Abstract
A novel method to produce pure nano-hydroxyapatite from silica suspension was devised. Its main advantage consists in allowing the control of the reaction rate of calcium and phosphate ions through the use of a chelate, namely EDTA. Further control of acidic conditions allows both the particle size and the shape to vary.
1. Introduction
The synthesis of calcium phosphates has attracted a great deal of attention in the last few decades, particularly hydroxyapatite (Hap), the main mineral component of human bones, its chemical formula being Ca10(PO4)6(OH)2. Its relevance as a material arises from the possibilities of using it in surgical implants and minimising the risk of rejection since it is a natural constituent of the human body. Additionally, Hap implants have the proven ability to stimulate the formation of natural bone Citation1 and to permit the invasion of both capillaries and muscular tissue into their porous structure Citation1,Citation2.
Generally speaking, the reported methods for producing Hap Citation1–4 can be classified as follows:
(a) Solution procedures Citation5,Citation6. Solutions of various sources for phosphate and calcium chemicals are employed and Hap crystallites are produced by precipitation. The method is very simple and involves inexpensive equipment. However, the control of crystallite size represents a difficulty and the presence of metastable phases in the final product of the reaction is perhaps the main disadvantage. In general, solution methods are among the most important methods for producing Hap as biomaterial at an industrial or semi-industrial scale.
(b) Solid state methods Citation7–10. These methods have the convenience of avoiding undesirable calcium phosphate phases and producing good quality Hap from very inexpensive raw materials. The main disadvantage in this case is the energy consumption involved in the procedure. The possibility of producing composites by in situ reactions also shows an interesting potential in the area of biomaterials.
(c) Hydrothermal systems Citation4,Citation11,Citation12. These procedures have been employed for producing industrial ceramics and materials, other than phosphate-based biocompatible compounds. Poor control of the variables and the production of only relatively large particles are the main disadvantages. However, the current technology allows the precise control of the thermodynamical parameters involved, and recently the possibility of producing nanometre-sized calcium phosphates has been reported. This field is expected to attract more attention in the near future.
(d) Low temperature methods Citation13–15. Low temperature methods for the production of calcium phosphates (including Hap) particulates at near human body temperature, in a simulated body fluid on top of an inorganic (silica) gel have been reported. This route opens exciting possibilities for discovering how ‘nature’ itself manages to produce ceramic materials at nearly room temperature.
(e) The sol–gel method. This is a technique which has received little attention. Reports using calcium salts and phosphoric acid esters have been published recently. However, Hap is obtained only at temperatures above 500°C Citation16,Citation17. This stresses the need for more work in this area.
Other methods used to obtain Hap coatings include electrochemical deposition and plasma sprayed Citation18–25.
A common disadvantage of the aforementioned methods is the difficulty of obtaining Hap particles homogeneous in size, since both the structure and the composition of Hap are very dependent on the reaction conditions. Thus, low crystallinity is typical and the presence of other calcium phosphate phases is common. Besides, most of these methods require either high temperatures or long reaction times Citation23–27.
The technique described in this work allows the controlled precipitation of pure Hap from a starting homogeneous solution of Ca+2 and ions at low temperature and low pressure conditions, and in short periods of time. It also allows the formation of nanometre-sized composites of Hap and silica.
2. Experimental
2.1. Synthesis route
2.1.1. Preparation of complexed solutions
Solutions were prepared with CaCl2 · 2H2O (Riedel-de Haën, analytical grade), Na2 EDTA · 2H2O (Aldrich Chemical Co., analytical grade) and K2HPO4 (Riedel-de Haën, analytical grade). One litre of the solution was prepared in order to obtain CaCl2 0.25 M, Na2 EDTA 0.25 M and K2HPO4 0.15 M at different pH values. About 200 mL of each solution were kept in a pressure reactor (Parr, model 4841) at 160°C and 100 PSI during 4 h. Samples with pH 6 and 9 are discussed.
2.1.2. Suspensions preparation
The starting solution for the sol–gel route is prepared as follows: 3.5 mL of tetraethyl orthosilicate (TEOS) (Aldrich Chemical Co., analytical grade), 1 mL of distilled water, 5 mL ethanol (Baker Analytical grade) and 0.5 mL of hydrofluoric acid (Baker, 40% wt.). After 2 min the sol was formed. This sol was put into 100 mL of the solution described in Section 2.1.1 and refluxed under stirring for 4 h. The white precipitate obtained was filtered and dried for 3 h at 110°C.
