Abstract
The study deals with thermal and optical properties of poly(methyl methacrylate) (PMMA) containing 2wt% calcium carbonate (CaCO3) nanofiller. It was found that the thermal conductivity increases with increasing temperatures, due to thermal activation of the phonons in the PMMA/CaCO3 nanocomposite. This enhancement in the thermal conduction is mainly attributed to the heat transferred by lattice vibrations as major contributors and electrons as minor contributors during thermal conduction. The optical properties were investigated as a function of wavelength and photon energy of UV radiation. The optical results obtained were analysed in terms of absorption formula for noncrystalline materials. It was found that the measured optical energy gap for the pure PMMA is greater than the PMMA/CaCO3 nanocomposite. The width of the energy tails of the localised states was calculated. Adding CaCO3 nanofiller into PMMA matrix may cause the localised states of different colour centres to overlap and extend in the mobility gap. This overlap may give an evidence for decreasing energy gap when adding CaCO3 nanofiller in the polymer matrix.
1. Introduction
Nanocomposites are a relatively new class of materials with ultrafine phase dimensions, typically of the order of a few nanometres. The objectives for preparation of enhanced performance nanomaterials are to obtain a homogeneous distribution of the nanoparticles within the polymer matrix, and to promote a strong interfacial adhesion between the matrix and the nanofiller. Uniform dispersion of the nanometre particles offers a major specific surface area enhancement, compared to conventional reinforcements of micrometre size. As a result, addition of small amount of a filler, compared to conventional composites, can induce dramatic changes in host matrix properties Citation1, Citation2.
Recently, nanoparticles attracted great interest because, when they are added to polymers, they create environmentally friendly polymers that can be utilised in the fabrications of advanced materials, especially in the biotechnology world where medical devices and packaging boxes are of great interest Citation3–5. Nanocomposites could dramatically induce improvements in mechanical and electrical properties, heat resistance, radiation resistance and other areas as a result of the nanometre-sized dispersion of the inorganic fillers in the organic matrix Citation6–10. An immense amount of research and development has been devoted to characterising and understanding the mechanical and physical properties of such nanocomposites. In recent years, studies on the optical, thermal and electrical properties of polymer nanocomposites have attracted much attention in view of their important applications in optical devices. The optical properties of polymers can be enormously modified by addition of nanoparticles, which react effectively with the host matrix due to their very large surface areas with respect to macro/microparticles. Thus, addition of such nanoparticles such as titanium dioxide (TiO2), potassium chlorate (KClO3), calcium carbonate (CaCO3), and potassium iodate (KIO3) to a polymeric system may be expected to enhance its optical and electrical performance Citation6.
Calcium carbonate and other layered silicates in nanosize dimension have been commonly used in preparation of highly performance nanocomposites with large specific surface areas. The nanometre-sized commercially available CaCO3 has been used merely to reduce the cost of expensive resins; however, the improvement in various properties such as the mechanical property of micrometre-size CaCO3-filled composites is believed to be poor due to the decrease of the polymer–filler interaction Citation6, Citation11.
In the study of physical properties of polymers, the optical absorption spectrum is one of the most important tools for understanding band structure and electronic properties of pure and filled polymers. This technique depends on the transition of some electrons from the valence band (VB) to the conduction band (CB), when photon energy is greater than the band energy. The transition is direct when the wave vector for the electron remains unchanged, but in the case of indirect transition, interaction with a lattice vibration (phonon) occurs (the minimum of the CB lies in a different part of K-space from the maximum of the VB) Citation12, Citation13. As a consequence, the nanocomposite properties are strongly influenced by the nature of the interface between inorganic and polymer matrices. A strong interfacial interaction between the inorganic nanoparticles and the polymer matrix can give rise to some unusual properties in these materials Citation14, Citation15.
Avella et al. Citation16 succeeded in the preparation of novel poly(methyl methacrylate) (PMMA)/CaCO3 nanocomposites by an in situ polymerisation process with a very fine dispersion and a good interfacial adhesion between the two phases. The exhibited performance of the examined properties of the nanocomposites as glass temperature, abrasion resistance, and morphology was quite different from the convential microcomposites. Furthermore, they claimed from the observed scanning electron microscope (SEM) morphology that the nanoparticles are completely covered by the PMMA matrix and the material appears as a one-phase system. To the best of our knowledge, little attention has been paid to the study of the thermal conductivity and optical properties of the CaCO3 nanocomposites. The present work is part of a systematic study of the effect of the addition of a nanofiller into a polymer matrix for the purpose of characterising the thermal and optical properties of PMMA/CaCO3 nanocomposites.
