Abstract
Polypyrrole (PPy)/Al2O3 nanocomposites were prepared by chemical polymerisation of pyrrole in the presence of Al2O3 nanoparticles using iron trichloride (FeCl3) as an oxidant. The obtained nanocomposites were characterised by Fourier transform infrared spectroscopy, Raman spectroscopy, scanning electron microscopy and thermal gravimetric analysis. Upon exposure to an electromagnetic wave in the X-band ranging from 8 to 12 GHz, PPy/Al2O3 composite was shown to be an effective electromagnetic absorbent. More than 53% of the incident microwave radiation was absorbed after passing through a thick composite coated textile.
1. Introduction
Numerous studies have been focused on the conjugated polymers such as polypyrrole, polyaniline, polythiophene and polyphenylene. These polymers are capable of exhibiting a significant level of electrical conductivity, hence they are termed conducting polymers. They have promising applications for batteries Citation1, electrochromic devices Citation2, sensors Citation3, light emitting diodes Citation4 and much more. Much research effort has been spent on producing composites or blends of conducting polymer film with some insulating polymers in order to overcome the drawbacks, such as poor processability and lack of essential mechanical properties, exhibited by these polymers. In this technique, a host of inorganic nanoparticles (such as silver, gold, titanium dioxide, aluminium oxide, zinium oxide, iron oxide and silica) is combined with a conducting polymer (polypyrrole, polyaniline, polythiophene) in an aqueous or organic medium. The resulting composites with the core-shell structures have the combination properties of their components, i.e. the conducting properties of the conducting polymer shell and some physical properties of the inorganic material core Citation5.
There is no doubt that good electromagnetic shielding materials must possess both high conductivity and high permeability. In these respects, metals are the best suited. However, apart from military applications, metals are being increasingly replaced by thermoplastics for housing commercial equipments, due to their flexibility, light weight and low cost. Metallised thermoplastic materials are now commonly used for shielding elements. In addition, several metallised fabrics have been developed that are suitable to provide protective clothing for people exposed to high-frequency electromagnetic fields Citation6. Another possibility involves the incorporation of electrically conductive fillers, in the form of fibres, into synthetic resins during the moulding stage Citation7. Intrinsically conducting polymers, such as polypyrrole (PPy) and polyaniline (PANi) are expected to be excellent conductive fillers to produce economical coating fabrics made of natural or synthetic fibres Citation1,Citation7.
In this study, PPy/Al2O3 nanocomposites were prepared by a chemical procedure using FeCl3 as an oxidant. The obtained nanocomposites were studied by Fourier transform infrared spectroscopy (FT-IR), Raman spectroscopy, scanning electron microscopy (SEM) and thermal gravimetric analysis (TGA). Upon exposure to an electromagnetic wave in the X-band ranging from 8 to 12 GHz, the electromagnetic shielding features of the textile coated PPy/Al2O3 composites were measured.
2. Experimental
2.1. Preparation of PPy/Al2O3 nanocomposites
Nanocomposites were prepared following a procedure described in Citation5,Citation8,Citation9. First, a dispersion was prepared by a mixture of 10.0 g Al2O3 (Reana, Budapest), 1.0 g sodium toluensulfonate and 1.0 ml pyrrole monomers in 40.0 ml distilled water. To absorb monomers on the surface of oxide particles, this dispersion was stirred for 30 min. Then 3.5 g FeCl3 (water-free, Fluka Chemie) was added to the oxide particle dispersion during stirring. The colour of the mixture was changed from grey to black. The oxide particles were covered by oxidised PPy shell, and PPy/Al2O3 composites were formed. After 2 h of stirring, the composites were cleaned with distilled water, filtered and dried at 40°C–50°C for 3 h under low pressure. The core-shell structure of the same nanocomposites is described in Citation5.
2.2. Characterisation of PPy nanocomposites
The morphology of nanocomposites particles was studied by SEM and TEM techniques. The SEM pictures were performed by JSM 53000. The chemical structure of the nanocomposites was characterised by FT-IR and Raman spectroscopy. FT-IR spectra were obtained on GBC Cintra 40-Nicolet Nexus 670 FT-IR. Raman spectra were measured by a laser Raman spectrophotometer (Ramalog 9I, USA). TGA was done by Ghimashu-50 H with scan rate of 10°C min−1 in atmospheric condition. For attenuation properties measurements, the PPy/Al2O3 nanocomposite was mixed with a resin (transparent material with electromagnetic microwave) and coated on a textile with a layer of 0.6 mm thickness. The electromagnetic shielding features of the nanocomposites in X-band from 8 to 12 GHz microwave were performed by HP8720D Network Analyzer (USA).