2.2. Characterisation techniques
Morphology and constituent elements of the samples were determined through a Low Vacuum Scanning Electron Microscope, JEOL-5900LV (LV-SEM), equipped with an energy dispersive X-ray analysis probe, Oxford (EDS). Samples were fixed to the aluminium holder via adhesive carbon foil.
X-ray diffraction analysis was performed with a powder diffractometer Siemens D5000, at 30 keV, 25 µA, scanning from 5° to 70° with 0.1° steps. Samples were previously ground until a very fine powder was obtained.
3. Results and discussion
Under standard conditions, calcium phosphate aqueous solutions are at the verge of supersaturation, due to their extreme insolubility. Thus, in very short time, precipitates such as Hap, tricalcium phosphate (TCP), octacalcium phosphate (OCP), dicalcium phosphate anhydrous (DCPA) and dicalcium phosphate dihydrate (DCPD) are produced. This is due to the poor control of the reactivity conditions of Ca ions during the reactions involved in all the synthesis methods described above. In fact, this is reflected in the corresponding phase diagrams, which show a rather narrow region, particularly in terms of pH, in which Hap can be produced without the presence of other phosphate phases. Thus, a way to control chemically the availability of reacting Ca ions during the changing conditions, in terms of pH and supersaturation, is necessary if pure Hap with specific crystallite sizes is to be produced.
Accordingly, a reservoir of calcium ion could be made available in the aqueous medium, as a Ca2+/EDTA soluble complex. As a matter of fact, with EDTA one is able to control the equilibrium of the reaction, the precipitation rate and even the size of the particles obtained, in addition to obtaining the desired phase.
By mixing Ca2+/EDTA/ in the proportion 5 : 5 : 3 one can obtain the Hap phase alone, because it is thermodynamically more stable. In order to precipitate it, the equilibrium of the reaction can be shifted conveniently by heating to temperatures ≥130°C. Varying the pH from acidic to basic solutions produces changes in particle size and the corresponding equilibrium shapes. shows a SEM micrograph of the particles obtained by this procedure at pH = 6; one can appreciate the very small needle-like structures, which grow radically from a nucleation centre. The size of these needles is about 1 µm long and less than 0.1 µm in thickness. corresponds to a XRD pattern taken from this sample. It shows very clearly the presence and high degree of crystallinity of the Hap produced.
Figure 1. SEM micrograph of sample prepared as described in the text at pH = 6. Hap needles about 1 µm long and 0.1 µm thick form bundles from a common origin. These bundles join one another and fill the space (marker = 1 µm).
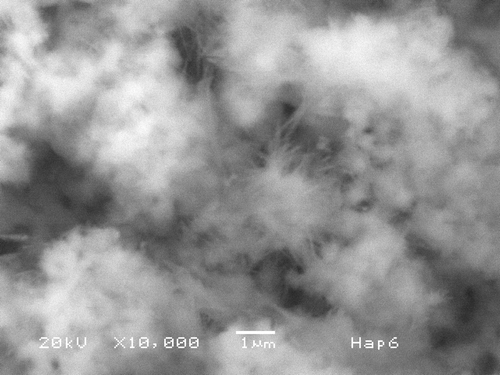
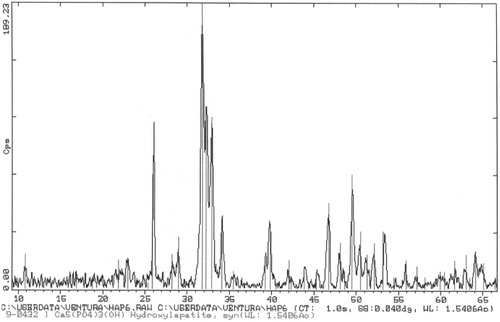
Figure 2. XRD from sample in shows the presence of Hap with very high crystallinity. Peaks for other calcium phosphates are not present.
