Abstract
ZnO nanoparticles have been synthesised by thermal decomposition of zinc acetate at ∼800°C. The structural characteristics and size distribution of ZnO nanoparticles have been investigated by X-ray powder diffraction (XRD) and small-angle X-ray scattering (SAXS), respectively. SAXS study reveals nanoparticles are of different sizes: namely 23 wt% of 8 nm, 19 wt% of 21 nm and 58 wt% of 51 nm. These ZnO nanoparticles possess yellow visible emission at 552 nm. The polydispersity and single emission peak at 552 nm in ZnO nanoparticles suggest that the yellow emission might be a bulk property instead of having a surface origin in nanostructured ZnO. The surface impurities are characterised by Fourier-transform infrared spectroscopy. The quenching of band edge emission in ZnO nanoparticles seems due to the presence of surface impurities.
Keywords:
1. Introduction
ZnO, due to its wide band gap of ∼3.37 eV Citation1 and large exciton binding energy (60 meV) Citation2 at room temperature, is a promising material for application in many kinds of devices. Understanding the roles of defects in ZnO nanoparticles is important for applications of these nanoparticles in devices. The photoluminescence (PL) spectra of ZnO nanocrystallines largely depend on synthesis methods, crystalline size and, probably most importantly, the defect contents in bulk and surfaces Citation3–10. For the green emission, oxygen vacancies Citation11, zinc vacancies Citation12 and impurities such as copper Citation13 are considered the most probable causes. Apart from the green emission, some other emission bands are also observed in ZnO. A yellow emission is often reported. Ohashi et al. Citation14 observed a yellow band in polycrystalline ZnO doped with aluminium. A yellow emission was also detected in undoped ZnO film by pulse laser deposition Citation15 and in lithium-doped ZnO film by spray pyrolysis Citation16. Some previous studies also suggested that a yellow emission had been induced by doping with Li or Na Citation17.
In this letter, we report a yellow visible emission in ZnO nanoparticles synthesised by thermal decomposition of zinc acetate. Small-angle X-ray scattering (SAXS) study was carried out for analysis of particle size distribution of ZnO nanoparticles. We discuss a possible mechanism for the yellow emission in ZnO nanoparticles.
2. Experimental section
2.1. Chemicals
The zinc acetate extra pure Zn(CH3COO)2 · 2H2O (98%) was from E. Merck Limited, Mumbai-400018, India. This chemical was directly used without special treatment.
2.2. Sample preparation
The zinc acetate dihydrate, Zn(CH3COO)2 · 2H2O was used as the precursor to make ZnO nanoparticles. A typical synthesis procedure is as follows: 1 g of Zn(CH3COO)2 · 2H2O was placed into a crucible and calcined at 800°C for 15 h in a tubular furnace with no special atmospheric environment. Finally, ZnO yellow photoluminescent nanoparticles were produced after decomposing solid material zinc acetate in air at ambient condition. Consequently, ZnO nanoparticles were obtained through a simple, low-cost and direct mass-scale production route.
2.3. Apparatus
The crystal structure of ZnO nanoparticles was characterised by X-ray diffraction (XRD, Rigaku D/MAX–2200H/PC, Cu Kα radiation). The PL spectra were studied by 325 nm radiation from He-Cd laser (KIMMON) and Mechelle 900 spectrograph. The FTIR spectra were recorded on a ABB FTLA 2000 FTIR spectrometer (Canada) in the wavenumber range of 4000–500 cm−1 on pellets obtained by mixing the sample in KBr.
The particle size distribution of the obtained ZnO nanoparticles was characterised by SAXS (Rigaku D/MAX–2200H/PC, Cu-Kα radiation).
