate and harvest. It is currently believed that hydrocarbons, especially botryococcenes and squalene, can play an important role in the development of alternate fuels as well as alternative feedstocks for other fossil-based products [Citation11–13]. Botryococcenes and squalene are hydrocarbons that have the potential to be converted into more sustainable fuel sources such as biodiesel and bio-ethanol, as well as into feedstocks for other products such as plastics and lubricants. As a result, the engine’s performance will be lower than that of a standard CI engine. Previously, a variety of non-edible vegetable oils with low and medium viscosity were tested as CI engine fuel [Citation14–17]. The search for the native energy source paved the way for the renewable energy sources available in their nation. Biofuel is identified as the best choice as it is renewable, biodegradable, and, highly viable in the local region. Among the various alternative energy sources, biofuel is the best option for the ever-increasing diesel engine [Citation18–21]. Algal oil is converted into biodiesel through a transesterification process. This process involves combining the algal oil with an alcohol, such as methanol or ethanol, and a catalyst, such as sodium hydroxide. This reaction breaks the triglycerides found in the oil into fatty acids and glycerol, which are then converted into biodiesel. Biodiesel is made from fatty acid methyl esters made from algae oil mixed with alcohol and an acid or a base. The biodiesel produced can be used in any diesel engine and has a higher energy content than fossil fuels. It also produces fewer emissions and is more sustainable. Biodiesel can be blended with petroleum diesel to create a fuel with a lower carbon footprint. The present research work seeks to find a novel renewable biofuel abundantly available in India, to take care of the issues addressed especially low viscous biofuel. The objective of this article is to use algae oil as a fuel in CI engines. As a result, experiments were conducted in different modes using nanoparticles in algae biodiesel to improve combustion and reduce the emission level of the engine exhaust.
2. Materials and methods
2.1. Biodiesel yield
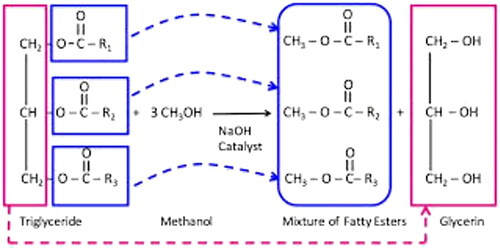
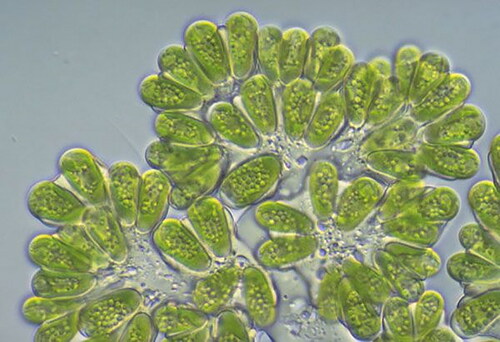
The potassium hydroxide reacts with the oil to produce soap, which helps to dissolve the oil. The methanol mixture helps to solubilise the soap so it can be washed away, taking the oil with it. It is heated to 50 °C, mixed with 20% methanol w/w and 0.5-1.5% potassium hydroxide KOH, and then reacted with the mixture [Citation22–24]. The methanol helps reduce the surface tension of the soap and water, allowing the soap to spread out and dissolve more easily. The potassium hydroxide helps to increase the pH of the solution, which helps to further dissolve the soap [Citation25]. The heat helps to increase the solubility of the soap, allowing it to be washed away more easily. A constant stirring of the solution for 120 min leads to a temperature of 65 °C. The methyl ester of biodiesel was detected in one of the phases, while the other phase did not. It was decanted into a separate pear and allowed to decant for 30 min [Citation26–28]. Biodiesel was extracted from the glycerin by washing it four times with purified water to remove soap residues and methanol, then boiling it and removing the absolute water. shows the transesterification process, shows the photo view of the Botryococcus braunii marine algae and shows the separation glycerol from biodiesel [Citation29–31]. shows the properties of CeO2 nanoparticles.
Table 1. Properties of CeO2 nanoparticles.