2. Experimental work
2.1. Material preparation
The PMMA/CaCO3 nanocomposites were prepared by an in situ polymerisation. Precipitated CaCO3 nanoparticles covered by stearic acid, commercialised under the trademark SOCAL (Solvay & Cie), with a mean size of about 40 nm as fillers, were used. The presence of stearic acid as organic coating on the nanofiller surface confers hydrophobic properties to CaCO3 that can improve its compatibility with the polymeric matrix. In particular, the experimental conditions of the sample preparation were as follows. Step 1–In a cylindrical reactor equipped with inlets for refrigeration, mechanical stirring, and nitrogen, kept in an oil bath at 90°C, methyl methacrylate containing 1wt% of dicumylperoxide, previously dissolved, was added. To this solution, 2wt% of CaCO3 nanoparticles was added. This solution was stirred mechanically at 90°C until a critical viscosity that corresponds to a prepolymerisation of the monomer was reached. It was observed that the time it takes to achieve a critical viscosity value was related to the amount of nanoparticles. Step 2–The viscous mixture was then inserted into a mold and kept at 100°C for 24 h to complete the polymerisation process. Finally, the nanocomposite so obtained was kept for 4 h at 140°C to be sure that the entire prepolymer fraction had been converted. Monomer polymerisation has been promoted by thermal decomposition of the organic peroxide during the blending. Generally, acrylic polymerisation is carried out in suspension, and the suspending medium is a salt-water solution. Due to the incompatibility of the nanoparticles with water, in this case methyl methacrylate polymerisation has been performed in bulk Citation13. To establish the degree of nanoparticle dispersion within the polymeric matrix, nanocomposite samples were cryogenically broken after immersion in liquid nitrogen and the fractured surfaces were observed by SEM Citation16. This analysis was carried out with a Philips SEM 501 electron microscope, and the scanning electron micrographs were taken on Au/Pd-coated fractured surfaces of the dumbbell specimens. The effects of CaCO3 nanoparticles on mechanical properties of PMMA/CaCO3 nanocomposites have been analysed and the data are summarised in Citation16. In and (b), the SEM micrographs of the fractured surfaces of the neat PMMA and nanocomposites containing 2wt% of the CaCO3 nanoparticles are shown as examples. From the figures it can be observed that the nanoparticle size ranges between 40 and 70 nm. The tiny small black dots refer to the nanofiller CaCO3 Citation16.
Figure 1. (a) SEM micrograph for neat PMMA polymer fractured surface. (b) SEM micrograph for PMMA/CaCO3 nanocomposite fractured surface.
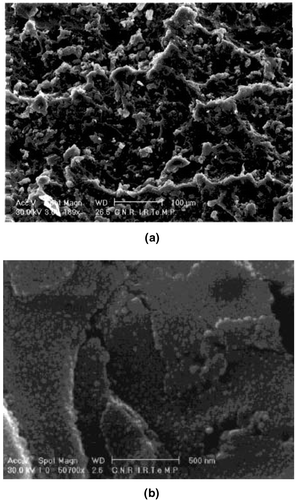
Table 1. Flexural properties and glass transition temperature of neat PMMA and PMMA/CaCO3 nanocomposite.
2.2. Thermal properties measurements
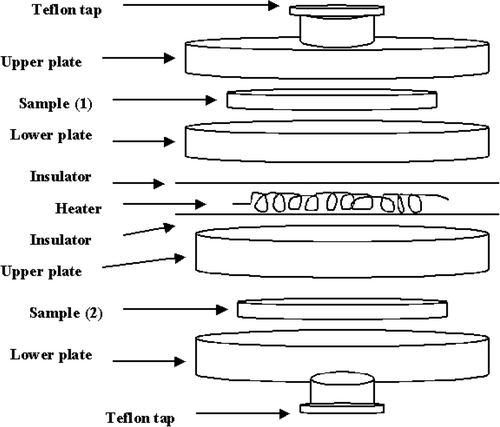
The measurements of the thermal conductivity were carried out using a computerised thermal conductivity apparatus (). This apparatus measures the thermal conductivity using Heat Flowmeter Steady-State Method. The specimen was sandwiched between two rubber sheets to remove the air gaps, and then it was placed between a hot plate and the heat flowmeter, which is attached to a cold plate. The hot and cold plates are maintained at constant temperatures, the temperature of the two plates was measured using surface thermocouples. The apparatus is surrounded by an insulating jacket to minimise the heat loss.