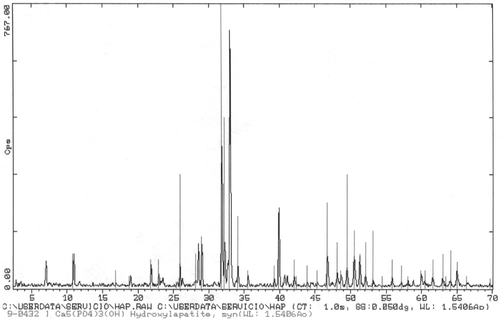
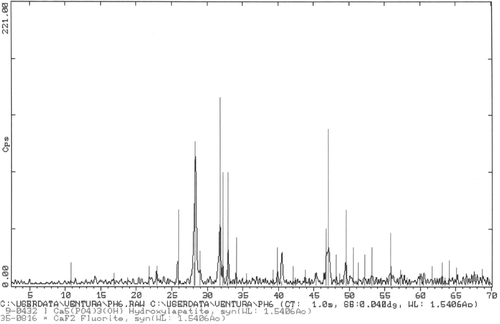
Another sample prepared by the same procedure but at pH = 9 is shown in ; needles are also formed but their size is of 10–15 µm in length and some up to 1 µm in thickness, which are definitively larger than those of pH = 6. corresponds to a X-ray diffractogram of the sample shown in ; again one can appreciate the high degree of crystallinity obtained and the purity of the phase.
Figure 3. SEM micrograph of sample prepared at pH = 9. Hap needles 12 µm long are observed, some as thick as 1 µm are present. Some form bundles from a common origin.

Figure 4. XRD of sample of shows the presence of Hap with very high crystallinity. No peaks for other calcium phosphates are present.
In general the higher the pH, the bigger the particles formed but the size distribution would also be broader. This effect is under further study and the details of the growth kinetics and its relationship to the controlled pH conditions, through a modified Avrami approach, are currently under way and will be reported separately.
Based on the results described in Section 2, and the reported growth of Hap on silica blocks produced by the sol–gel route Citation14,Citation15, a variation was devised as explained in Section 2. The standard sol–gel procedure was followed as usual, but while the materials are still in the sol state, a Hap solution prepared as explained was added under reflux. A composite is then obtained, consisting in amorphous silica particles with particles of crystalline Hap. The method allows for the variation of the pH. shows a backscattered SEM picture of sample prepared at pH = 6. The SiO2 particles are present and Hap elongated crystallites are embedded in the SiO2 matrix. The crystallites are about 5 µm long and 1 µm thick, much larger than the sample in , which also corresponds to pH = 6. is a X-ray diffractogram of the sample in . The presence of Hap and its high degree of crystallinite are very clear. However, the peaks at 28° and 47° could be explained by the presence of KCl or CaF2. In this diffractogram, the broad reflection corresponding to SiO2 was suppressed with the background.
Figure 5. Backscattered electrons micrograph from sample prepared at pH = 6 shows elongated crystallites about 5 µm long and 1 µm thick embedded in a SiO2 matrix. Hap crystallites appear isolated, not in bundles.
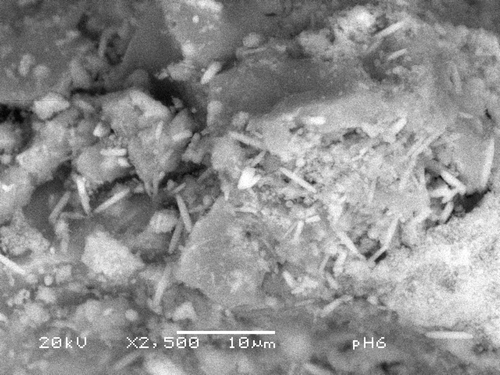
Figure 6. XRD from sample in . It shows that the needles are crystalline Hap, although a couple of extra peaks are present. These could be due to CaF2 or KCl2.
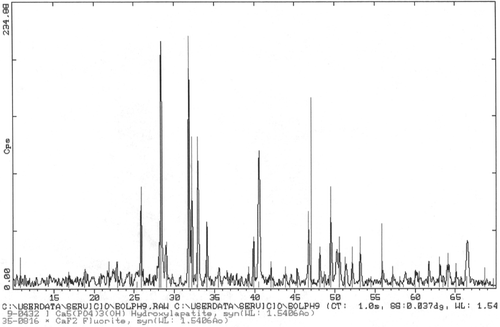
shows a SEM electrograph of the sample prepared with the procedure similar to the sample in , but at pH = 9. Bars are about 8 µm × 1 µm and SiO2 particles show dimensions which vary from 1 to 5 µm. One can also observe that some crystallites form groups and interesting twins are observed. Again, the higher the pH, the bigger the particles formed with this process as with the former one. The hexagonal cross section of the crystallites evidences the equilibrium shape, which reflects, in turn, the thermodynamical conditions of the reaction. shows an X-ray diffractogram of the sample in . Hap is present in a very crystalline phase, but spurious peaks at 28° and 47° appear again. The broad reflexion corresponding to SiO2 was suppressed.
