Abstract
Polymeric micelle or self-aggregate formations have received much attention as carriers for drug delivery systems because of their high efficiency for drug delivery. Our recent results in the formation of nano-aggregates using chemically modified chitosan encouraged us to investigate the possibility of applying them as drug carriers. Therefore, we investigated a novel chitosan-based nano-aggregate as a drug carrier for sustained protein release. Thermosensitive chitosan–Pluronic (CP) was prepared by grafting monocarboxy Pluronic F127 onto the chitosan using 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide and N-hydroxysuccinimide. Bovine serum albumin (BSA)-loaded CP nano-aggregate was prepared by the direct dissolution method based on thermosensitive property of the CP copolymer. The physico–chemical properties of CP nano-aggregates were characterised by critical aggregate concentration, dynamic light scattering, and ζ-potential. In vitro release studies were investigated with BSA as a model protein at 37°C. BSA released from the CP nano-aggregates for about 6 h. The results suggest that CP nano-aggregates can potentially be efficient nanocarriers for protein delivery.
1. Introduction
Over the past few years, there has been intensive research interest in protein delivery because new therapeutic proteins have been developed via biotechnology. However, protein drugs have some limits in their application, including low stability and poor passage through biological membranes Citation1. To resolve these problems, various protein delivery carriers have been developed. In particular, self assembled nanoparticles such as micelles and aggregates have received great attention in protein delivery system development due to their easy modification.
Pluronic, tri-block copolymers consist of polyethylene oxide (PEO) and polypropylene oxide (PPO) blocks arranged in a basic structure: PEO–PPO–PEO. These amphiphilic copolymers that are composed of hydrophilic and hydrophobic parts can form micelles or aggregates with each characteristic structure in aqueous solution. The hydrophobic parts serve as a space to incorporate proteins, genes, and water-insoluble drugs. The hydrophobic part allows drugs to keep their activity because drugs in the space are protected from biological conditions.
Chitosan, the cationic polysaccharide derived from chitin, has been widely used in biomedical and pharmaceutical applications Citation2. The amine group of chitosan improves biocompatibility and binding affinity with negative charged proteins, drugs, and DNA Citation3. Chitosan was chosen for using in drug carriers due to its recognised muco-adhesivity and ability to improve the penetration of drugs through the mucosal surface Citation4.
In this study, we report new nano-aggregates of chitosan–Pluronic (CP) for controlled delivery with bovine serum albumin (BSA) as a model protein. The protein-loaded nano-aggregate was prepared by direct dissolution methods. Their physico–chemical properties were characterised by various methods. In vitro release study was investigated and the release kinetics of BSA was discussed.
2. Experimental
2.1. Materials
Chitosan (150 kDa, 75–85% deacetylated), 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide (EDC), N-hydroxysuccinimide (NHS), succinic anhydride, 4-morpholineethanesulfonic acid, 4-dimethylaminopyridine and BSA were purchased from Sigma-Aldrich (St. Louis, MO, USA). The Pluronic (12, 600 Da) was supplied from BASF Corp. PEG (3400 Da, pharmaceutical grade) was obtained from Polyscience Inc. All other chemicals and solvents were used without further purification.
2.2. Preparation of BSA-loaded CP nano-aggergates
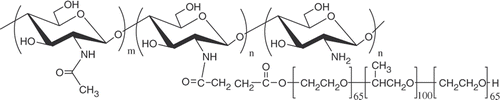
In our previous study, we reported thermosensitive CP copolymer as a hydrogel for tissue engineering Citation5. The CP copolymer was synthesised by grafting monocarboxylated Pluronic F127 onto chitosan back bone using EDC and NHS as coupling reagents. shows the chemical structure of the CP copolymer. In this study, we used two kinds of CP copolymer having different grafting percentage (GP) based on the weight ratio of chitosan to Pluronic Citation5. GP (%) was calculated by the difference of weights before and after grafting reaction according to following equations:
BSA-loaded CP nano-aggregates were prepared by a direct dissolution method Citation6. First, 35 mg CP copolymer was dissolved in 35 mL distillated water at below 4°C, followed by addition of BSA (6 mg/mL in 0.01 M PBS) and incubation at 37°C for 24 h. The unloaded BSA was completely removed using salt-out methods, in which an aqueous PEO solution (10 wt% solution) was added to the mixture and filtered. BSA-loaded CP nano-aggregate solution was sonicated for 30 min by BRANSON 5510 sonicator to give stable aggregates. Finally, BSA-loaded nano-aggregate solution was lyophilised to obtain dried product.
