Abstract
This paper reports the reversible aggregation of porphyrin molecules in a thin solid film. A commercially available molecule, p-meso-tetra(4-pyridyl)porphyrin (H2TPyP(4)), was used for these studies. Optical absorption spectroscopy of the solution and solid state forms of this molecule show that even in the solid state the molecules have enough mobility to undergo the movement required to form J-aggregates. Resonance light scattering (RLS), fluorescence spectroscopy and atomic force microscopy (AFM) are used to support these results.
1. Introduction
Porphyrins, cyanines and other related molecules have been the source of intense research for many years. They are structurally similar to chlorophyll, a naturally occurring molecule with many analogues, which is part of all photosynthetic units (PSUs) Citation1. The PSU of purple bacteria, for example, consists of two light harvesting (LH) anntennae and a reaction centre (RC). Absorption of photons by the LH units and subsequent electron transfer to the RC, results in very efficient charge-separation (∼95% quantum efficiency) Citation2. Porphyrins, like their natural counterpart, have extremely high extinction cross-sections (∼250,000 M−1 cm−1), their absorption and emission maxima can be tuned throughout the visible spectrum (for example by changing the metal ion they complex), and their optical properties vary substantially depending on their aggregation state Citation3. As a result, porphyrins are considered for a very diverse range of applications such as molecular photovoltaic and optoelectronic devices Citation4, Citation5, optical sensors Citation6, and photodynamic cancer therapy Citation7. Here we present a method to reversibly aggregate porphyrins in thin films.
Solid state forms of porphyrin are of technological relevance because of their use in a number of applications Citation8, Citation9. Many studies have been carried out to characterise the morphology of porphyrin thin films Citation10, Citation11, and a few have dealt with their optical properties Citation12–14. Recently, a method to reversibly change the aggregation state of the porphyrins in a solid film was presented Citation8. It was based on the exposure of the film to vapours of acids and bases. It was suggested that this could be used for sensing purposes.
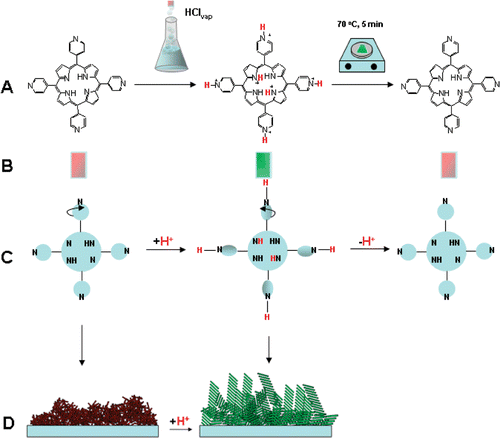
Here we describe a similar method to reversibly switch porphyrin aggregates in solid state. Films of H2TpyP(4), , were cast onto glass slides and forced into J-aggregates by exposure to HCl vapours. The original state could be restored by heating at 70°C. summarises the results presented in this paper.
Scheme 1. A. Exposure of porphyrin film to vapours of HCl induces aggregation. This is reversed by heating briefly on a hotplate. B. Colour change in this film after exposure to acid. C. The change in configuration of the porphyrin upon protonation. D. Representation of change in film morphology due to porphyrin aggregation (see AFM, ).
In solution, it is known that well-solvated (hereafter called monomeric) free-base porphyrins can form electronically coupled aggregates with distinct optical properties. In extreme cases they form H- or J-aggregates where the porphyrin molecules stack head-to-head or head-to-tail with a blue or red-shift of the Soret band respectively. Methods to control the molecules’ aggregation state range from the use of surfactants Citation15, to the control of the solutions’ ionic strength or pH Citation16–19. It should be noted that generally these experiments have been carried out in water or biphasic mixtures of solvents Citation20. Only limited work has been done on the aggregation of porphyrins in homogeneous organic solutions, where aggregation could be achieved through the addition of acids or through the UV irradiation of chlorinated solvents Citation21, Citation22.