3. Results and discussion
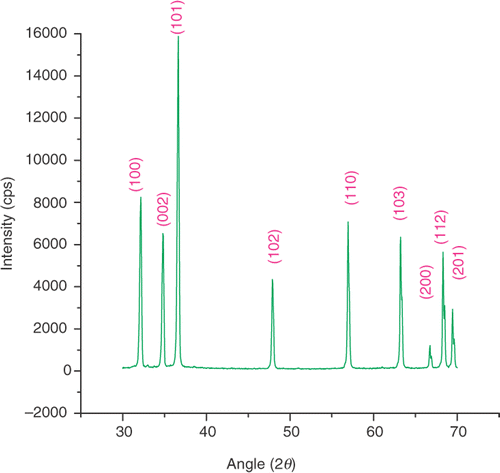
shows the XRD pattern of ZnO nanoparticles synthesised by thermal decomposition of zinc acetate. All the XRD peaks match very well with standard ZnO of wurtzite structure (P63 mc) (JCPDS 36-1451), with the measured lattice constants of c and a of this hexagonal phase being 5.207 and 3.25 Å, respectively (c/a = 1.60). The broadened peaks indicate that the sizes of the particles are in the nanorange. In order to achieve more confirmative information, the Debye–Scherrer formula Citation18:
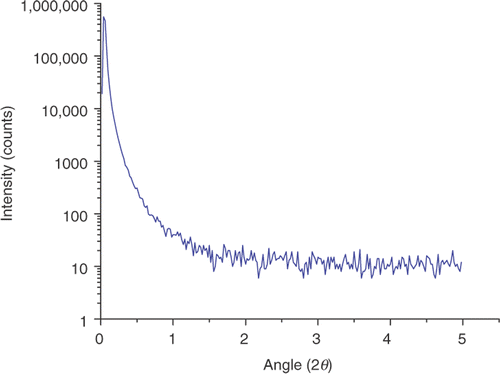
Small-angle X-ray scattering is one of the best techniques to study particle size distribution of nanoparticles Citation19. As is well known, particle size distribution is an important feature of nanopowders. shows the SAXS pattern of ZnO nanoparticles. The particle size distributions are obtained through Guinier plot of the SAXS profile of ZnO nanoparticles, as it is well known that the size of the particles can be estimated from the slope of the Guinier plot Citation20. The ZnO sample consists of nanoparticles of different sizes such as 23 wt% of 8 nm, 19 wt% of 21 nm and 58 wt% of 51 nm. Particle size distributions analysis was done by the grain size analysis program provided with Rigaku D/MAX-2200H/PC.
Figure 2. Small-angle X-ray scattering pattern of ZnO nanoparticles synthesised by thermal decomposition method.
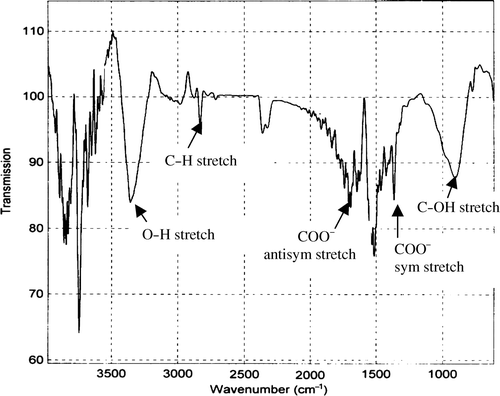
shows FTIR spectra of ZnO nanoparticles synthesised by the thermal decomposition method. A series of absorption peaks from 500 to 4000 cm−1 can be found, corresponding to the vibration mode of impurities. To be more specific, a broadband at 3200–3500 cm−1 is assigned to the OH stretching mode of hydroxyl group. Peaks between 2830 and 3000 cm−1 are due to C–H stretching vibration of alkane groups. The peaks observed at 1630 and 1384 cm−1 are due to the asymmetrical and symmetrical stretching of the zinc carboxylate (COO−), respectively. One absorption band due to C–OH bond is observed at 890 cm−1. These impurities identified by FTIR analysis mainly exist near the surface of the ZnO nanoparticles. These surface impurities such as hydroxyl, carboxylate and alkane serve as nonradiative recombination. For example, surface hydroxyl groups quench the band edge emission in ZnO nanoparticles Citation3.