2.2. Engine test set up
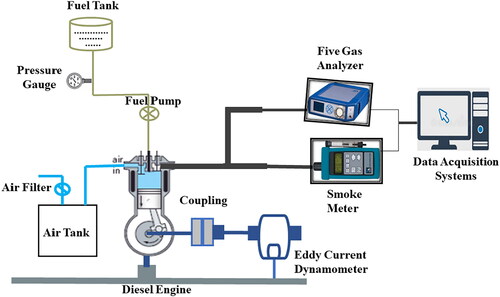
The dynamometer is used to measure the torque and speed generated by the engine and to regulate the engine load. This allows the engine to be tested under various conditions and helps to identify any issues with the engine performance [Citation32]. The dynamometer is connected to the engine and the engine is then run at a variety of speeds, loads, and temperatures. The data collected from the dynamometer is used to generate a graph of the engine’s performance, which can then be used to identify any potential problems or areas for improvement. All of these devices are connected to your computer [Citation1, Citation34–36]. This allows technicians to monitor the engine’s performance in real-time, so they can take immediate action if any issues arise. The data collected can also be used to create more accurate models and simulations of the engine, which can be used to optimise its design and performance. This combination of components allows the user to monitor the fuel and air flow in the system, as well as measure the fuel and air pressure, in order to ensure the engine is running optimally and efficiently [Citation36–38] All of these readings are taken in real time, allowing the user to make adjustments to the fuel and air flow as needed in order to keep the engine running at peak performance. The readings also provide data that can be used to diagnose and troubleshoot any potential issues with the engine.
A constant speed of 1500 rpm was used throughout the experiments. This was done to ensure that the results were consistent. This also ensured that any changes in the data could be attributed to the variables being tested, rather than the speed at which the experiment was conducted [Citation39]. Testing was conducted after the engine had reached a stable state. By maintaining a constant speed, any changes in the data can be accurately attributed to the variables under test. This is in contrast to the speed at which the experiment was conducted. This helps to ensure that the results are reliable and that any changes can be confidently attributed to the variables being tested [Citation40]. A 200-bar injection pressure was set for the fuel. Keeping the injection pressure constant ensures that any changes in the test results can be attributed to the fuel itself, rather than changes in the injection pressure. Therefore, the results can be trusted and can be used to draw reliable conclusions. Engine output was varied in steps from 25% to 100% under the single-fuel mode. By keeping the injection pressure constant, any changes in the test results are attributed to the fuel and not the injection pressure, ensuring accuracy and reliability [Citation41]. This allows the engine output to be varied with accuracy and confidence in the results, enabling reliable conclusions to be drawn. The DAQ system recorded pressure crank angle data for 100 consecutive cycles [Citation24]. The data was used to calculate the torque, power, and mean effective pressure of the engine, and the results showed that the engine’s performance was stable and consistent across the test period. This indicates that the DAQ system was able to accurately measure and record the engine’s output over time. The DAQ system was able to measure the pressure at each crank angle in the engine and compare it to the average pressure of the same crank angle over time [Citation42]. From this data, the variance in pressure at each crank angle was calculated, which allowed for a more accurate analysis of the engine’s output. During the first phase of the test, based fuels were compared for their emission characteristics, performance, and combustion behaviour at variable loads of 1500 rpm [Citation43]. This allowed engineers to identify the best fuel for a given engine configuration by comparing the pressure variances at each crank angle with the base fuel’s. This allowed them to identify the fuel that produced the most consistent pressure output over time and at different loads, which was essential for optimal engine performance. shows the schematic drawing of the engine setup. shows the range and accuracy of various instruments.
Table 2. Range and accuracy of various instruments.
3. Result and discussion
3.1. Performance
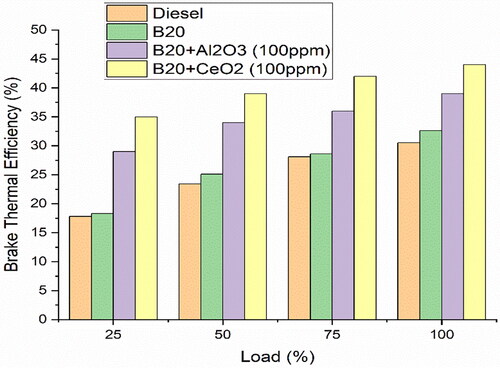
illustrates that the BTE of the research fuels decreased as the load increased. This indicates that the BTE of research fuels is affected by the amount of fuel used. A comparison of BTE for the research fuels with load is shown in . The transesterification phase helps to reduce the viscosity and density of the B20 + 100ppm CeO2 blend, which in turn helps to improve combustion by reducing the amount of unburned fuel that is left in the exhaust [Citation44]. This helps to increase efficiency and reduce emissions. CeO2 increases the stability of the fuel mixture and prevents it from forming soot. The nanoparticles of CeO2 also decrease the viscosity of the fuel which allows for a more efficient combustion process. The combustion process is also sped up due to the increased burning speed, resulting in more of the energy being transferred to productive work. The BTE improves considerably as a result of CeO2 additives.