Figure 7. SEM micrograph of sample prepared as in Section 2.2.2 at pH = 9. Hap bars are observed as growing on top of the SiO2 particles. Bars are about 8 µm × 1 µm and SiO2 particles show dimensions in the order of 5 µm. The hexagonal cross section is clearly observed.
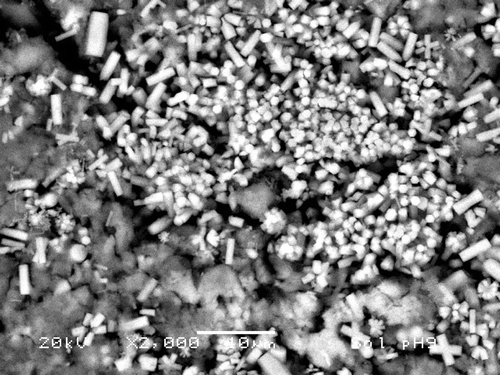
Figure 8. XRD from sample in . It shows that the bars correspond to crystalline Hap, although other peaks are present. These could be due to CaF2 or KCl2.
It is thought that the in situ production of silica promotes the formation of Hap at lower temperatures and with a much larger crystallites size. It seems that the presence of the SiO2 has a nucleation effect, which can be controlled through the chemistry of the silica gel.
As for the mechanical properties of these materials, the resulting powder is very stable and homogeneous and it should be sintered to produce bodies of this nanocomposite Citation28. However, since Hap itself is a rather weak material, the presence of a silica glass component will quite likely add mechanical strength to the final composite, in particular for powder coating technologies, as it has been reported elsewhere Citation29.
4. Concluding remarks
The use of a convenient complexing agent allows us to control properly the reaction conditions of extremely reactive ions, such as those involved in the synthesis of Hap, which represents a particularly cumbersome system, since the corresponding phase diagram shows a very narrow zone where Hap can be produced free of other phosphate compounds. This zone is highly pH-dependent, and pH itself changes drastically as the reaction proceeds. In addition to the practical application of this methodology to the present case, the use of multifunctional complexing agents opens interesting possibilities for synthesising metastable inorganic compounds.
Acknowledgements
A. Sáenz acknowledges the financial support obtained from ICTP via CLAF-M for research visits to Mexico. We are indebted to Dr Ventura Rodriguez-Lugo of CUV-BUAP and Dr Gilberto Mondragon-Galicia, of Instituto Nacional de Investigaciones Nucleares (ININ), Mexico for their technical support and useful discussions.
References
- Park , JB . 1984 . Biomaterials Science and Engineering , New York : Plenum Press .
- Ravaglioli , A and Krajewski , A . 1992 . Bioceramics; Materials Properties and Applications , London : Chapman & Hall .
- Khon , DH and Ducheyne , P . 1991 . “ Materials science and technology: A comprehensive treatment ” . In Medical and Dental Materials , Edited by: Cahn , RW , Haasen , P , Kramer , EJ and Williams , DF . Vol. 14 , 47 – 69 . Cambridge : VCH .
- Yoshimura , M and Suda , H . 1994 . Hydroxyapatite and Related Materials , Edited by: Brown , PW and Constantz , B . 48 – 71 . Boca Raton : CRC Press .
- Monma , H and Kamiya , Y . 1987 . Preparation of hydroxyapatite by the hydrolysis of brushite . J. Mat. Sci. , 22 : 4247 – 4250 .
- Fang , Y and Agrawal , DM . 1992 . Ultrasonically accelerated synthesis of hydroxyapatite . J. Matr. Res. , 7 : 2294 – 2302 .
- Black , J . 1992 . Biological Performance of Materials, Fundamentals of Biocompatibility , 2nd , 3 New York : Marcel Dekker Inc .
- LeGeros , RZ . 1988 . Calcium phosphate materials in restorative dentistry: a review . Adv. Dent. Res. , 2 : 164 – 180 .
- Arita , I , Wilkinson , D , Mondragron , M and Castaño , VM . 1995 . Chemistry and sintering behaviour of thin hydroxyapatite ceramics with controlled porosity . Biomaterials , 16 : 403 – 408 .
