Abstract
Covalent functionalisation of nanodiamond has been carried out by employing several methods. One of them involves the reaction of acid-treated nanodiamond with thionyl chloride followed by reaction with a long-chain aliphatic amine to produce the amide derivative. The second method involves reaction of acid-treated nanodiamond with an organosilicon or organotin reagent such as hexadecyltrimethoxysilane, dibutyldimethoxytin, and perfluoro-octyltriethoxysilane. The products of covalent functionalisation produce excellent dispersions in CCl4 and toluene. SiO2–and SnO2–covered nanodiamond are obtained by heating the nanodiamond coated with the organosilane and the organotin reagents, respectively. By interaction of nanodiamond with surfactants such as sodium bis(2-ethylhexyl) sulphosuccinate (AOT), Triton X-100 (TX-100), polyvinyl alcohol (PVA), cetyltrimethylammonium bromide (CTAB), and tert-octylphenoxy poly(oxyethylene)ethanol (IGEPAL) gives good dispersions in water, the best dispersion with the lowest surfactant concentration being obtained with IGEPAL.
1. Introduction
Nanodiamond is a carbon-based nanomaterial with potential technological applications which has attracted increasing attention in the last few years Citation1,Citation2. Synthesis of nanodiamond has been accomplished by several methods and the material is a commercial product. Surface modification of nanodiamond has been carried out by various means. Non-covalent functionalisation of nanodiamond has been attempted by absorption of certain molecules such as amino acids and proteins Citation3–5. One of the methods employed for covalent surface modification is to treat nanodiamond with acids or oxidising agents to create carboxyl and hydroxyl groups on the surface Citation1,Citation6,Citation7. Reaction with fluorine, ammonia and such gases has also been carried out to produce chemical changes on the surfaces Citation8,Citation9. Other reactions used are the reaction of silanes with hydroxylated nanodiamonds or reaction of halogenated nanodiamonds with amines and Grignard reagents Citation8,Citation10. One of the purposes of such functionalisation would be to produce new materials, especially composites. Even though many of these papers report reactions of nanodiamond surfaces with several chemicals or compounds, covalent functionalisation of nanodiamond is not fully accomplished Citation1. Furthermore, clear evidence for functionalisation has not been provided by spectroscopic, microscopic, and other physical measurements. Solubilisation of functionalised nanodiamond in different solvents has also not been reported. In view of the importance of functionalising nanodiamond and its subsequent solubilisation in polar and nonpolar solvents as well as water, we have carried out careful investigations making use of the experience with carbon nanotubes and graphene Citation11–13. We have achieved covalent functionalisation of nanodiamond through the preparation of a long-chain amide derivative and also by the reaction with organosilicon and organotin reagents. Noncovalent functionalisation with surfactants gives good water dispersions of nanodiamond.
2. Experimental
Nanodiamond with phase purity higher than 98% and an average particle size of around 5 nm (Tokyo Diamond Tools, Tokyo, Japan) was used for the studies. It was first acid treated to create surface carboxyl and hydroxyl groups. For this purpose, 100 mg of the nanodiamond was refluxed with 5 mL conc. HNO3 and 45 mL conc. H2SO4 for 12 h Citation14. The acid-treated nanodiamond was washed with distilled water and dried under vacuum. The acid-treated nanodiamond was reacted with thionyl chloride for 12 h in a nitrogen atmosphere to generate surface–COCl groups. This was reacted with an excess of oleyl amine refluxed for 12 h, washed with toluene, and dried under vacuum overnight.