3.2. Combustion
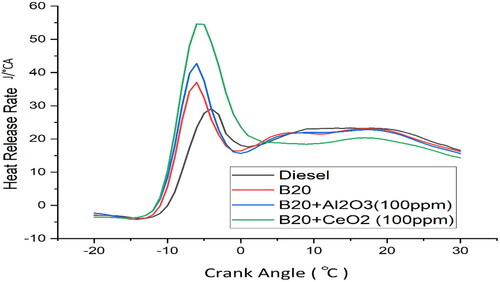
shows that the higher the CA, the higher the HRR, indicating a positive correlation between the two variables. This suggests that as the compression ratio increases, the efficiency of the fuel also increases [Citation45]. The peak HRR for B20 + 100ppm CeO2 is observed at 100% load. This is due to the increased compression ratio resulting in higher thermal efficiency, leading to increased fuel efficiency and higher HRR at the same load. The increased compression ratio of B20 + 100ppm CeO2 leads to increased combustion pressure and temperature, which results in improved thermal efficiency [Citation46]. The higher viscosity of B20 also causes more fuel to remain in the cylinder, resulting in increased fuel efficiency due to extended combustion duration. The increased compression ratio and extended combustion duration allow for higher HRR at the same load. The higher viscosity of B20 leads to poor atomisation and lower premixed combustion [Citation26]. The increased compression ratio and extended combustion duration create more complete combustion, resulting in higher HRR. The higher viscosity of B20 makes it more difficult for the fuel to be atomised and mixed with the air, resulting in incomplete combustion and lower HRR. The addition of 100 ppm CeO2 helps to improve the atomisation of the fuel and increase the HRR. The slower flame speed also reduces the heat release rate in the combustion chamber, which increases the cooling rate and thus, a higher overall efficiency. As a result, B20 + 100ppm CeO2 has higher thermal efficiency and lower emissions than other fuels. The viscosity of B20 + 100ppm CeO2 slows down the diffusion rate of the fuel, allowing for more time for the fuel to mix and react with the oxygen in the combustion chamber, resulting in more complete combustion [Citation24]. This increases the heat release rate (HRR), which in turn increases the efficiency of the engine. This means that more fuel is able to burn during the premixed combustion phase, leading to more efficient combustion and a higher HRR. The CeO2 nano additives reduce the viscosity of the fuel, which means that it is able to burn more easily and more quickly. This also increases the cetane number, which is a measure of how quickly the fuel ignites in the combustion chamber. Therefore, the use of CeO2 nano additives in the B20 blend leads to more efficient combustion and increased engine performance.
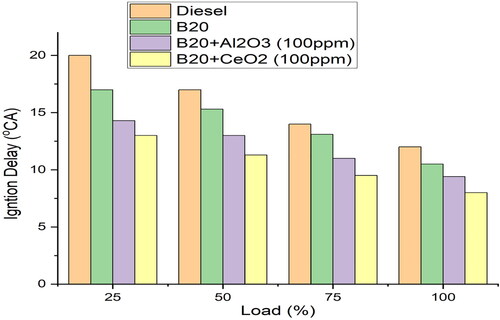
As shown in , the ID increases with load, meaning that the fuel-air mixture takes longer to ignite as the load increases. This is because a higher load causes more heat to be generated, which makes the fuel-air mixture harder to ignite. This is likely because the addition of CeO2 (100 ppm) acts as an ignition enhancer, meaning that it increases the temperature of the fuel-air mixture and makes it easier to ignite [Citation32]. In addition, CeO2 helps to reduce the formation of soot and other combustion by-products, as it increases the flame temperature and reduces the overall combustion time. This leads to a cleaner and more efficient combustion, resulting in higher thermal efficiency of the engine. The addition of the CeO2 decreases the surface tension of the fuel, allowing the fuel to spread out more easily and reducing the viscosity. This allows for better atomisation in the combustion chamber, which results in better vaporisation and air-fuel mixing formation for improved combustion. The addition of CeO2 acts as a catalyst, increasing the rate at which the fuel reacts with the oxygen in the combustion chamber [Citation38]. This allows for more complete combustion which results in higher power output and efficiency. The 8°CA ignition delay is an indication of the fuel’s ability to react quickly and completely with the oxygen in the combustion chamber.