2.3. Determination of critical aggregate concentration
In order to determine the critical aggregate concentration (CAC) value of CP nano-aggregates, fluorescence measurement was carried out using pyrene as a probe. Aliquots of pyrene solution in acetone (1.2 × 10−7 M, 5 mL) were added to vials and the acetone was evaporated. Then polymer solutions with various concentrations in the range from 0.02 to 10 g L−1 were added to the labeled vials. The polymer solutions were incubated at 37°C for 24 h. The intensity of pyrene was plotted as a function of logarithm to the curve at the inflection with the horizontal tangent through the plots at low concentrations Citation7.
2.4. Characterisations of CP nano-aggregates
The size distribution of the nano-aggregates was measured by dynamic light scattering (DLS) (FPAR-1000, Photal, Japan) equipped with a He–Ne laser source at a wavelength of 633 nm at 37°C. Sample solutions were filtered with 0.45 µm prior to measurement. The mean diameter was evaluated from the Marquat method. To evaluate the loading amount of BSA, bicinchoninic acid assay was carried out. In addition, ζ-potential was measured to confirm their surface charge of CP nano-aggregates.
2.5. In vitro release test
The release kinetics of BSA from CP nano-aggregates was investigated. To the dried nano-aggregates, 1 mL of PBS was added to make aggregate solutions. The aggregate solution was then placed in a dialysis bag, which was immersed in a large vial containing warmed PBS (49 mL, pH 7.4, 37°C). The released medium (5 mL) was withdrawn at predetermined time intervals and replaced with an equivalent volume of fresh PBS.
3. Results and discussion
3.1. Characterisations of CP nano-aggregates
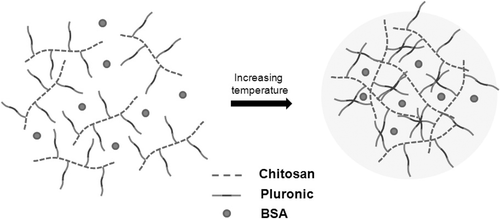
Chitosan–Pluronic copolymers with grafting yields of 1000% (CP1K) and 1500% (CP1.5K) were synthesised and characterised. The prepared CP copolymers formed nano-aggregates by increasing temperature in aqueous solution. shows schematic expression of BSA-loaded CP nano-aggregate formation.
The formation of the aggregates was investigated by the determination of CAC by a fluorescence method using pyrene. The emission spectra were recorded from 360 to 420 nm with 339 nm of excitation wavelength. As the concentration of the polymer solution increased, the fluorescence intensity increased at 395 nm because the pyrene was incorporated into hydrophobic part in the CP nano-aggregates. The intensity value remained below the CAC. Above that concentration, the intensity value was considerably increased (data not shown). The CAC values were 0.10 and 0.06 g L−1, respectively, as concerned with their GPs. As the GP increased, the CAC value decreased because the hydrophobic portion of the CP nano-aggregates increased Citation8. The CAC value, size, and surface charge of the CP nano-aggregate are shown in .
Table 1. The CAC value and size of the CP nano-aggregates.
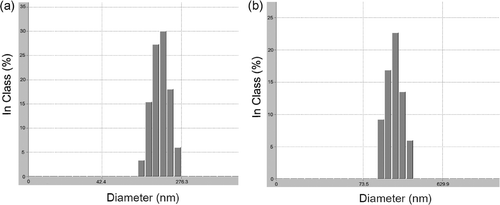
The particle size is an important factor in drug delivery systems (DDS). The particles larger than 5–7 µm are filtrated in the lung or spleen Citation9. To avoid this problem, particle size should be small enough (below 200 nm) for long circulation. exhibits the size distribution of CP nano-aggregate.
In the DLS measurements results, the size of the BSA-unloaded CP nano-aggregates was 80–110 nm in accordance with their GP.
In general, copolymers with larger molecular weight form larger aggregates. Therefore, the size of nano-aggregate of CP1.5K was larger. In the case of the BSA-loaded nano-aggregate, the size increased up to ∼160 nm. This result can be explained by the loading amount.
The BSA content entrapped in the CP aggregates was calculated from the weight of the initial drug loaded to the CP aggregates and the amount of drug incorporated from the following equation:
Table 2. BSA loading amount of the CP nano-aggregates.