To coat nanodiamond with the organosilicon or organotin reagents hexadecyltrimethoxysilane (HDTMS), dibutyldimethoxytin (DBDMT), and perfluoro-octyltriethoxysilane (PFOTES) purchased from Sigma Aldrich, were used without any further purification. A 20 mL of dry toluene was taken with 30 mg of the nanodiamond. To this the organosilane or organotin reagents (1:1 molar ratio) was added in a nitrogen atmosphere. The mixture was refluxed for 12 h, after which the mixture was cooled, the residue washed with dry toluene and then with water–acetone mixture (20% of H2O). The functionalised nanodiamond was dried in vacuum for a day under ambient temperature. Dispersion studies were carried by sonication of the functionalised nanodiamonds in an organic solvent for 5 min. Excess nanodiamond was removed by centrifugation and the dispersion kept undisturbed to check stability
Surfactant-induced noncovalent dispersions of nanodiamond were obtained as follows. Surfactant solutions of 20 mM strength were prepared in different solvents. A known quantity of the nanodiamond (5 mg) was dispersed in 20 mL of the solution and sonicated in a water bath for 1 h. The dispersion was allowed to stand for 24 h and then characterised. In the case of IGEPAL, 1 mM solution was prepared in water to which this 5 mg of acid-treated nanodiamonds was added and sonicated for 30 min. The dispersion thus obtained was left undisturbed for 24 h to study the stability of the dispersion.
In order to prepare the PVA–nanodiamond composite, the required quantity of PVA and nanodiamond were added to warm water (50 mL) and the mixture was heated at 70°C until the polymer dissolved forming a dispersion of the nanodiamond. The dispersion was dried in a Petri dish at 50°C over a period of 3 days Citation15. The PMMA–nanodiamond composite was prepared by dissolving PMMA and HDTMS-coated nanodiamond in toluene by sonication. The film was grown on water in a Petri dish maintained at 50°C for a day. For TEM studies, the PMMA-nanodiamond film was dissolved in toluene and a drop of this was dried over holey carbon copper grid.
Organosilane or organotin-coated nanodiamond was characterised by various techniques. Infrared spectra were recorded using Bruker IFS 66v/S spectrometer. X-ray diffraction (XRD) patterns were recorded using Cu Kα radiation on a Rich-Siefert XRD-3000-TT diffractometer. FESEM images were obtained using a FEI NOVA NANOSEM 600. Transmission electron microscope (TEM) images were obtained with a JEOL JEM 3010, operating with an accelerating voltage of 300 kV. Raman spectra were secured with Jobin Yvon LabRam HR spectrometer with 514 nm Ar laser.
3. Results and discussion
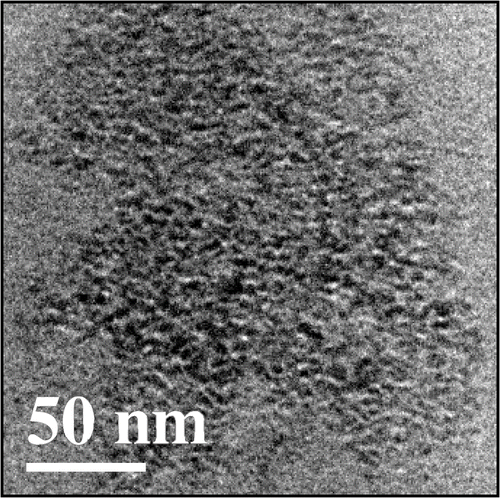
Acid-treated nanodiamond has carboxyl and hydroxyl groups on the surface as revealed by the IR spectrum shown in . The spectrum shows carbonyl band at 1720 cm−1and hydroxyl band at 3400 cm−1. shows the IR spectrum of the amide derivative of nanodiamond, which shows the amide I and II bands at around 1700 cm−1 and amide II band at 1540 cm−1, respectively and the bands due to alkyl groups at 2850 and 2950 cm−1. In and , we compare the TEM image of the starting material with that of amide derivative. From the TEM image it is seen that the pristine nanodiamond has an average diameter of 5 nm. The diameter is not affected by amidation but the EDAX spectrum at the inset of shows the presence of nitrogen. The amide-derivatised nanodiamonds were soluble in nonpolar solvents such as toluene and CCl4. In , we show the photograph of the dispersions of amide-derivatised nanodiamonds in nonpolar solvents taken after 4 h. The XRD pattern of the amide-derivatised residue after evaporating the solvent gave characteristic (111) and (220) reflections of diamond.[placement id="F0001"][placement id="F0002"][placement id="F0003"]
Figure 1. IR spectraof the (a) as-obtained, (b) amide-derivatised, (c) HDTMS-coated, (d) DBDMT-coated, (e) PFOTES-coated, (f) silica-coated and (g) tin oxide-coated nanodiamond.