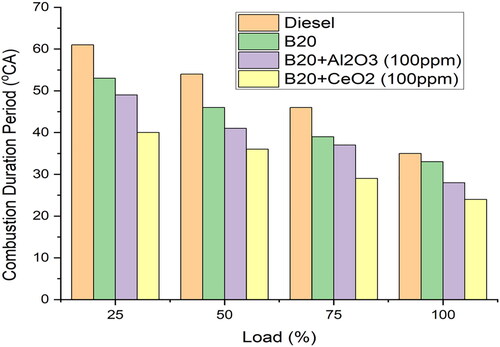
is a graph that shows how long the combustion process takes with a full load condition. It shows that the combustion duration is shorter when the load is greater, and longer when the load is lower [Citation18]. The addition of cerium oxide particles to the B20 blend helps to improve the atomisation of the fuel, resulting in a more even air-fuel mixture. This improved atomisation leads to more efficient combustion, resulting in a shorter combustion duration. The shorter combustion duration allows for a more complete and efficient combustion of the fuel, leading to increased power output and fuel efficiency [Citation37]. Additionally, the improved atomisation of the fuel ensures a more even air-fuel mixture, leading to reduced emissions. A full load combustion of B20, B20 + 100ppm Al2O3, and B20 + 100ppm CeO2 is found to last 33, 28, and 24°CA, respectively [Citation23]. This is due to the shorter combustion duration reduces heat losses due to incomplete combustion and allows for more complete combustion of the fuel. This results in higher power output and fuel efficiency as well as lower emissions. The addition of cerium oxide further reduces the combustion duration, leading to even greater improvements in power output and fuel efficiency.
3.3. Emission
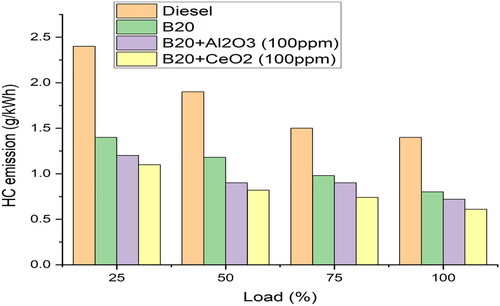
shows the variations of HC with Load. This indicates that the addition of 100 ppm of cerium oxide to B20 fuel leads to a reduction in HC emissions. This is due to the cerium oxide’s ability to act as a catalyst, which increases the fuel’s combustibility and reduces the amount of HC that is emitted [Citation22]. This is due to the fact that the addition of CeO2 increases the thermal stability of the fuel and improves its ignition characteristics. This allows for better combustion, which results in lower levels of unburned hydrocarbons and lowers HC emissions. This in turn reduces the HC emissions at both part and full load conditions. Adding CeO2 to the fuel helps to improve the ignition properties of the fuel. This leads to a shorter ignition delay, which allows for more complete combustion of the fuel. The more complete combustion of the fuel results in fewer unburned hydrocarbons being emitted into the exhaust, leading to a decrease in HC emissions. By shortening the ignition delay, the fuel is able to be ignited and burned more quickly [Citation17]. This leads to a more efficient burn, resulting in fewer unburned hydrocarbons being emitted into the exhaust. The use of CeO2 helps to create a stable and homogeneous flame front, which furthers the complete combustion of the fuel and helps to reduce HC emissions. This means that the fuel burns more quickly, resulting in fewer exhaust gases being produced. Since the fuel is carbon-free, this leads to lower levels of hydrocarbons being released into the atmosphere, making it a much cleaner and more efficient fuel option.