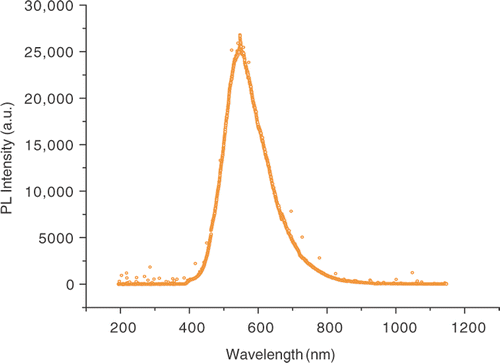
In ZnO nanoparticles, the visible emissions are still a subject of controversy and different explanations have been proposed, all based on defect effect. The most referred defects in ZnO are Zn i , Vo, O i , VZn, OZn, etc. Citation21. The defects Vo, Zn i act as shallow donor states and VZn, O i , OZn as deep level acceptor states in the band gap. These defects are very sensitive to higher temperature Citation22. illustrates the PL spectrum of ZnO nanoparticles synthesised at ∼800°C under photon excitation of 325 nm. The PL spectrum revealed that the ZnO nanocrystals have a yellow visible emission band at ∼552 nm, whereas no UV band edge emission ∼384 nm was observed. The quenching of excitonic transition at ∼384 nm of ZnO nanoparticles seems due to presence of surface impurities in ZnO nanoparticles. Van Dijken et al. Citation23 reported a change in green luminescence intensity when the size of the ZnO nanoparticles was changed. However, the size of our sample is larger than the sample investigated by van Dijken (0.7–1 nm) Citation23. Therefore, trapped electron tunnelling to the surface can be a significant source contributing to the green luminescence in ZnO nanoparticles, but probably not for the particles of much larger size, as in our sample (23 wt% of 8 nm, 19 wt% of 21 nm and 58 wt% of 51 nm). It is because the probability of tunnelling recombination between a surface trapped hole and ionised oxygen vacancy centre decreases rapidly versus the distance, due to the limited wave function overlapping between trapped electrons and holes. Furthermore, there exist fewer surface states per volume for a larger particle, which also discourages surface recombination. Therefore, we believe that the majority of carriers responsible for the yellow visible emission in our sample is trapped within the ZnO nanoparticles. The origin of such a yellow visible emission in ZnO nanoparticles may be the recombination of a shallow trapped electron with a deeply trapped hole Citation24. In this scheme, the photogenerated hole is first trapped at the surface of the nanoparticles by surface defects such as O2−/O− and then migrates to vacancy levels located deep in the ZnO nanoparticles, leading to the formation of a deep hole trapped levels above the valence band. The yellow emission at ∼552 nm occurs when the photogenerated hole trapped in the deep oxygen vacancy recombines with the photogenerated electron trapped in the shallow level located just below the conduction band ().
Figure 5. Schematic illustration of (a) band edge emission at ∼384 nm and (b) yellow visible emission at ∼552 nm from ZnO nanoparticles.
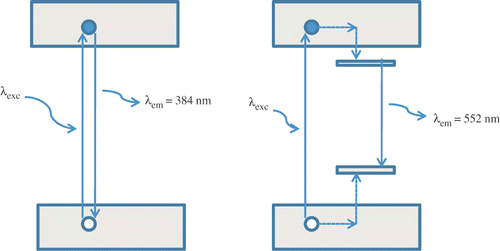
The ZnO sample consists of nanoparticles of different sizes, such as 23 wt% of 8 nm, 19 wt% of 21 nm and 58 wt% of 51 nm; but no significant size effects are observed in the PL spectrum. Since the particle sizes discussed are in the weak exciton confinement regime, the sizes are much larger than the exciton Bohr radius: approximately 2.34 nm in ZnO, where exciton wave function coherence may be expected to achieve some enhancement of oscillator strength, where bulk ZnO properties may begin to be approached, and yet where surface trapping, quenching, and other dead-layer effects can still be significant. Therefore, yellow luminescence is not due to the red-shift effect that occurs as nanoparticle size increases, but involves the recombination of a shallow trapped electron with a deep trapped hole due to the presence of oxygen vacancies. These traps appeared to be size independent and are considered as bulk defects. The yellow luminescence in GaN is also a subject of much debate, as several studies have suggested that is originates at surface states Citation25–27 while other studies suggested have a bulk state Citation28. The results suggested in this study may prove useful in resolving this controversy.