2. Results and discussion
2.1. Solution state characterisation
2.1.1. Optical absorption spectroscopy
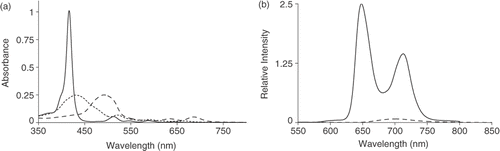
shows the optical absorption spectra of a chloroform solution of H2TPyP(4) (10−5 M) in its monomeric, tetra-protonated and aggregated states. The spectral features of the monomeric porphyrin are characterised by a Soret (λmax = 418 nm) and four Q-bands. Gradual addition of HCl shows a red-shift in the Soret with a clear intermediate (λmax = 450 nm), assigned to H4TpyP(4)4+. The J-aggregate is formed upon further addition of HCl, leading to the addition of two more protons yielding H6TpyP(4)6+. This is characterised by a further red-shift in the Soret band (λmax = 470 nm), while the four Q-bands become two indicating a symmetry change, D4h → D2h. J-aggregates of porphyrins can be generated using UV irradiation of chlorinated solvents or addition of HCl. This is also true in the specific case of H2TPyP(4). The mechanism for the J-aggregation of H2TPyP(4) under acidic conditions has been postulated Citation23. What seems clear is that the band at 450 nm is assigned to the protonation of the four outer pyridyl rings, H6TpyP(4)4+, as the four Q-band system is maintained. Upon protonation of the inner pyrollene-like nitrogens aggregation is complete. The meso-substituted moieties in the free base porphyrin are converted from a non-planar configuration to one that is co-planar with the inner porphine macrocycle. This planarisation reduces the steric hindrance between the meso-groups allowing the porphyrin molecules to stack head-to-tail forming J-aggregates. Fluorescence emission spectroscopy, , has shown that there is a decrease in emission of the aggregate, probably due to a decrease in the lifetime of the photo-excited state Citation24. A similar aggregation mechanism should also, in principle, be possible in solid state.
Figure 2. (a) Optical absorption of H2TPyP(4) in chloroform showing monomeric (solid line), tetra-protonated (dotted line) and the aggregated (dashed line) states. (b) The fluorescence spectra of H2TPyP(4) in de-gassed chloroform showing the monomeric (solid line) an aggregated (dashed line) states.
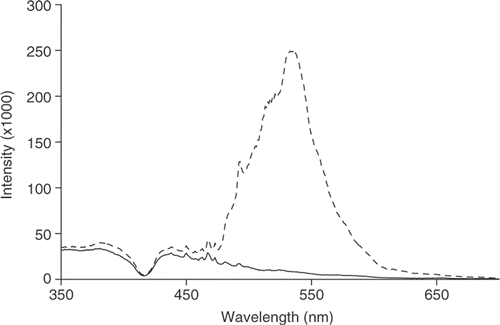
2.1.2. Resonance light scattering (RLS)
RLS was used to confirm the existence of J-aggregates. It is known that, for electronically coupled molecular aggregates, close to the wavelength of absorption of the aggregate there is a very large scattering peak Citation25. confirms both the monomeric and aggregated states of the porphyrin in solution.
2.2. Solid state characterisation
2.2.1. Optical absorption spectroscopy
Films of H2TPyP(4) showed a Soret band with an absorbance at 423 nm and four Q-bands (). When the films were exposed to HCl vapours changes in both their optical properties and in their morphology were observed. The optical properties identified were almost identical for both the solution and solid state. It is therefore reasonable to assume that J-aggregation in the solid state also occurred.
Figure 4. Optical absorption of H2TPyP(4) in solid state showing monomeric (solid line), tetra-protonated (dotted line) and the aggregated (dashed line) states.
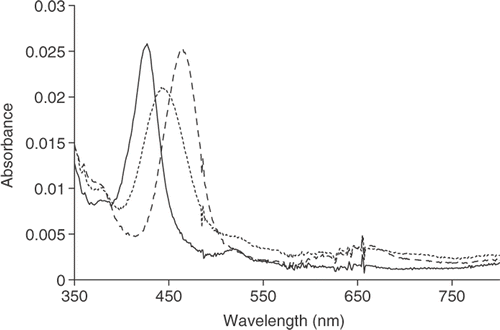
The Soret band red-shifted (488 nm), while, at the same time, the four Q-bands became two bands, indicating the formation of J-aggregates in the film.
2.2.2. Atomic force microscopy
Upon exposure to HCl vapours, the overall morphology of the films undergoes a dewetting-like phenomenon where smooth films bead-up . This is consistent with a change in intermolecular conformation leading to an increase in local density. Films of aggregates were then heated using either a heat gun for a few seconds or by placing them in an oven at 70°C for 5–10 minutes. Their optical properties returned to the original casting state (). Further heating (24 hours at 100°C) was needed to return the film to its original morphology.
Figure 5. AFM of H2TPyP(4) (a) before and (b) after exposure to HCl vapours. (Insets are photographs of the cast films before and after exposure of HCL).