- Arita , I , Wilkinson , D and Castano , VM . 1994 . Synthesis and processing of hydroxyapatite ceramic tapes with controlled porosity . J. Mater. Sci. Mater. , 6 : 19 – 23 .
- Lavenia , C . 1991 . Calcium phosphate ceramics as bone substitute . Am. Ceram. Soc. Bull. , 70 : 95 – 100 .
- Helmus , MN . 1991 . Overview of biomedical materials . MRS Bulletin , 16 : 33 – 38 .
- Brinker , CJ and Scherer , GW . 1990 . Sol–Gel Science , San Diego : Academic Press .
- Rivera , E , Bonilla , M , Hernández , R , Rodríguez , R and Castaño , VM . 1997 . Effect of chemical additives on the growth of hydroxyapatite on silica gel . J. Mater. Synth. Proc. , 5 : 153 – 160 .
- Rivera , E , Brostow , W , Rodríguez , R and Castaño , VM . 2001 . Growth of hydroxyapatite on silica gels in presence of additives: kinetics and mechanism , Vol. 4 , 222 – 230 . Mater. Res. Innov .
- Bres , E and Hardouin , P . 1998 . Les matériaux en phosphate de calcium. Aspects fondamentaux, Sauramps medical , Montpellier .
- Mao , C , Li , H , Cui , F , Feng , Q and Ma , C . 1999 . The functionalization of titanium with EDTA to induce biomimetic mineralization of hydroxyapatite . J. Mat. Chem. , 9 : 2573 – 2582 .
- Ducheyne , P , Beight , J , Cuckler , J , Evans , B and Radin , S . 1990 . Effect of calcium phosphate coating characteristics on early post-operative bone tissue ingrowth . Biomaterials , 11 : 531 – 540 .
- Encinas , M , Aguayo , S , Castillo , S , Castillón , F and Castaño , VM . 2008 . Synthesis and characterization of hydroxyapatite-wollastonite composite powders by sol-gel processing . Int. J. Appl. Ceram. Technol , 5 : 401 – 411 .
- Li , Y , Li , D and Weng , W . 2008 . Preparation of nano carbonate-substituted hydroxyapatite from an amorphous precursor . Int. J. Appl. Ceram. Technol , 5 : 442 – 448 .
- Gauthier , A and Thomassin , JH . 2007 . Synthesis of hydroxyapatite during glassy matrix dissolution: influence of their chemical composition . Int. J. Appl. Ceram. Technol. , 4 : 367 – 377 .
- Mavropoulos , E , Rocha , NCC , Moreira , JC , Rossi , AM and Soares , GA . 2004 . Characterization of phase evolution during lead immobilization by synthetic hydroxyapatite . Mater. Charact. , 53 : 71 – 78 .
- Queiroz , AC , Santos , JD , Monteiro , FJ and Prado da Silva , MH . 2003 . Dissolution studies of hydroxyapatite and glass-reinforced hydroxyapatite ceramics . Mater. Charact. , 50 : 197 – 202 .
- Hing , K . 2005 . Bioceramic bone graft substitutes: influence of porosity and chemistry . Int. J. Appl. Ceram. Technol. , 2 : 184 – 199 .
- Tas , AC . 2007 . Porous, biphasic CaCO3-calcium phosphate biomedical cement scaffolds from calcite (CaCO3) powder . Int. J. Appl. Ceram. Technol. , 4 : 152 – 163 .
- Ruhe , PQ , Kroese-Deutman , HC , Wolke , JGC , Spauwen , PHM and Jansen , JA . 2004 . Bone inductive properties of rhBMP-2 loaded porous calcium phosphate cement implants in cranial defects in rabbits . Biomaterials , 25 : 2123 – 2132 .
- Simon , CG , Guthrie , WF and Wang , FW . 2004 . Cell seeding into calcium phosphate cement . J. Biomed. Mater. Res. , 68 : 628 – 639 .
- Sáenz , A , Montero , M and Castaño , VM . 2002 . Preparation of silica-hydroxyapatite nanometric composites . Phys. Stat. Solidi (b) , 230 : 347 – 350 .
- Arce , H , Montero , M , Sáenz , A and Castaño , VM . 2007 . Hydroxyapatite coatings on metals from calcium-EDTA-phosphate homogeneous solutions . Int. J. Mater. Prod. Technol. , 30 : 408 – 418 .