Thermal conductivity represents the amount of heat energy conducted per unit time through a unit area (perpendicular to the direction of heat transfer) when the temperature gradient across the heat-conducting element is one unit. Conduction of heat in insulating solids can be due to thermally excited lattice vibrations (phonons), thus the thermal conductivity is caused by phonon conductivity as a major contributor as a result of the molecular vibration of the lattice where there are little free electrons in the nanocomposite Citation16,Citation17. The thermal conductivity (k) can be found from the familiar relation Citation18:
Under steady-state condition, the temperature gradient becomes (ΔT/ΔL), and the heat flow (Δq/Δt) becomes Citation18:
The quasi-state temperature difference ΔTs were found to be identical for the thermocouple pairs on one side, and those on the other sides. This gives support to the assumption of symmetry on both sides of the heat flux. The values of thermal conductivity (k), at each temperature, were calculated from the relation:
2.3. Optical absorption measurements
The most direct and the simplest method for probing the band structure of materials is to measure the absorption spectrum. In the absorption process, a photon of known energy excites an electron from a lower to a higher energy state. Studying the changes in transmitted radiation, one can decide the types of possible transitions an electron can make. Fundamental absorption refers to band-to-band or to exciting transitions. The fundamental absorption manifests itself by a rapid rising in absorption, known as the absorption edge, and can be used to determine the optical energy gap. Absorption is expressed in terms of a coefficient α(ν), which is defined as the relative rate of decrease in the light intensity.
The optical absorbance A(ν) spectra of nanocomposite were collected at room temperature in the wavelength range 200–800 nm with UV-visible spectrophotometer. The absorption coefficient α(ν) was calculated from the absorbance A(ν). After correction for reflection α(ν) was calculated using the relation:
The relationship between fundamental absorption and optical energy gap is given by the relation:
At high absorption coefficient levels for noncrystalline materials can be related to the energy of the incident photon as follows:
3. Results and discussions
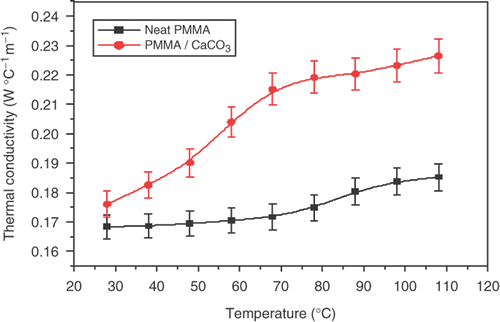
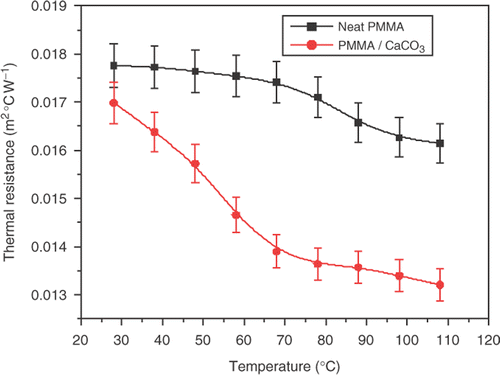
shows the variation of thermal conductivity versus temperature for PMMA/CaCO3 composite. It is clearly shown that the thermal conductivity increases with increasing temperatures, due to thermal activation of the phonons in the PMMA/CaCO3 composite. We observed that the thermal conductivity was affected most by the addition of 2wt% CaCO3 to neat PMMA polymer. It was found that the thermal conductivity of neat PMMA increased from 0.168 W m−1K−1 to 0.175 W m−1K−1 with the addition of 2wt% CaCO3, which indicates that the composite conduct heat is better than neat PMMA. We must point out the enhancement in the thermal conduction is mainly attributed to the heat transferred by lattice vibrations as (major contributors) and electronic contribution as (minor contributors) during thermal activation. The increase of thermal conductivity to PMMA with temperature is typical to that of the amorphous polymers. The variation of thermal resistance versus temperature shown in , in which it decreases with increasing temperature.