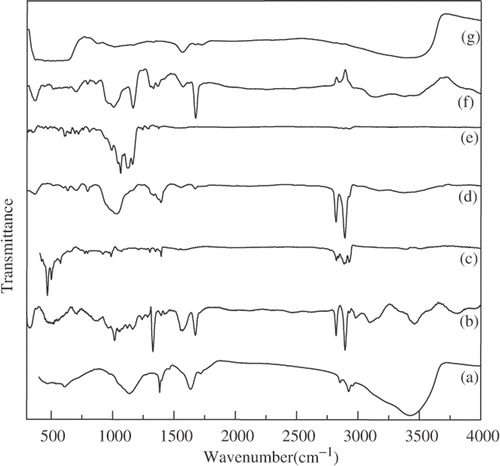
Functionalisation of nanodiamond with organosilane and organotin reagents was established through IR spectroscopy. In , we show the IR spectra of organosilane- and organotin-coated nanodiamonds. The HDTMS-coated nanodiamond shows a band at 1100 cm−1 due to the Si–O stretching band along with bands due to the alkyl groups around 2850 and 2950 cm−1 Citation16. DBDMT-coated nanodiamond shows the characteristic Sn–O stretching band in the 500–600 cm−1 region along with C–H stretching bands for the alkyl groups Citation17. The product of the reaction between acid-treated nanodiamond with PFOTES shows strong C–F stretching bands in the region 1130–1230 cm−1 region in the IR spectrum as can be seen from . The likely reactions between acid-treated nanodiamond (ND) and organosilane- and organotin-reagents are as follows:
Figure 2. TEM images of the (a) as-obtained nanodiamond with the inset showing the particle size distribution, (b) amide-derivatised nanodiamond with the EDAX pattern as the inset, (c) HDTMS-coated nanodiamond with the EDAX pattern as the inset and (d) DBDMT-coated nanodiamond with the EDAX patter as the inset.
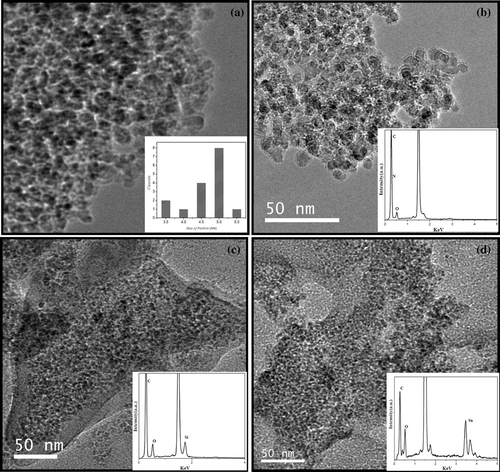
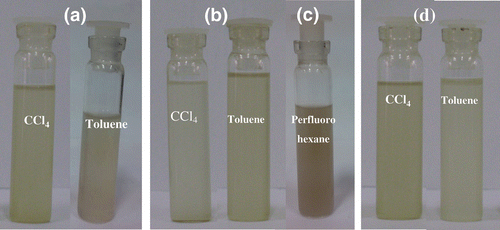
TEM images of HDTMS and DBDMT-coated nanodiamond are shown along with the EDAX spectra in , respectively. The presence of the organosilane and organotin coating is also confirmed by EDAX analysis. Thus, the EDAX spectrum of HDTMS-coated nanodiamond shows the presence of silicon and that of the DBDMT-coated nanodiamond shows the presence of tin. In , we show the photographs of the dispersions of the HDTMS, PFOTES and DBDMT-coated nanodiamond, taken 4 h after the preparation. Among the two solvents, CCl4 gives better dispersions. Interestingly, we could obtain stable dispersions of nanodiamond in the highly nonpolar perfluorohexane by using PFOTES. It appears that functionalisation of nanodiamond with the long-chain oleyl amine or treatment with HDTMS and DBDMT and PFOTES renders the surface hydrophobic which in turn induces the dispersion in nonpolar solvents. The XRD patterns of HDTMS- and DBDMT-coated nanodiamond obtained after evaporating the solvent showed the characteristics peak due to (111) and (220) reflections.