4. Conclusion
We have investigated the particle size of ZnO nanoparticles by SAXS study. These nanoparticles are of different sizes: namely 23 wt% of 8 nm, 19 wt% of 21 nm and 58 wt% of 51 nm. There is no size-dependent emission peak in the PL spectrum of ZnO nanoparticles synthesised by thermal decomposition at ∼800C. The yellow emission band seems due to the recombination of a shallow trapped electron with a deep trapped hole due to the presence of oxygen vacancies, and quenching of band edge emission seems due to the presence of surface impurities such as hydroxyl, carboxylate, etc., confirmed by FTIR analysis.
Acknowledgements
The authors are very grateful to Dr M. Kumar for the PL experiments. This work was financially supported by DST, India.
References
- Bagnall , DM , Chen , YF , Zhu , Z , Yao , T , Koyama , S , Snen , M and Goto , T . 1997 . Optically pumped lasing of ZnO at room temperature . Appl. Phys. Lett. , 70 : 2230 – 2232 .
- Look , DC . 2001 . Recent advances in ZnO materials and devices . Mater. Sci. Eng. B , 80 : 384 – 387 .
- Zhou , H , Alves , H , Hofmann , DM , Kriegseis , W , Meyer , BK , Kaczmarczyk , G and Hoffmann , A . 2002 . Behind the weak excitonic emission of ZnO quantum dots: ZnO/Zn(OH)2 core-shell structure . Appl. Phys. Lett. , 80 : 210 – 212 .
- Xiong , G , Wilkinson , J , Lyles , J , Ucer , KB and Williams , RT . 2003 . Luminescence and stimulated emission in zinc oxide nanoparticles, films and crystals . Radiat. Eff. Defect Solids , 158 : 83 – 88 .
- Priller , H , Hauschild , R , Zeller , J , Klingshirn , C , Kalt , H , Kling , R , Reuss , F , Kirchner , Ch and Waag , A . 2005 . Temperature-dependent luminescence dynamics in ZnO nanorods . J. Lumin. , 112 : 173 – 176 .
- Hong , S , Joo , T , Park , WI , Jun , YH and Yi , G-C . 2003 . Time-resolved photoluminescence of the size-controlled ZnO nanorods . Appl. Phys. Lett. , 83 : 4157 – 4159 .
- Hirai , T , Harada , Y , Hashimoto , S , Itoh , T and Ohno , N . 2005 . Luminescence of excitons in mesoscopic ZnO particles . J. Lumin. , 112 : 196 – 199 .
- Millers , D , Grigorjeva , L , Lojkowski , W and Strachowski , T . 2004 . Luminescence of ZnO nanopowders . Radiat. Meas. , 38 : 589 – 591 .
- Gu , Y , Kuskovsky , I , Yin , M , O’Brien , S and Neumark , GF . 2004 . Quantum confinement in ZnO nanorods . Appl. Phys. Lett. , 85 : 3833 – 3835 .
- Shalish , I , Temkin , H and Narayanamurti , V . 2004 . Size-dependent surface luminescence in ZnO nanowires . Phys. Rev. B , 69 : 245401
- Zhang , SB , Wei , S-H and Zunger , A . 2001 . Intrinsic n-type versus p-type doping asymmetry and the defect physics of ZnO . Phys. Rev. B , 63 : 075205
- Kohan , AF , Ceder , G , Morgan , D and Van de Walle , CG . 2000 . First-principles study of native point defects in ZnO . Phys. Rev. B , 61 : 15019 – 15027 .