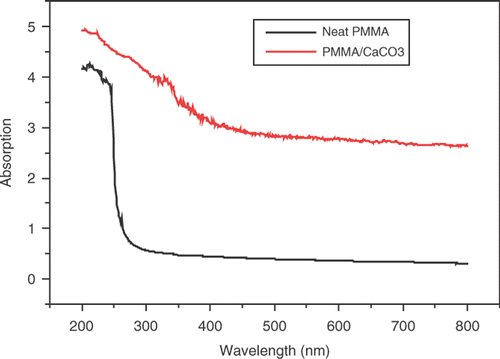
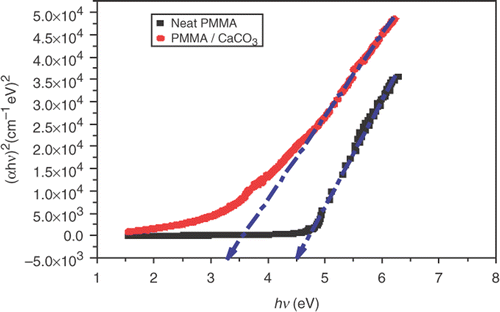
Optical absorption studies on neat PMMA and PMMA/CaCO3 nanocomposite as shown in were carried out to obtain energy-band gaps of the samples, the optical spectra were recorded at room temperature.
The plots of (αhν)2 versus photon energy hν were used to evaluate the optical gaps. A typical plot is shown in Citation23, Citation24. The best fitting to the absorption spectra in Equation (Equation9) gave r = (1/2), meaning the electron transition allowed direct transition for these nanocomposite samples.
It can be seen that the plot is linear in the region of strong absorption near the fundamental absorption edge. Thus, the absorption takes place through direct transition. The band gap obtained by extrapolating the linear part to zero of the ordinate is also indicated in the figure. The band gaps, E g, were evaluated in this way and the slope gives the value of constant β. The factor β depends on the transition probability and can be assumed to be constant within the optical frequency range.
Adding CaCO3 in PMMA matrix may cause the localised states of different colour centres to overlap and extend in the mobility gap. This overlap may give us an evidence for decreasing energy gap when adding CaCO3 in the polymer matrix Citation25.
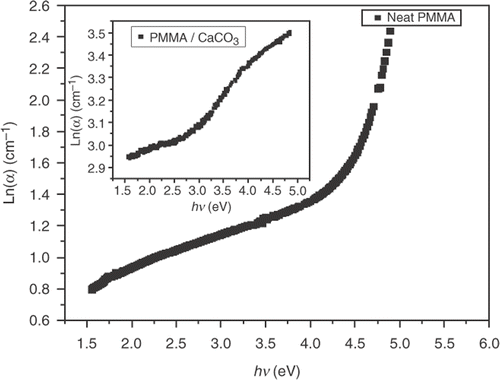
The Urbach formula Equation (Equation10) was used to calculate the width of the urbach tail of the localised states due to the defect levels in the transition gap Citation20. The linear dependence of ln α versus hν is presented in . The reciprocal of the slopes of the curves yields the magnitude of width of the band tail, ΔE, and the value of ΔE for pure PMMA is 0.221 eV and 0.318 for PMMA/CaCO3 composite. It is observed that the Urbach tail for pure PMMA is less than that for PMMA/CaCO3 composite. summarises the obtained optical data.
Table 2. The obtained optical data of neat PMMA and PMMA/CaCO3.
4. Conclusions
In the present study, thermal and optical properties of PMMA/CaCO3 nanocomposite were investigated and the following conclusions can be obtained:
| 1. | The thermal conductivity increases with increasing temperatures. | ||||
| 2. | The thermal conductivity of neat PMMA increased with the addition of 2wt% CaCO3 nanofiller, which indicate that the composite conduct heat is better than neat PMMA. | ||||
| 3. | The enhancement in the thermal conduction is mainly attributed to the heat transferred by lattice vibrations as (major contributors) and electronic contribution as (minor contributors) during thermal activation. | ||||
| 4. | The best fitting to the absorption spectra gave r = (½), meaning it allowed direct transition for these samples. | ||||
| 5. | Adding CaCO3 nanofiller in PMMA matrix may cause the localised states of different colour centres to overlap and extend in the mobility gap. This overlap may give us an evidence for decreasing energy gap when adding CaCO3 in the polymer matrix. | ||||
| 6. | It is observed that the Urbach tail for pure PMMA is less than that for PMMA/CaCO3 composite. | ||||
References
- Giannees , EP . 1996 . Polymer layered silicate nanocomposites . Adv. Mater. , 8 : 29 – 35 .
- Avella , M , Cosco , S , Di Lorenzo , ML , Di Pace , E and Errico , ME . 2005 . Influence of CaCO3 nanoparticles shape on thermal and crystallization behavior of isotactic polypropyelene based nanocomposites . J. Thermal Anal. Calorimetry , 80 : 131 – 136 .
- Gersten , J and Smith , F . 2001 . The Physics and Chemistry of Solids, Nanostructures , 383 New York : John Wiley and sons, INC .