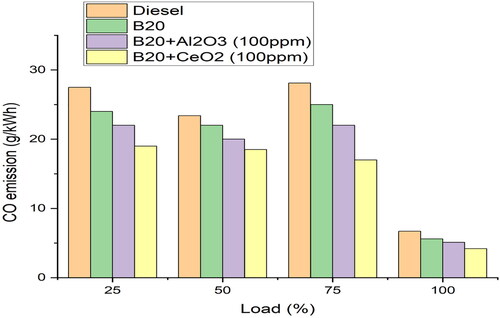
From , it can be seen that the CO emission increases with the increase in the full load. This is because when the load increases, more fuel is consumed, leading to higher CO emission. In addition, B20 has a higher cetane number than other blends, which is indicative of a slower combustion rate. This leads to an incomplete combustion of the fuel and, as a result, higher CO emissions at full load. The slow combustion rate of B20 fuel helps to reduce the amount of oxygen available for complete combustion, leading to higher carbon monoxide emissions [Citation8]. Adding a small amount of cerium oxide (CeO2) to B20 fuel helps to reduce these emissions by promoting more complete combustion. The reduced carbon content in the fuel input increases the efficiency of the combustion process. The high flame velocity of hydrogen helps to further increase the efficiency by allowing for complete combustion of the fuel, resulting in a more efficient engine.
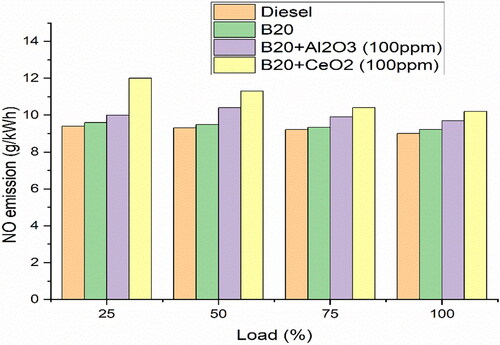
shows the variation of NO emissions with load for various test fuels. B20 + 100ppm CeO2 emits higher NO emissions than B20. This is due to the CeO2 particles helping to reduce the combustion temperature, which increases the nitrogen oxide emissions. The higher combustion temperature of B20 fuel without CeO2 particles causes less NO emissions [Citation40]. The higher in-cylinder temperature resulting from the low viscosity of B20 and more oxygen availability leads to improved combustion efficiency, which leads to an increased formation of NO (nitric oxide). Furthermore, the low cetane number of B20 + 100ppm CeO2 enables a longer combustion period, which further increases the premixed combustion phase.
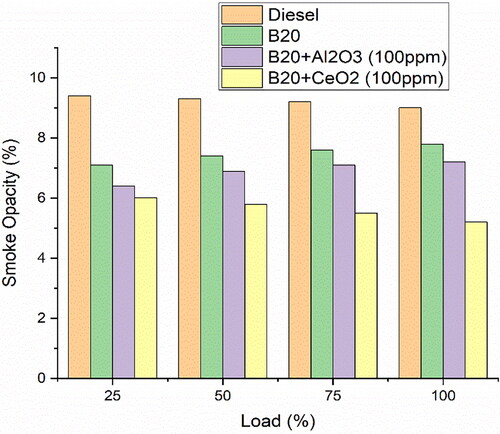
shows the relationship between the amount of fuel used and the amount of smoke emitted from the engine. The higher the load, the more smoke is emitted. The larger droplets produced by B20 can lead to droplet impingement on the walls of the combustion chamber, which can result in incomplete combustion and increased smoke opacity [Citation23]. Additionally, the higher viscosity of B20 results in a slower evaporation rate that can cause incomplete combustion and more smoke. The addition of 100 parts per million of CeO2 to the B20 blend improves the atomisation of the fuel, which reduces the viscosity of the fuel droplets. This, in turn, reduces the amount of soot that is formed as it is more easily vaporised, thus leading to lower smoke levels.
4. Conclusions
In the present study, diesel exhibits poor combustion characteristics compared to other blends. The high boiling point and viscosity of diesel make it harder to vaporise and burn compared to other fuels. This can lead to incomplete combustion and higher emissions. The higher boiling point and viscosity of diesel make it to take longer for it to vaporise and burn. This results in longer ignition delays because it takes longer for the fuel to ignite. A lower heat release rate (HRR) and a longer combustion duration may also result in incomplete combustion and higher emissions. The transesterification increases the fuel’s volatility, which reduces ignition delays, increases HRR, and decreases combustion duration due to transesterification. The result is a cleaner and more efficient fuel that has fewer incomplete combustions and emissions. The addition of cerium oxide (CeO2) to the fuel blend increases its reactivity, resulting in a shorter ignition delay and higher heat release rate. It ensures better combustion and reduces emissions, making the fuel more efficient and cleaner to burn. In addition to acting as a catalyst, CeO2 nanoparticles accelerate the chemical reaction of fuel combustion. The result is a higher combustion rate and increased cylinder pressure, which in turn results in an earlier ignition. In comparison with other blends, this blend had the highest viscosity and density, as well as the lowest heat release and shortest ignition delay. Fuel efficiency and combustion performance will be better with this blend than with other blends.