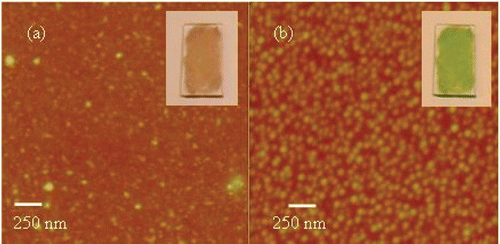
It should be noted that RLS measurement of the thin films were not possible, as the concentration was too low. It was found that the film morphology varied from fractal to smooth depending on the hydrophilicity of the glass substrate, on the speed of drying and mostly on humidity. In general under ambient conditions, using dry solvent, smooth films could be obtained (). Optical spectra that closely resembled those of the molecules in solution were obtained, when smooth films were cast.
Our experiments show that porphyrins in thin films have enough mobility to undergo conformational changes that lead to aggregation states similar to the ones in solution. It should be noted that the recovery of the optical properties upon heating is considerably faster than the recovery of the morphology. Optical properties probe very short range order (i.e. a few chromophores), while morphology depends on molecular arrangement on a longer range. When the molecules in the film disaggregate there is a weakening of the intermolecular attraction forces that were balancing surface tension to generate islands, resulting in an overall tensile stress. Given enough time and thermal induced mobility, the molecules reform a smooth film to minimise interfacial tension. The system described here can be cycled without the addition of new chemicals. Films have been cycled up to 10 times showing reversibility with no apparent molecular decomposition
3. Conclusions
In conclusion, here we have presented a method for the reversible aggregation of thin solid films of porphirins that allows us to tune the films’ optical properties to specific parts of the visible spectrum and to reversibly erase the changes by heating or by exposure to HCl vapours. The system described here can be cycled, without the addition of new chemicals, up to ten times showing no apparent molecular decomposition.
4. Experimental
All chemicals were purchased for Aldrich and used as received. All optical absorption spectra were carried out on a diode array Agilent spectrometer.
4.1. Solution state preparation and aggregation
Solutions of approximately 2 × 10−8 M in chloroform were used. Aggregation was induced by addition of a dilute solution of HCl (10 µL HCl in 5 ml of chloroform). Drops of this solution were added until the endpoint of aggregation (as monitored by the optical absorption spectrum) was reached.
4.2. Solid state sample preparation and aggregation
4.2.1. Subrate preparation
Glass microscope slides were cut into pieces 2 cm by 1 cm. They were cleaned by first immersing them in a freshly prepared piranha solution for 30 min after which time they were rinsed with MilliQ water. Subsequently, they were immersed in a solution of H2O : NH4OH : H2O2 (5 : 1 : 1) for 30 minutes at 80°C, and thoroughly rinsed with MilliQ water. They were then dried using purified dust free air.
4.2.2. Sample casting
Solutions of approximately 5 × 10−6 M were prepared in anhydrous chloroform for casting. 10–13 drops of the solution were dropped onto the cleaned glass slides until the surface was just covered. Drying of the film was carried in an evaporation chamber over chloroform vapours. All casting was carried out a room temperature.
Aggregation in the solid state was induced by holding the sample over the neck of a bottle of HCl (37%) for 1–2 seconds. To reverse the aggregation the sample was heating with a heat gun for ∼30 s or left in an oven at 70°C for a longer period of time.
Tapping Mode AFM images were obtained using a Digital Instrument MultiMode Nanoscope IIIa and a J scanner. All images shown in the paper were performed using Veeco Nanoprobe™ tips (Model #: RTESP; Length: 125 µm, Resonance Frequency ∼300 kHz).
Acknowledgements
The financial support of the National Science Foundation (NIRT DMR-0303973) and of the Petroleum Research Fund are kindly acknowledged. FS is grateful to 3M and to DuPont for young faculty awards.
Notes
†Dedicated to Áine Casserley, 18 June 2007–26 March 2008.
References
- Pearlstein , RM . 1987 . Photosynthesis , 299 – 317 . Amsterdam : Elsevier .
- van Oijen , AM . 1999 . Unraveling the electronic structure of individual photosynthetic pigment-protein complexes . Science , 285 : 401
- Smith , KM . 1986 . Porphyrins and Metalloporphyrins , Amsterdam : Elsevier .
- Imahori , H and Fukuzumi , S . 2004 . Porphyrin- and fullerene-based molecular photovoltaic devices . Advan. Funct. Mater. , 14 : 525 – 536 .