3. Results and discussion
3.1. Morphology of nanocomposite particles
A SEM image of the nanocomposite particles is shown in , and depicts the surface morphology of a pressed pellet made from Al2O3 covered by PPy (in oxidised state). The average size of composite particle is around 100 nm while that of Al2O3 particle is around 50–60 nm. This indicates that the surface of the Al2O3 particles is covered by a PPy shell with a thickness of about 20–25 nm.
3.2. FT-IR spectra and Raman scattering
The chemical structure of PPy nanocomposites was characterised by FT-IR spectroscopy and Raman scattering. The FT-IR spectra are of good quality and the infrared bands are well defined. The oxidised PPy are characterised by a very large absorption band located in the spectral domain between 4000 and 2500 cm−1, which is characteristic of the OH groups belonging to residual water molecules trapped in the polymer matrix. These bands have already been observed in the case of PPy electrochemically synthesised in aqueous sodium tartrate, and in some solids containing water of crystallisation, corresponding to the so-called ‘lattice water’ Citation10. In comparison with the well-known IR spectrum of PPy obtained in organic media on noble metal electrodes such as Pt, all the PPy characteristic bands are observed in the spectra. The bands situated at 884 and 873 cm−1 are attributed to the out-of-plane vibration of the C–H bonds. The sharp band at 1408 cm−1 corresponds to the in-plane deformation of the N–H bonds. The band at 1040 cm−1 in the spectra is attributed to the in-plane deformation of the C–H bonds. The stretching vibration of the nucleus C=C bonds corresponds to the very intense IR bands located at 1664 and 1629 cm−1 for the oxidised states of PPy. Finally, the intensity of the sharp bands located at 1710 cm−1 depends on the oxidation degree of the polymer. Indeed, this band is weak in the reduced form and is intensified in the oxidised form. On the basis of these data, the band can be attributed to carbonyl groups fixed in the β position of some pyrrole rings.
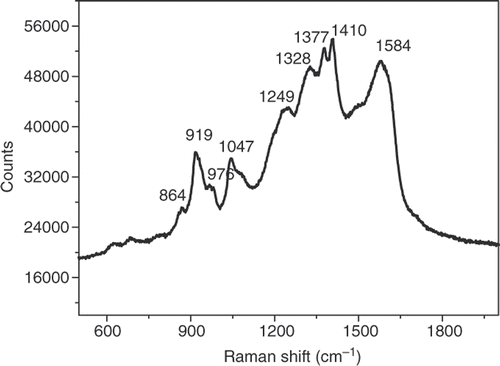
presents the Raman spectrum of PPy/Al2O3 nanocomposites measured at 633 nm laser with 2.5 mW power. gives the assignments of some Raman bands and compares the frequencies of the various vibration modes collected on the PPy/Zn spectra with those theoretically calculated by Faulques et al. Citation11. According to the theoretical values, the vibration frequency of the C=C bonds of the PPy in the oxidised form is greater than that in the reduced form ().
Table 1. Wavenumbers and assignments of Raman bands of PPy/Al2O3 nanocomposites.
3.3. Thermal analyses
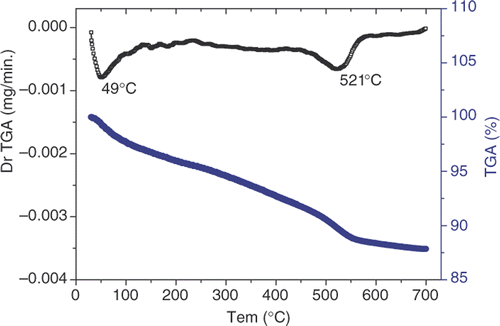
Thermal analyses of PPy/Al2O3 composites are shown in . Below 120°C, the weight reduces due to the vaporisation of water inside the samples. It is also the source of the wide band between 4000 and 2500 cm−1 in the IR spectra. This reduction is evidence that PPy is in an oxidised state and that this results in the higher absorption of water. In the range 100°C–330°C, the weight loss is less, corresponding to the decomposition of redundant monomers and oligomers. At 521°C, the change in weight is attributed to the decomposition of the oxidised PPy.