Disclosure statement
The authors report that there are no competing interests to declare.
Data availability statement
The data used to support the findings of this study are included in the article.
Additional information
Notes on contributors
S. Karthikeyan
Dr. S. Karthikeyan is presently working as Professor in the Mechanical Engineering Department at Syed Ammal Engineering College, Ramanathapuram, Tamilnadu, India. He is having 20 years of teaching experience. He has produced three Ph.D. scholars and presently guiding 5 Ph.D. scholars. He has published 50 (SCI) and 36 (Scopus) research papers in International and National Journals. His specialized area is Thermal engineering, Biofuel, Engine, and solar technology. He received many research funds from AICTE, DRDO, and TNSCST. He finished his doctoral research in Engine Testing at Anna University, Tamilnadu, India, in 2015. He obtained his post-graduation in Anna University, Chennai from 2004 to 2007 and a Bachelor of Engineering at Madurai Kamaraj University, Tamilnadu, India, from 1991 – 1995 from the same college. Currently, he is act as a guest editor of different journals like Elsevier, Springer, Hindawi, etc…
T. Dharmaprabhakaran
Mr. T. Dharmaprabhakaran is presently working as Assistant Professor in the Mechanical Engineering Department at Syed Ammal Engineering College, Ramanathapuram, Tamilnadu, India. He is having 6 years of teaching experience. He has published 5 (SCI) and 5 (Scopus) research papers in International and National Journals. His specialized area is Thermal engineering, Biofuel, Engine, and solar technology.
Ekrem Yanmaz
Dr. Ekrem Yanmaz is presently working as a Professor at Nisantasi University, Faculty of Architecture-Engineering, Department of Electric And Electronics, Ayazağa, Maslak-İstanbul, TURKEY. He is having 37 years of teaching experience. He has published 120 research papers in International and National Journals. His specialized area is High-Temperature Superconductors, Magnesium, Diboride, Nanomagnetics Materials.
Sana Sulaiman Hamid
Dr. Sana Sulaiman Hamid, He is from Iraq, He is 29 years old, He has received his PhD. from Turkey, and a Master degree from Iraq, Baghdad. He has many research papers on the subject of data aggregation, wireless sensor, BEE, optimisation technique, right now He is a Faculty on Al-Farahidi University, Communication Technical Engineering, Baghdad, Iraq.
T. Bothichandar
T. Bothichandar Working as a Faculty in Industrial Engineering Department, AMBO UNIVERSITY, AMBO, ETHIOPIA, He is having 6 years of teaching experience. He has published 20 research papers in International and National Journals. His specialized area is Thermal engineering and Manufacturing.
References
- Daneshvar E, Wicker RJ, Show PL, et al. Biologically-mediated carbon capture and utilization by microalgae towards sustainable CO2 biofixation and biomass valorization—a review. Chem Eng J. 2022;427:1.
- Karthikeyan S, Periyasamy M, Prathima A, et al. Combustion analysis of single-cylinder CI engine fueled with S. marginatum macro algae biofuel- diesel blends. Elsev Mater Today Proceed. 2020;33(7):3475–14.
- Yadav PS, Gautam R. Numerical and experimental analysis on spray characteristics of biodiesel (waste cooking oil) using pressure swirl atomizer. Environ Prog Sustain Energy. 2022;41(3):e13761.
- Zhang W, Deng Z, Wang Q, et al. Experimental research on chemical desorption based on CO2-rich absorption solutions. Int J Green Gas Con. 2021;109:103356.
- Faruque MO, Razzak SA, Hossain MM. Application of heterogeneous catalysts for biodiesel production from microalgal oil—a review. Catalysts. 2020;10(9):1025.
- Karthikeyan S, Periyasamy M, Prathima A, et al. Agricultural tractor engine performance analysis using Stoechospermum marginatum microalgae biodiesel. Elsev Mater Today Proceed. 2020;33(7):3438–3442.
- Karthikeyan S, Periyasamy M, Prathima A, et al. Effect of biosolar fuels and S. marginatum algae biofuel on equivalence ratio in diesel engine with EGR. Elsev Mater Today Proceed. 2020;33(7):3443–3448.