3.2. In vitro BSA release profiles
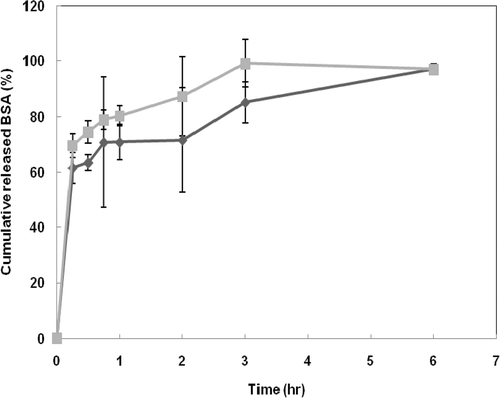
exhibits the release profiles of BSA from the CP nano-aggregates. The release behaviours of BSA showed similar profiles in both samples. However the BSA release rate from the nano-aggregate of CP1K was slightly slower than that of CP1.5K, resulting from difference of chitosan portion in aggregate structures Citation3. The burst release of BSA was shown during initial 45 min in both cases. The initial burst can be derived from the BSA loaded into the outer space, according to previous reports Citation4. After 45 min, the release rate of BSA from CP1.5K nano-aggregate was faster than that from the CP1K nano-aggregate. Almost the total amount of BSA was released from both CP nano-aggregates within 6 h. This release rate result is very fast compared with other DDSs. Particulate systems using biodegradable polymers, via emulsion methods, for protein delivery give robust matrices for encapsulation and delivery of protein to target sites, resulting in long release times. Polymeric micelle systems also show a relatively slow release rate due to the presence of a densely packed inner core of hydrophobic segments, although they are faster than systems of biodegradable polymers. However, CP nano-aggregates could be loosely packed self-assembled structures due to comb-like CP chains.
4. Conclusions
We prepared nano-aggregates of CP conjugates as a protein carrier by direct dissolution with BSA as a model protein. Our results demonstrated that the CP nano-aggregates have considerable potential as an injectable protein carrier for controlled drug delivery.
Acknowledgements
This research was supported from the Korea Science and Engineering Foundation (KOSEF) grant funded by the Korea government (MOST) (R01-2004-000-10164-0) and the ‘GRRC’ Project of Gyeonggi Provincial Government.
References
- Lee , SH , Zhang , Z and Feng , SS . 2007 . Nanoparticles of poly(lactide)-tocopheryl polyethylene glycol succinate (PLA-TPGS) copolymers for protein drug delivery . Biomaterials , 28 : 2041 – 2050 .
- Agnihotri , SA , Mallikarajuna , NN and Aminabhavi , TM . 2004 . Recent advances in chitosan-based micro- and nanoparticles in drug delivery . J. Control. Release , 100 : 5 – 28 .
- Yongmei , X and Yumin , D . 2003 . Effect od molecular structure of chitosan on protein delivery properties of chitosan nanoparticles . Int. J. Pharm. , 250 : 215 – 226 .
- Illum , L . 1998 . Chitosan and its use as a pharmaceutical excipient . Pharm. Res. , 47 : 1326 – 1331 .
- Chung , HJ , Go , DH , Bae , JW , Jung , IK , Lee , JW and Park , KE . 2005 . Synthesis and characterization of Pluronic grafted chitosan copolymer as a novel injectable biomaterial . Curr. Appl. Phys. , 5 : 485 – 488 .
- Allen , C , Maysinger , D and Eisenberg , A . 1999 . Nano-engineering block copolymer aggregates for drug delivery . Colloids Surf. B: Biointerfaces , 16 : 3 – 27 .
- Kabanov , AV , Nazarova , IR , Astafieva , IV , Batrakova , EV , Alakhov , VY , Yaroslavov , AA and Kavanov , VA . 1995 . Micelle formation and solubilisation of fluorescent probes in poly(oxyethylene-b-oxypropylene-b-oxyethylene) solutions . Macromolecules , 28 : 2303 – 2314 .
- Gao , Z and Eigenberg , A . 1993 . A model of micellization for block copolymers in solutions . Macromolecules , 26 : 7353 – 7360 .
- Stolnik , S , Illum , L and Davis , SS . 1995 . Long circulating microparticle drug carriers . Adv. Drug Deliv. Rev. , 16 : 195 – 214 .