- Batraton , M . 2003 . Synthesis, Fictionalization and Surface Treatment of Nanoparticles, Chap. 8 , California : American Scientific Publishers .
- Zhoa , Y and Lin , Y . 2004 . Optical and dielectric properties of a nanostructured NbO2 thin film prepared by thermal oxidation . Phys. D Appl. Phys. , 37 : 3392 – 3395 .
- Sawada , H , Shikauchi , Y , Kakehi , H , Katoh , Y and Miura , M . 2007 . Preparation and applications of novel fluoroalkyl end-capped oligomers/calcium carbonate nanocomposites . Colloid. Polym. Sci. , 285 : 499 – 506 .
- Zanetti , M , Lomakin , S and Camino , G . 2000 . Polymer layered silicate nanocomposites . Macromol. Mater. Eng. , 279 : 1 – 9 .
- Schmid , G . 2004 . Nanoparticles–from Theory to Application , Weinheim : Wiley-VCH .
- Ajayan , PM , Schadler , LS and Braun , PV . 2003 . Nanocomposite Science and Technology , Weinheim : Wiley-VCH .
- Gomez-Romero , P and Sanchez , C . 2004 . Functional Hybrid Materials , Weinheim : Wiley-VCH .
- Chan , CM , Wu , J , Li , JX and Cheung , YK . 2002 . Polypropylene/calcium carbonate nanocomposites . Polymer , 43 : 2981 – 2992 .
- Murri , R , Schiavulli , L , Pinto , N and Ligonzo , T . 1992 . Urbach tail in amorphous gallium arsenide films . J. Non-Cryst. Solids , 139 : 60 – 66 .
- Urbach , F . 1953 . The long-wavelength case of photographic sensitivity and of the electronic absorption of solids . Phys. Rev. , 92 : 1324
- Qiang , X , Chunfang , Z , JianZun , Y and Yuan , CS . 2004 . The effects of polymer-nanofiller interactions on the dynamical mechanical properties of PMMA/CaCO3 composites prepared by microemulsion template . J. Appl. Polym. Sci. , 91 : 2739 – 2749 .
- Bose , S , Pandey , R , Kulkarni , MB and Mahanwar , PA . 2005 . Effect of talc and synthetic sodium aluminum silicate (SSAS) on the properties of poly (methyl methacrylate) . J. Thermoplast. Compos. Mater. , 18 : 393 – 405 .
- Avella , M , Errico , ME and Martuscelli , E . 2001 . Novel PMMA/CaCO3 nanocomposites abrasion resistant prepared by an in situ polymerization process . Nano Lett. , 1 : 213 – 217 .
- Sakandiou , EP , Van Den Berg , HR and Ten Seldam , CA . 1996 . Thermal conductivity of Methan in the critical region . J. Chem. Phys. , 1058 ( 23 ) : 10535 – 10555 .
- Tabor , D . 1995 . Gasses, Liquids and Solids , London : Arrowsmith .
- Katsure , T and Kamal , MR . 1987 . Electrical and thermal properties of polypropylene filled with steel fiber . Adv. Polymer Tech. , 5 : 193 – 202 .
- Daris , EA and Mott , N . 1970 . Conduction in noncrystalline systems. V. Conductivity, optical absorption, and photoconductivity in amorphous semiconductors . Phil. Mag. , 22 : 903 – 922 .
- Al-Ani , SKJ and Zihlif , AM . 1996 . Optical properties of epoxy-glass microballoons composite . Optic. Mater. , 59 : 69 – 73 .
- Mott , NF and Davis , EA . 1971 . Electronic Processes in Non-crystalline Materials , Oxford , , England : Clarendon Press .
- Ma , X , Zhou , B , Deng , Y , Sheng , Y , Wang , C , Pan , Y and Wang , Z . 2008 . Study on CaCO3/PMMA nanocomposite microspheres by soapless emulsion polymerization . Colloid. Surface A: Physicochem. Eng. Aspect. , 312 : 190 – 194 .
- Medeiros , SK , Albuquerque , EL , Maia , FF Jr , Caetano , EWS and Freire , VN . 2007 . Electronic and optical properties of CaCO3 calcite, and excitons in Si@CaCO3 and CaCO3@SiO2 core-shell quantum dots . J. Phys. D: Appl. Phys. , 40 : 5747 – 5752 .
- Zidan , HM and Abu-Elnader , M . 2005 . Structural and optical properties of pure PMMA and metal chloride-doped PMMA films . Physica B , 355 : 308 – 317 .