- Karthikeyan S, Periyasamy M, Prathima A. Combustion analysis of CI engine using pilot dual fuel blends. Elsev Mater Today Proceed. 2020;33(7):3254–3259.
- Karthikeyan S, Periyasamy M, Prathima A. Performance characteristics of CI engine using Chlorella vulgaris microalgae oil as a pilot dual fuel blends. Elsev Mater Today Proceed. 2020;33(7):3277–3282.
- Kant Bhatia S, Kant Bhatia R, Jeon JM, et al. An overview on advancements in biobased transesterification methods for biodiesel production: oil resources, extraction, biocatalysts, and process intensification technologies. Fuel. 2021;285:119117.
- Rizwanul Fattah IM, Ong HC, Mahlia TMI, et al. State of the art of catalysts for biodiesel production. Front Energy Res. 2020;8:1–17.
- Changmai B, Vanlalveni C, Ingle AP, et al. Widely used catalysts in biodiesel production: a review. RSC Adv. 2020;10(68):41625–41679.
- Aga WS, Fantaye SK, Jabasingh SA. Biodiesel production from Ethiopian ‘Besana’ – Croton macrostachyus seed: characterization and optimization. Renew Energy. 2020;157:574–584.
- Hamzah NHC, Khairuddin N, Siddique BM, et al. Potential of Jatropha curcas L. as biodiesel feedstock in Malaysia: a concise review. Processes. 2020;8(7):786.
- Nair JN, Murthy YVVS, Javed S. Combustion and emission characteristics of light duty diesel engine fueled with transesterified algae biodiesel by K2CO3/ZnO heterogeneous base catalyst. Energy Sour A Recov Util Environ Effects. 2020:1–13.
- Nanthagopal K, Kishna RS, Atabani AE, et al. A compressive review on the effects of alcohols and nanoparticles as an oxygenated enhancer in compression ignition engine. Energy Convers Manag. 2020;203:112244.
- Karthikeyan S, Periyasamy M, Prathima A. Emission analysis of CI engine fueled by pilot dual fuel blends. Elsev Mater Today Proceed. 2020;33(7):3248–3253.
- Karthikeyan S, Periyasamy M, Prathima A. Biodiesel from microalgae environmental aspects. Elsev Mater Today Proceed. 2020;33(7):3664–3667.
- Prathima A, Karthikeyan S, Radhi Devi K, et al. Investigations on the binary mixtures of organic additives in lubricating oil-SAE15W40 through ultrasound velocity measurements. Res. J. Chem. Sci. 2013;3(5):43–46.
- Ning L, Duan Q, Chen Z, et al. A comparative study on the combustion and emissions of a non-road common rail diesel engine fueled with primary alcohol fuels (methanol, ethanol, and n-butanol)/diesel dual fuel. Fuel. 2020;266:117034.
- Shrivastava P, Salam S, Verma TN, et al. Experimental and empirical analysis of an IC engine operating with ternary blends of diesel, Karanja and Roselle biodiesel. Fuel. 2020;262:116608.
- Uslu S, Aydın M. Effect of operating parameters on performance and emissions of a diesel engine fueled with ternary blends of palm oil biodiesel/diethyl ether/diesel by Taguchi method. Fuel. 2020;275:117978.
- Yesilyurt MK, Yilbasi Z, Aydin M. The performance, emissions, and combustion characteristics of an unmodified diesel engine running on the ternary blends of pentanol/safflower oil biodiesel/diesel fuel. J Therm Anal Calorim. 2020;140(6):2903–2942.
- Prathima A, Karthikeyan S, Shanthi M, et al. Environmental effect of lubricity additives through dielectric molecular parameters. Elsev Mater Today Proceed. 2020;33(7):3658–3663.
- Zhen X, Wang Y, Liu D. Bio-butanol as a new generation of clean alternative fuel for SI (spark ignition) and CI (compression ignition) engines. Renew Energy. 2020;147:2494–2521.
- EL-Seesy AI, Waly MS, He Z, et al. Influence of quaternary combinations of biodiesel/methanol/n-octanol/diethyl ether from waste cooking oil on combustion, emission, and stability aspects of a diesel engine. Energy Convers Manag. 2021;240:114268.
- Gillani SE, Ikhlaq M, Khan MU, et al. Effects of butanol blending and fumigation with Jatropha biodiesel on combustion, performance, and emissions of diesel engine. Int J Environ Sci Technol. 2021;18(4):819–834.