- Di Natale , C . 2000 . Porphyrins-based opto-electronic nose for volatile compounds detection . Sens. Actuat. B -Chem. , 65 : 220 – 226 .
- Okura , I . 2002 . Overview of optical sensors using porphyrins . J. Porph. Phthalocyan. , 6 : 268 – 270 .
- Bonnett , R . 1995 . Photosensitizers of the porphyrin and phthalocyanine series for photodynamic therapy . Chem. Soc. Rev. , 24 : 19 – 33 .
- De Luca , G . 2006 . Sensing behavior of tetrakis(4-sulfonatophenyl)porphyrin thin films . Chem. Mat. , 18 : 2005 – 2007 .
- Chowdhury , A and Pal , AJ . 2001 . Alternating current light-emitting devices based on Langmuir–Blodgett films of a porphyrin derivative: space charges in device operation . Thin Solid Films , 385 : 266 – 270 .
- Fagadar-Cosma , E . 2007 . Design of hybrid nanomaterials based on silica-porphyrin. AFM characterization . J. Optoelect. Advan. Mater. , 9 : 1878 – 1882 .
- Duong , B , Arechabaleta , R and Tao , NJ . 1998 . In situ AFM/STM characterization of porphyrin electrode films for electrochemical detection of neurotransmitters . J. Electroanal. Chem. , 447 : 63 – 69 .
- De Luca , G . 2006 . Control over the optical and morphological properties of UV-deposited porphyrin structures . Chem. Mater. , 18 : 5429 – 5436 .
- Scolaro , LM . 2005 . Unusual optical properties of porphyrin fractal J-aggregates . Chem. Commun. , 24 : 3018 – 3020 .
- Nagahara , T . 2005 . Morphological and spectroscopic properties of thin films of self-assembling amphiphilic porphyrins on a hydrophilic surface as revealed by scanning near-field optical microscopy . J. Phys. Chem. B , 109 : 19839 – 19844 .
- Maiti , NC , Mazumdar , S and Periasamy , N . 1998 . J- and H-aggregates of porphyrins with surfactants: Fluorescence, stopped flow and electron microscopy studies . J. Porphyrins Phthalocyanines , 2 : 369 – 376 .
- Hatano , T . 2004 . New morphology-controlled poly(aniline) synthesis using anionic porphyrin aggregate as a template and proton-driven structural changes in the porphyrin aggregate . Bull. Chem. Soc. Japan , 77 : 1951 – 1957 .
- Jiang , SG and Liu , MH . 2004 . Aggregation and induced chirality of an anionic meso-tetraphenylsulfonato porphyrin (TPPS) on a layer-by-layer assembled DNA/PAH matrix . J. Phys. Chem. B , 108 : 2880 – 2884 .
- Siskova , K , Vlckova , B and Mojzes , P . 2005 . Spectral detection of J-aggregates of cationic porphyrin and investigation of conditions of their formation . J. Molec. Struct. , 744 : 265 – 272 .
- Wangfuengkanagul , N and Tabata , M . 2005 . Change in conformation of J-aggregate 5,10,15,20-tetrakis(4-sulfonatophenyl)porphyrin (H2TPPS) by addition of nonionic surfactant (Triton X-100) . Chem. Lett. , 34 : 38 – 39 .
- Pasterna , RF . 1973 . Aggregation of nickel(Ii), copper(Ii), and zinc(Ii) derivatives of water-soluble porphyrins . Inorg. Chem. , 12 : 2606 – 2611 .
- Akins , DL , Zhu , HR and Guo , C . 1996 . Aggregation of tetraaryl-substituted porphyrins in homogeneous solution . J. Phys. Chem. , 100 : 5420 – 5425 .
- Scolaro , LM . 2003 . Porphyrin deposition induced by UV irradiation . J. Am. Chem. Soc. , 125 : 2040 – 2041 .
- De Luca , G , Romeo , A and Scolaro , LM . 2005 . Role of counteranions in acid-induced aggregation of isomeric tetrapyridylporphyrins in organic solvents . J. Phys. Chem. B , 109 : 7149 – 7158 .
- De Luca , G , Romeo , A and Scolaro , LM . 2006 . Counteranion dependent protonation and aggregation of tetrakis(4-sulfonatophenyl)porphyrin in organic solvents . J. Phys. Chem. B , 110 : 7309 – 7315 .
- Pasternack , RF and Collings , PJ . 1995 . Resonance light scattering: A new technique to study chromophore aggregation . Science , 269 : 935