Abstract
Efficient functional coupling of neuronal cells and electronic sensors could result in hybrid bidirectional communication between neurons and computers (L.J. Breckenridge et al., Advantages of using microfabricated extracellular electrodes for in vitro neuronal recordings, J. Neurosci Res. 42 (1995), pp. 266–276; G. Zeck and P. Fromherz, Noninvasive neuroelectronic interfacing with synaptically connected snail neurons immobilised on a semiconductor chip, PNAS 98 (2001), pp. 10457–10462). Such systems could enable us to gain insight into the mechanisms of neuro-degenerative diseases like Parkinson's and Alzheimer's disease in vitro or could be used to improve the function and efficiency of devices used in vivo, for example in Deep Brain Stimulation devices that are already used in the treatment of Parkinson's disease. One of the major challenges for the development of reliable neuro-electronic systems is to perform extracellular recordings of action potentials with a high signal-to-noise ratio. The poor quality of these recordings is caused by the culture medium, which is present in the cleft between the cell membrane and the sensor surface (P. Fromherz, Neuroelectronic interfacing: semiconductor chips with ion channels, nerve cells and brain, in Nanoelectronics and Information Technology, R. Waser, ed., Wiley–VCH, Berlin, 2003, pp. 781–810; G. Zeck and P. Fromherz, Noninvasive neuroelectronic interfacing with synaptically connected snail neurons immobilised on a semiconductor chip, PNAS 98 (2001), pp. 10457–10462). In this article, we describe a method allowing a reduction of the distance between the membrane and the surface by combining surface chemistry and topography. We have developed a specialised surface chemistry, based on small laminin-derived peptides, which applied onto the topographical structures, triggers their engulfment by the cell membrane in a phagocytosis-like event. In the phagocytotic pit, the distance between the cell membrane and the sensor surface is believed to be minimal. We describe the surface chemistry used for the controlled immobilisation of the small peptides on the surface of the needle-like structures that are manufactured on the surface of electronic devices. PC12 neuro-blastoma cells and genetically-modified HeLa cells have been used to investigate the interaction between the cell membrane and the peptide functionalised topographical structures. The membrane–surface interaction was examined by means of electron microscopy and fluorescence microscopy.
1. Introduction
Integration of nerve cells with microelectronics has been subjected to extensive research in the last few years Citation1. Neuro-electronic hybrid devices are considered to become a useful tool in the research of neuro-degenerative diseases like Alzheimer's disease or Parkinson's disease. They offer the possibility to perform long-term studies of the electrical activity of each individual neuron in an entire predefined neuronal network in a non-invasive manner. This is a great advantage compared to standard electrophysiological tools like the patch clamp technique. However, in order to record neuronal signals efficiently, tight coupling between the sensor and the cell membrane is one of the major requirements. The electrical coupling between the cell and the sensor is capacitive. The capacity of the cell membrane changes during an action potential and this is picked up by the sensor. However, the cell membrane is not perfectly attached to the sensor and a small gap exists, which is filled with cell culture medium, creating a leaky path. Moreover, due to their inherent mobility, the position of the cell body changes with respect to the recording site. In the past, several attempts were made to cage the neurons above the sensor using polyimide pillars or by guiding the neurons using predefined networks in elastomeric polymers such as PDMS or agarose Citation2–4. However, the forces which are exerted on the cell bodies during the formation of the synapses are large enough to pull these away from the sensor and thereby decrease the signal-to-noise ratio of the recordings. The viability and function of the neurons in such artificial environments are a major point of discussion.
We present an approach where a combination of topography and specialised surface functionalisation is used in order to induce a biological phenomenon, namely phagocytosis, around the topographical cues in order to improve the coupling.
Phagocytosis is a common cellular mechanism to internalise large particles. In a normal cell such as a macrophage, this process starts with the interaction between a particle and certain cell membrane receptors that can induce phagocytosis Citation5. Upon binding to these receptors, they will recruit the actin cytoskeleton and form a phagocytotic pit and finally engulf the particle inside the cell. In our application, this means that ligands should be available on the needles. They react with cell membrane receptors and induce phagocytosis. The cell will try to internalise the needle, creating a very tight seal.