Figure 3. Photographs of dispersions of (a) amide-derivatised, (b) HDTMS-coated (c) PFOTES-coated and (d) DBDMT-coated nanodiamonds in nonpolar solvents.
On heating the organosilane-coated nanodiamond at 400°C for 1 h, silica-coated nanodiamond was obtained. The presence of silica coating was confirmed by the EDAX spectrum which show the presence of silicon. The IR spectrum shows that the Si–O stretching band at 1100 cm−1 is retained while the bands due to the alkyl groups disappear. The silica coating is, however, X-ray amorphous. Heating the organotin-coated nanodiamond produces SnO2-coated nanodiamond. This is confirmed by the EDAX spectrum which show (g) showing the band due to Sn–O stretching. The XRD pattern of the SnO2-coated nanodiamond showed peaks characteristic of tetragonal SnO2 which crystallises in the space group P42 /mnm (a = 4.71 Å, c = 3.17 Å, JCPDS card no: 01-0657).
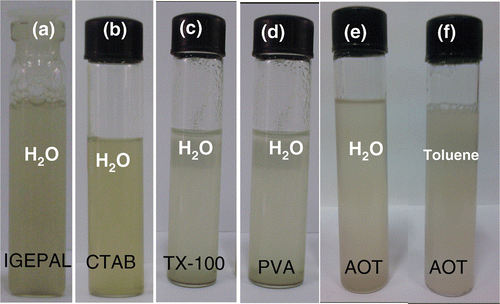
Nanodiamond treated with acids readily disperses in water. However, the dispersions are not stable for long periods. In order to obtain stable dispersions of nanodiamond in water, noncovalent functionalisation has been carried out using various surfactants. show photographs of surfactant-induced dispersions of acid-treated nanodiamond in water taken 24 h after preparation. The surfactants which hold the nanodiamonds in water for long periods are sodium AOT,Triton X-100, PVA, CTAB, and IGEPAL. Interestingly, AOT gives stable dispersions in toluene as shown in . All these water dispersions were stable for a week. In the case of the surfactants, except IGEPAL, a concentration of 20 mM was required to produce stable dispersions. In the case of IGEPAL stable dispersions in water could be obtained at a low concentration of about 1 mM. The dispersion was also stable over longer durations compared to other surfactants.[placement id="F0004"]
Figure 4. Photographs of dispersions of nanodiamond induced by (a) IGEPAL, (b) CTAB, (c) TX-100, (d) PVA and (e) AOT in water and (f) AOT in toluene.
We have carried out some preliminary investigations on the preparation of nanodiamond–polymer composites using the nanodiamond dispersions in water and toluene. It has been possible to obtain films of the nanodiamond–PVA composite by mixing aqueous solutions of PVA and acid-treated nanodiamond. Similarly, the poly (methyl methacrylate) (PMMA) composite was obtained by mixing toluene solutions of PMMA and HDTMS-coated nanodiamond in toluene. In , we show a TEM image of the PMMA–nanodiamond composite.[placement id="F0005"]
4. Conclusion
In conclusion, nanodiamond can be functionalised both by covalent and noncovalent means. The covalently functionalised nanodiamond can easily be solubilised in nonpolar solvents such as CCl4 and toluene. Surfactant-wrapped nanodiamond forms good dispersions in water. The dispersions of nanodiamond would be of use in the preparation of nanodiamond–polymer composites.