3.4. Shielding effectiveness measurements
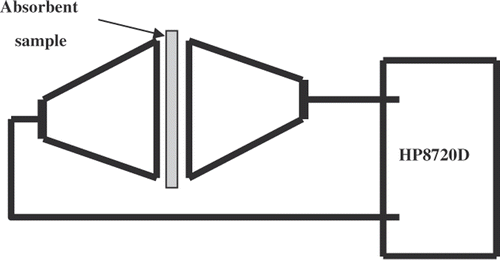
The PPy/Al2O3 coated textile was cut into test samples to fit the waveguide sample holders for the shielding efficiency (SE) measurements. The scheme of SE measurement is shown in . The sample holder is kept between two coaxial transmission lines with an interrupted inner conductor and a flanged outer conductor and then connected to HP8720D Network Analyzer. Using an uncoated textile as the reference sample, the network analyzer is calibrated to 0 dB and the calibration is stored for load measurement. From the measured data, absorption coefficient A and EMI SE of the test samples are evaluated. The SE is calculated using the ratio of the power of the incident wave to that of the transmitted wave, as in the following formula Citation11:
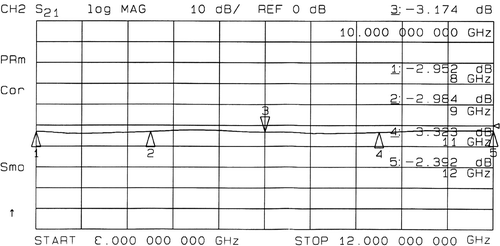
The SE of the PPy/Al2O3 coated textile is given in . The result reveals that a SE around −3.3 dB (53%) is obtained. However, for most industrial applications, a SE of −30 dB is useful because it prevents 99.9% of electromagnetic flow.
4. Conclusion
In this article, PPy/Al2O3 nanocomposites were prepared by a chemical process. The morphology of composite particles was studied by SEM and TEM techniques showing that they were in order of nanoscale. The aluminium oxide cores were covered by a thin polypyrrole shell. The chemical structure of PPy in nanocomposites was characterised by FT-IR and Raman spectra. The PPy/Al2O3 nanocomposites showed features comparable to those of the PPy films prepared by electropolymerisation on metal electrodes. The thermal analysis showed that PPy in the nanocomposites was still stable at 521°C. The shielding effectiveness of the PPy nanocomposite-coated fabric measured in the range 8–12 GHz was in the order of −3.3 dB (53%). However, for industrial applications the attenuation value was a modest value. The SE of these nanocomposites must be improved for practical applications.
References
- Li , N , Lee , Y and Ong , LH . 1992 . A polyaniline and Nafion® composite film as a rechargeable battery . J. Appl. Electrochem. , 22 : 512 – 516 .
- Chandler , GK and Pletcher , D . 1985 . The electrochemistry of conducting polymers . R. Soc. Chem. Lond. , 10 : 117 – 150 .
- Brady , S , Lau , KT , Megill , W , Wallace , GG and Diamond , D . 2005 . The development and characterization of conducting polymeric-based sensing devices . Synth. Met. , 154 ( 1–3 ) : 25 – 28 .
- Garten , F , Vrijmoeth , J , Schlatmann , AR , Gill , RE , Klapwijk , TM and Hadziioannou , G . 1996 . Light-emitting diodes based on polythiophene: Influence of the metal work function on rectification properties . Synth. Met. , 76 ( 1–3 ) : 85 – 89 .
- Vu , QT , Pavlik , M , Hebestreit , N , Rammelt , U , Plieth , W and Pfleger , J . 2005 . Nano-composites based on titanium dioxide and polythiophene: Structure and properties . React. Funct. Polym. , 65 : 69 – 77 .
- Avloni , J , Ouyang , M , Florio , L , Henn , AR and Sparavigna , A . 2007 . Shielding effectiveness evaluation of metallized and polypyrrole-coated fabrics . J. Thermoplast. Compos. Mater. , 20 ( 3 ) : 241 – 254 .
- Chung , DDL . 2001 . Electromagnetic interference shielding effectiveness of carbon materials . Carbon , 39 ( 2 ) : 279 – 285 .
- Vu , QT , Pavlik , M , Hebestreit , N , Pfleger , J , Rammelt , U and Plieth , W . 2005 . Electrophoresis deposition of nanocomposites formed from polythiophene and metal oxides . Electrochim. Acta , 51 : 1117 – 1124 .
- Plieth , W and Hebestreit , N . German Patent Application No. 19919261.8
- Bazzaoui , EA , Aeiyach , S and Lacaze , PC . 1994 . Low potential electropolymerization of thiophene in aqueous perchloric acid . J. Electroanal. Chem. , 364 ( 1–2 ) : 63 – 69 .
- Faulques , A , Wallnoefer , W and Kuzmany , H . 1989 . Vibrational analysis of heterocyclic polymers: A comparative study of polythiophene, polypyrrole, and polyisothianaphtene . J. Chem. Phys. , 90 ( 12 ) : 7585 – 7595 .