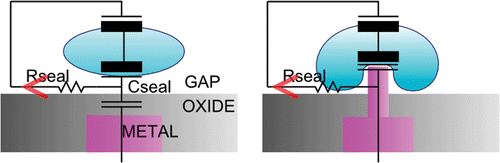
The use of this phagocytosis-like event offers three major differences compared to the previously-discussed methods of caging or using flat sensors ().
-
It makes sure that the contact area of the sensor is enlarged. This increases the signal-to-noise ratio.
-
The distance between the cell membrane and the sensor surface is minimal in such a phagocytotic pit.
-
The neuron is anchored above the sensor using intracellular pillars instead of extracellular ones.
Figure 1. Schematic comparison of a neuron–sensor interface using a flat sensor and a sensor with needles.
First, we have fabricated the topographical structures using deep UV lithography. The most important aspect is the functionalisation of the needles, since this should evoke the cell to engulf the needles in a phagocytosis-like event. Therefore, we use a small laminin-derived peptide namely PA22-2. This 19-mer is derived from the A chain of laminin with amino acid sequence: CSRARKQAASIKVAVSADR. This peptide is known for its ability to improve the cell adhesion and to stimulate the neurite outgrowth Citation6,Citation7. In previous work we showed that phagocytosis-like events were observed when using fluorescent PA22-2 functionalised microbeads Citation8. This surface chemistry has been transferred to the small gold needles on chips and we could visualise the membrane engulfment around the needles using electron microscopy and fluorescence imaging.
2. Material and methods
2.1. Surface chemistry
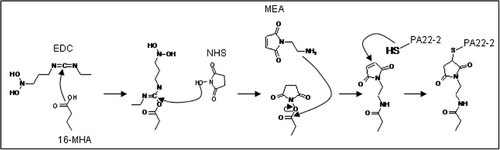
The PA22-2 peptide is synthesised by Pepscan N.V. (The Netherlands). The peptide is designed in such way that it posses a unique N-terminal cysteine. This allows us to control the peptide orientation on the surface. First, a Self Assembled Monolayer (SAM) is used to create the necessary functional groups on the gold surface. The SAM is directly formed on a gold surface by 3 h incubation in a 100% 10 mM 16-mercaptohexadecanoic acid (16-MHA, Sigma) solution Citation9. In the second step, the resulting carboxyl surface is transformed into a maleimide surface using first 0.1 M n-(3-diethylaminopropyl)-n-ethyl-carbodiimide (EDC, Sigma) and 0.4 M n-hydroxy-succinimide (NHS, Sigma), and second, a 50 mM 2-maleimidoethylamine (MEA, Molecular Biosciences) solution in a 10 mM Na borate buffer pH 8.5. The activation of the carboxyl groups with EDC/NHS is necessary to couple the maleimide cross-linker. The active maleimide groups on the surface are used to couple the peptides covalently. A schematic representation of the surface functionalisation can be found in .
In order to confer the peptide immobilisation on the chips, the same surface chemistry is applied. After the coupling, the peptide is fluorescently labelled using an Alexa-555 succinimidyl ester derivative (Invitrogen). The dye reacts well with the NH3 groups of the peptide. The dye is dissolved in 100 mM Na bicarbonate buffer and applied on the chip for at least 1 h at room temperature. Hereafter the sample is washed with PBS buffer. The results are examined using an Axioskop FS2 Mot (Zeiss) upright microscope equipped with an LSM5 Pascale confocal laser scanning unit.
2.2. Cell cultures
HeLa cells are cultured in DMEM/F12 (1 : 1) supplemented with 10% foetal calf serum (FCS). Cells are seeded in six-well plates at 150,000 cells/well. Stable Telencephalin (TLN) expressing HeLa cells are generated by co-transfection of pSG5FLTLN with a plasmid carrying the neomycin resistance gene and selected in the presence of 500 µg mL−1 G418 (Invitrogen).