- Karthikeyan S, Periyasamy M, Prathima A, et al. Performance and exhaust emissions of a CI engine using Bi2O3 nano blends as an alternate Caulerpa racemosa algae oil biofuel. Elsev Mater Today Proceed. 2020;33(7):3265–3270.
- Kipkorir D, Nturanabo F, Tewo R, et al. Properties of waste-distilled engine oil and biodiesel ternary blends. Heliyon. 2021;7(8):e07858.
- Prathima A, Karthikeyan S, Mahalakshmi S, et al. Environmental effect of ZOCO (ZrO2/Ceo2) nano composite in methyl ester from canola oil through the performance and emission studies on IC engine. Elsev Mater Today Proceed. 2020;33(7):3203–3207.
- Sudalaiyandi K, Alagar KR, Manoj Praveen VJ, et al. Performance and emission characteristics of diesel engine fueled with ternary blends of linseed and rubber seed oil biodiesel. Fuel. 2021;285:119255.
- Comitre AA, Vaz BS, Costa JAV, et al. Renewal of nanofibers in Chorella fusca microalgae cultivation to increase CO2 fixation. Bioresour Technol. 2021;321:124452.
- El-Gendy NS, Nassar HN. Biosynthesized magnetite nanoparticles as an environmental opulence and sustainable wastewater treatment. Sci Total Environ. 2021;774:145610.
- Karthikeyan S, Periyasamy M, Prathima A, et al. Emission analysis of the diesel engine using Stoechospermum marginatum, brown marine algae with Al2O3 nano fluid. Elsev Mater Today Proceed. 2020;33(7):4047–4053.
- He X. Polyvinylamine-based facilitated transport membranes for post-combustion CO2 capture: challenges and perspectives from materials to processes. Engineering. 2021;7(1):124–131.
- Hemmati F, Bahrami A, Esfanjani AF, et al. Electrospun antimicrobial materials: advanced packaging materials for food applications. Trends Food Sci Technol. 2021;111:520–533.
- Karthikeyan S, Periyasamy M, Prathima A. Combustion analysis of a CI engine with Caulerpa racemosa algae biofuel with nano additives. Elsev Mater Today Proceed. 2020;33(7):3324–3329.
- Karpagam R, Jawaharraj K, Gnanam R. Review on integrated biofuel production from microalgal biomass through the outset of transesterification route: a Cascade approach for sustainable bioenergy. Sci Total Environ. 2021;766:144236.
- Lu C, Shi X, Liu Y, et al. Nanomaterials for adsorption and conversion of CO2 under gentle conditions. Mater Today. 2021;50:385–399.
- Pattanayak DS, Pal D, Thakur C, et al. Bio-synthesis of iron nanoparticles for environmental remediation: status till date. Mater Today. 2021;44:3150–3155.
- Rodas-Zuluaga LI, Castañeda-Hernández L, Castillo-Vacas EI, et al. Bio-capture and influence of CO2 on the growth rate and biomass composition of the microalgae Botryococcus braunii and Scenedesmus sp. J CO2 Util. 2021;43:101371.
- Rostamabadi H, Falsafi SR, Rostamabadi MM, et al. Electrospraying as a novel process for the synthesis of particles/nanoparticles loaded with poorly water-soluble bioactive molecules. Adv Colloid Interface Sci. 2021;290:102384.
- Sudagidan M, Yildiz G, Onen S, et al. Targeted mesoporous silica nanoparticles for improved inhibition of disinfectant resistant listeria monocytogenes and lower environmental pollution. J Hazard Mater. 2021;418:126364.
- Karthikeyan S, Periyasamy M, Prathima A, et al. Emission analysis of CI engine fueled with S. marginatum macro algae biofuel – diesel blends. Elsev Mater Today Proceed. 2020;33(7):3458–3463.
- Karthikeyan S, Periyasamy M, Prathima A, et al. Performance analysis of diesel engine fueled with S. marginatum macro algae biofuel-diesel blends. Elsev Mater Today Proceed. 2020;33(7):3464–3469.
- Karthikeyan S, Prathima A, Periyasamy M. Characteristics studies on Stoechospermum marginatum, brown marine algae with Al2O3 nanofluid. Elsev Mater Today Proceed. 2020;33(7):3746–3750.