References
- Krueger , A . 2008 . The structure and reactivity of nanoscale diamond . J. Mater. Chem. , 18 : 1485 – 1492 .
- Krueger , A . 2008 . Diamond nanoparticles: jewels for chemistry and physics . Adv. Mater. , 20 : 2445 – 2449 .
- Yu , S-J , Kang , M-W , Chang , H-C , Chen , K-M and Yu , Y-C . 2005 . Bright fluorescent nanodiamonds: no photobleaching and low cytotoxicity . J. Am. Chem. Soc. , 127 : 17604 – 17605 .
- Nguyen , T-T-B , Chang , H-C and Wu , VW-K . 2007 . Adsorption and hydrolytic activity of lysozyme on diamond nanocrystallites . Diamond Relat. Mater. , 16 : 872 – 876 .
- Huang , L-CL and Chang , H-C . 2004 . Adsorption and immobilization of cytochrome c on nanodiamonds . Langmuir , 20 : 5879 – 5884 .
- Osswald , S , Yushin , G , Mochalin , V , Kucheyev , SO and Gogotsi , Y . 2006 . Control of sp 2 /sp 3 carbon ratio and surface chemistry of nanodiamond powders by selective oxidation in air . J. Am. Chem. Soc. , 128 : 11635 – 11642 .
- Loktev , VF , Makal'ski , VI , Stoyanova , IV , Kalinkin , AV and Likholobov , VA . 1990 . Surface modification of ultradispersed diamonds . Carbon , 29 : 817 – 819 .
- Liu , Y , Gu , Z , Margrave , JL and Khabashesku , VN . 2004 . Functionalization of nanoscale diamond powder: fluoro-, alkyl-, amino-, and amino acid-nanodiamond derivatives . Chem. Mater. , 16 : 3924 – 3930 .
- Spitsyn , BV , Davidson , JL , Gradoboev , MN , Galushko , TB , Serebryakova , NV , Karpukhina , TA , Kulakova , II and Melnik , NN . 2006 . Inroad to modification of detonation nanodiamond . Diamond Relat. Mater. , 15 : 296 – 299 .
- Krueger , A , Liang , Y , Jarre , G and Stegk , J . 2006 . Surface functionalisation of detonation diamond suitable for biological applications . J. Mater. Chem. , 16 : 2322 – 2328 .
- Rao , CNR and Govindaraj , A . 2006 . “ Nanotubes and Nanowires ” . In RSC Series on Nanoscience , London : Royal Society of Chemistry .
- Subhramanium , KS , Vivekchand , SRC , Govindaraj , A and Rao , CNR . 2008 . A study of graphenes prepared by different methods: characterization, properties and solubilization . J. Mater. Chem. , 18 : 1517 – 1523 .
- Gomathi , A , Hoseini , SJ and Rao , CNR . Functionalization and solubilization of inorganic nanostructures and carbon nanotubes by employing organosilicon and organotin reagents . J. Mater. Chem. (submitted) ,
- Ushizawa , K , Sato , Y , Mitsumori , T , Machinami , T , Ueda , T and Ando , T . 2002 . Covalent immobilization of DNA on diamond and its verification by diffuse reflectance infrared spectroscopy . Chem. Phys. Lett. , 351 : 105 – 108 .
- Vivekchand , SRC , Ramamurty , U and Rao , CNR . 2006 . Mecahnical properties of inorganic nanowires reinforced polymer-matrix composites, . Nanotechnology , 17 : S344 – S350 .
- Benny , TH and Wong , SS . 2006 . Silylation of single-walled carbon nanotubes . Chem. Mater. , 18 : 4827 – 4839 .
- Siciliano , P . 2000 . Preparation, characterisation and applications of thin films for gas sensors prepared by cheap chemical method . Sensors and Actuators B , 70 : 153 – 164 .