PC12 cells are cultured in laminin (sigma)-coated dishes using RPMI 1640 medium (Invitrogen) supplemented with 10% horse serum and 5% FCS. For the coating, a 2 µg cm− 2 of laminin in HBSS solution is applied to the dish 20 min prior to the culture. Cells are seeded on the substrates at a density of 100,000 cells well−1. After 12 h, the medium is changed to medium containing only 1% horse serum and 0.5% FCS. Nerve Growth Factor 7s (Sigma) and Forskolin (Sigma) are added to a respective concentration of 100 µg mL−1 and 50 µM.
2.3. Scanning electron microscopy
For scanning electron microscopy (SEM), the cells are fixed using 2.5% gluteraldehyde. The cells are kept in this solution for 2.5 h at room temperature. They are dehydrated using an ethanol series containing 30, 50, 70, 90 and 100% ethanol. The samples are incubated two times for 10 min in each of these concentrations. Hereafter they are subjected to critical point drying. This allows the cells to maintain their original shape.
3. Results
3.1. Processing of the needles
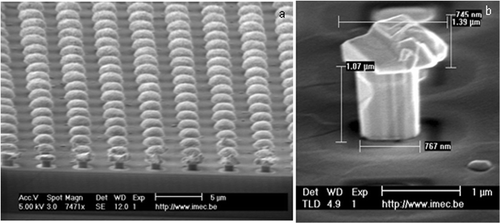
Nails with dimensions from hundreds of nanometers up to millimeter sizes can be processed by standard CMOS techniques. Once the necessary bond paths are processed in metal, a thick oxide layer is deposited. Vias are etched in this layer using Deep Reactive Ion Etching (DRIE) techniques. The width of the vias will determine the final thickness of the needle. In our case, the diameter varies between 250 nm and 1 µm. Hereafter the needles are formed using Au electroplating. Finally, they are released either by removing the surrounding oxide with buffer HF for 2.5 min or by a RIE etch. The final needle structure is represented in .
3.2. Coupling of PA22-2
In order to prove the coupling of the peptide on the gold needles of the chip, the peptide was labelled with an Alexa 555 dye after immobilisation on the surface. The result of such a labelling can be observed in .
Figure 4. Coupling of the peptide on the gold needles of the chip: (a) brightfield image of the chip and (b) fluorescent image of the same area.
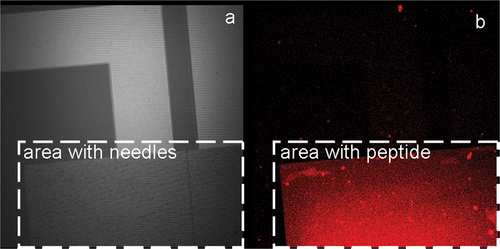
As illustrated in the area with the needles, as observed in the brightfield picture () and the signal of the Alexa 555 () are overlapping perfectly. This means that peptide is present in the same area and thus can be immobilised on the gold needles.
3.3. Phagocytosis experiments
The initial experiments were carried out using HeLa cells because they offer a higher throughput compared to primary cultures of hippocampal neurons. HeLa cells do not express the receptor, which was identified in neurons to cause phagocytosis, namely Telencephalin (TLN) or ICAM-5 Citation10. Therefore, these cells were genetically modified using a plasmid encoding full length TLN together with GFP. This allowed us also to study the interaction between the needles and the cells, using fluorescence microscopy. Upon culturing these cells on top of Alexa 555 labelled PA22-2 on the chips, Z-stacks could be made at 0.5 µm intervals. shows the result of such a Z-stack.
Figure 5. Z-stack of TLN expressing HeLa cells cultured on top of Alexa 555 labelled PA22-2 on top of the needles. The small insets form a schematic representation of the experimental set-up with the confocal plane of the picture as a dashed line. The consecutive pictures are taken at 0.5 µm intervals.