- Garces , NY , Wang , L , Bai , L , Giles , NC , Halliburton , LE and Cantwell , G . 2002 . Role of copper in the green luminescence from ZnO crystals . Appl. Phys. Lett. , 81 : 622 – 624 .
- Ohashi , N , Nakata , T , Sekiguchi , T , Hosono , H , Mizuguchi , M , Tsuumi , T , Tanaka , J and Haneda , H . 1999 . Yellow emission from zinc oxide giving an electron spin resonance signal at g = 1.96 . Jpn. J. Appl. Phys. , 38 : L113 – L115 .
- Wu , XL , Siu , GG , Fu , CL and Ong , HC . 2001 . Photoluminescence and cathodoluminescence studies of stiochiometric and oxygen-deficient ZnO films . Appl. Phys. Lett. , 78 : 2285 – 2287 .
- Ortiz , A , Falcony , C , Hernandez , J , Garcia , M and Alonso , JC . 1997 . Photoluminescent characteristics of lithium doped zinc oxide films deposited by spray pyrolysis . Thin Solid Films , 293 : 103 – 107 .
- Schirmer , OF and Zwingel , D . 1970 . The yellow luminescence of zinc oxide Solid . Solid State Commun. , 8 : 1559 – 1563 .
- Maleki , M , Ghamsari , MS , Mirdamadi , Sh and Ghasemzadeh , R . 2007 . A facile route for preparation of CdS nanoparticles . Semicond. Phys. Quantum Electron. Optoelectron. , 10 : 30 – 32 .
- Yadav , RS and Pandey , AC . 2007 . Small angle X-ray scattering and photoluminescence study of ZnO nanoparticles synthesized by hydrothermal process . J. Exper. Nanosci. , 2 : 177 – 182 .
- Guinier , A and Fournet , G . 1955 . Small-Angle Scattering of X-Rays , New York : Wiley .
- Lin , B , Fu , Z and Jia , Y . 2001 . Green luminescent center in undoped zinc oxide films deposited on silicon substrate . Appl. Phys. Lett. , 79 : 943 – 945 .
- Kang , HS , Kang , JS , Pang , SS , Shim , ES and Lee , SY . 2003 . Variation of light emitting properties of ZnO thin films depending on post-annealing temperature . Mater. Sci. Eng. B , 102 : 313 – 316 .
- van Dijken , A , Makkinje , J and Meijerink , A . 2001 . The influence of particle size on the luminescence quantum efficiency of nanocrystalline ZnO particles . J. Lumin. , 92 : 323 – 328 .
- Kahn , ML , Cardinal , T , Bousquet , B , Monge , M , Jubera , V and Chaudret , B . 2006 . Optical properties of zinc oxide nanoparticles and nanorods synthesized using an organometallic method . Chem. Phys. Chem. , 7 : 2392 – 2397 .
- Shalish , I , Kronik , L , Segal , G , Shapira , Y , Eizenberg , M and Salzman , J . 2000 . Yellow luminescence and Fermi level pinning in GaN layers . Appl. Phys. Lett. , 77 : 987 – 989 .
- Shalish , I , Kronik , L , Segal , G , Shapira , Y , Zamir , S , Meyler , B and Salzman , J . 2000 . Grain–boundary-controlled transport in GaN layers . Phys. Rev. B , 61 : 15573 – 15576 .
- Shalish , I , de Oliveira , CEM , Shapira , Y and Salzman , J . 2000 . Hall photovoltage deep-level spectroscopy of GaN films . Phys. Rev. B , 61 : 205313
- Saarinen , K , Laine , T , Kuisma , S , Nissila , P , Hautojarvi , P , Dobrzynski , L , Baranowski , JM , Pakula , K , Stepniewski , R , Wojdak , M , Wysmolek , A , Suski , T , Leszeynski , M , Grzegorg , I and Porowski , S . 1997 . Observation of native Ga vacancies in GaN by positron annihilation . Phys. Rev. Lett. , 79 : 3030 – 3033 .