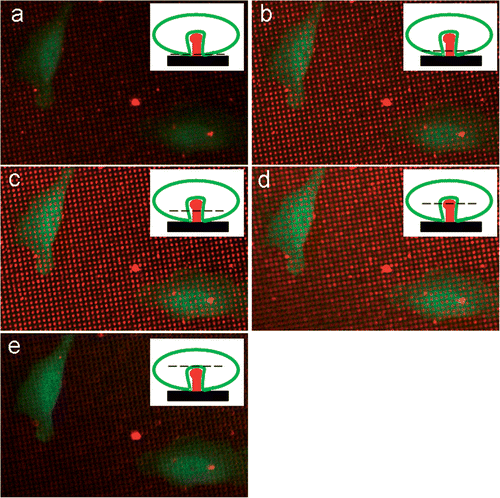
In , we can observe the fluorescence at the two wavelengths during a Z-stack. The interval between the pictures was set at 0.5 µm. represents the bottom plane of the chip. The red fluorescent signal of the needles is weak but we can see also the bottom cell membrane. In , the confocal plane is moved up respectively by 0.5, 1 and 1.5 µm. Here, the red fluorescent signal of the needles is very clear and the green fluorescent signal is absent at the place of the needles. This means that the membrane is folded around the needles. If we move the confocal plane another 0.5 µm (), the confocal plane is above the needles and the cell is seen as an uninterrupted green layer. These data suggest that the cell membrane is completely surrounding the needles. These samples were also dried and examined using SEM. The following figures were obtained ().
Figure 6. SEM pictures of HeLa cells growing on needles: (a) overview picture of a cell growing on a needle-bed. (b) Detailed picture of a cell extension that engulfs several needles.
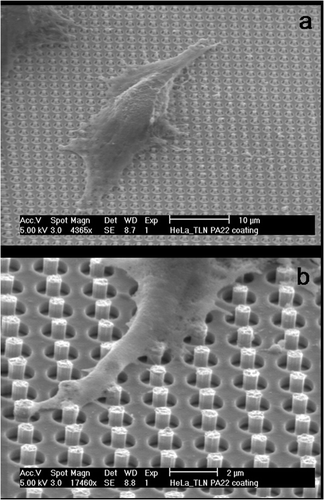
From it can be observed that the HeLa cells grow on top of the needles. If more closely examined, it can be seen that the extensions of the cells engulf the needles.
In the second step, PC12 neuro-blastoma cells were used as a model system for the hippocampal neurons. These cells were fixated and dried for SEM examination. The adhesion of PC12 cells on nails is shown in .
Figure 7. SEM pictures of PC12 cells growing on PA22-2-coated gold needles: (a) overview of the entire cell as it grows on the needles. (b) Detailed view showing a strong interaction between the cell membrane and the needles that becomes visible through the bending of the needles.
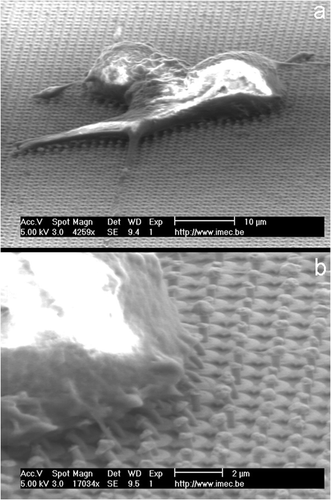
As observed from , there is a strong interaction between the needles and the cell membrane. The cell membrane is strongly attached. This figure even shows that the needles are bending at the edge of the cell membrane. This clearly indicates the existence of a strong and stable interaction between the cell membrane and the needles. However, SEM does not allow us to observe ultra-thin cross-sections of the membrane–nail interface. For this purpose, transmission electron microscopy (TEM) should be better suited.
4. Summary
Neuro-electronic hybrid devices are very promising tools to investigate the origin and possible cures for neuro-degenerative diseases and give us insight in basic cellular processes such as cell adhesion, communication and motility. The major advantages compared to the standard electrophysiological techniques are the non-invasive character and the high-throughput possibilities. This makes it possible to follow the activity of an entire network of neurons over an extended period of time.
However, there are still a couple of problems that need to be solved. The major problem is the low signal-to-noise ratio of the extracellular recordings due to the poor cell adhesion.
In this article, we present a technique which uses a needle-like sensor-surface to improve the coupling. The idea that the needle could be engulfed by the cell in a phagocytosis-like event would create a very tight seal and increase the contact area between the sensor and the cell membrane. This will increase the signal-to-noise ratio of the extracellular recordings. At the same time, the needles will act as anchor and immobilise the cells above the sensor.
We could successfully manufacture gold needles of micron-size dimensions using standard lithographic techniques. Those needles are functionalised with a small laminin-derived peptide, namely PA22-2. This peptide is known to improve the cell adhesion and stimulate the neurite outgrowth. In this article, we prove that the peptide is immobilised on top of the needles.
In the second step, HeLa cells are genetically modified to express the receptor for phagocytosis, which is also expressed in neurons. Those TLN expressing HeLa cells also expressed GFP. Using a Z-stack on the fluorescent needles, it was possible to demonstrate that the cell membrane of the HeLa cells was folded in between the needles. This is a clear indication for the appearance of phagocytosis-like events around the needles. SEM examination of those samples also revealed that the cells reached out to the needles and that the cell membrane is folded around those needles. This is also a clear indication for the appearance of phagocytosis-like events.
PC12 cells were cultured on these chips because they are more closely related to hippocampal neurons, which represent the final goal. Using these cells, the same events could be observed. Using the SEM, we were able to show that the needles were bended by the cell. This implies a very strong interaction between the cell membrane and the needle surface.
In the future, TEM studies of the interface between the cell membrane and the sensor surface should be performed. TEM should enable us to measure the dimensions of the cleft formed between the cell membrane and the needle surface. This is in contrast with SEM where only information about the boundaries of the cell membrane can be obtained. The disadvantage of TEM is the long preparation time and the fact that it is very labour intensive with a very low success rate.
References
- Heer , F , Franks , W , Blau , A , Tashini , S , Ziegler , C , Hierlemann , A and Baltes , H . 2004 . CMOS microelectrode array for monitoring electrogenic cells . Biosens. Bioelectron. , 20 : 358 – 366 .
- Heller , DA , Garga , V , Kelleher , KJ , Lee , TC , Mahbubani , S , Sigworth , LA , Randall Lee , T and Rea , MA . 2005 . Patterned networks of mouse hippocampal neurons on peptide-coated gold surfaces . Biomaterials , 26 : 883 – 889 .
- Park , TH and Shuler , ML . 2003 . Integration of cell culture and microfabrication technology . Biotechnol. Prog. , 19 : 243 – 253 .
- Stenger , DA , Gross , GW , Keefer , EW , Shaffer , KM , Andreadis , JD , Ma , W and Pancrazio , JJ . 2001 . Detection of physiologically active compounds using cell-based biosensors . Trends in Biotech. , 19 : 304 – 309 .
- DeMali , KA and Burridge , K . 2003 . Coupling membrane protrusion and cell adhesion . J. Cell Science , 116 : 2389 – 2397 .
- Edgar , D , Timpl , R and Thoenen , H . 1988 . Structural requirements for the stimulation of neurite outgrowth by two variants of laminin and their inhibition by antibodies . JCB , 106 : 1299 – 1306 .
- Tashiro , K , Sephel , GC , Weeks , B , Sasaki , M , Martin , GR , Kleinman , HK and Yamada , Y . 1989 . A synthetic peptide containing the IKVAV sequence from the A chain of laminin mediates cell attachment, migration and neurite outgrowth . JBC , 264 : 16174 – 16182 .
- Van Meerbergen , B , Raemaekers , T , Winters , K , Braeken , D , Bartic , C , Spira , M , Engelborghs , Y , Annaert , WG and Borghs , G . 2007 . Improving neuronal adhesion on chip using a phagocytosis-like event . J. Exp. Nanosci. , 2 : 101 – 114 .
- Mrksich , M . 2000 . A surface chemistry approach to studying cell adhesion . Chem. Soc. Rev. , 29 : 267 – 273 .
- Esselens , C , Oorschot , V , Baert , V , Raemaekers , T , Spittaels , K , Zheng , H , Saftig , P , De Strooper , B , Klumperman , J and Annaert , W . 2004 . Presenilin 1 mediates the turnover of telencephalin in hippocampal neurons via an autophagic degradative pathway . JCB , 166 : 1041 – 1054 .