Jordan CJ, and Xi ZX. Discovery and development of varenicline for smoking cessation. Expert Opin Drug Discov. 2018 Mar 28:1-12. doi: 10.1080/17460441.2018.1458090
When the article was first published online, it contained a number of errors. These have now been corrected in both the print and online versions as below.
Abstract
Areas Covered:
The authors discuss the rationale, preclinical and clinical development of varenicline for
smoking cessation. They cover the development of varenicline as a partial agonist at α4β2 receptors, the primary neural substrate for nicotine reward. Then, they discuss evidence from preclinical studies indicating varenicline’s efficacy in blocking nicotine reward, followed by clinical trials demonstrating safety and efficacy in sustaining abstinence in smokers. Finally, they cover post-market surveillance, including caution in heavy machine operators, putative cardiovascular risk, and the repealed warning for adverse neuropsychiatric events.
Article highlights
3rd point
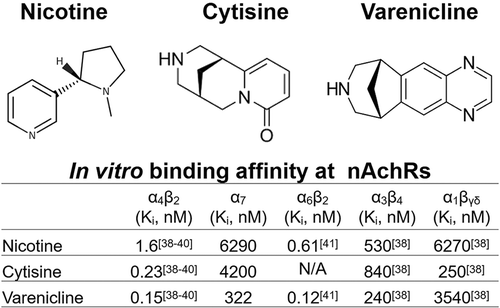
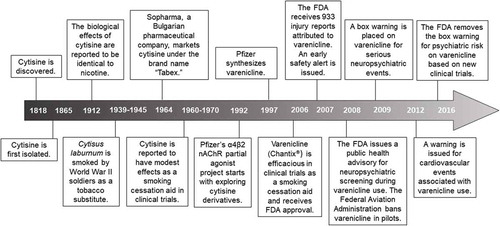
Varenicline was developed by Pfizer as a selective partial agonist at the α4β2 receptor based on derivatives of nicotinic natural products, including cytisine, a plant-derived alkaloid which was used as a tobacco substitute during World War II.
Section 2.4
First sentence
Varenicline tartrate was originally developed as a smoking cessation agent by Pfizer in 1997, based on derivatives of nicotinic natural products, including cytisine (Figure 2). However, the cytisine structure did not lead to a viable drug candidate but the bicyclic template led to drug-like nAChR partial agonists, including varenicline [38].
Section 3.2.
2nd Paragraph, line 16
In a pooled analysis of the work by Jorenby and colleagues [61] and Gonzales and colleagues [65], varenicline was more than doubled the rate of smoking cessation at one year with comparable or fewer side effects and adverse events [71,72]
Table 2 has been replaced
Table 2. Clinical Trials Supporting Varenicline for Smoking Cessation
Section 4.2.
First Sentence
In addition to post-marketing reports regarding caution for neuropsychiatric events, the varenicline package insert advises patients to use caution driving or operating machinery or engaging in other potentially hazardous activities until they know how Chantix may affect them.
Expert Opinion
Second Sentence: The strategy of the discovery project for varenicline synthesis began with derivatives of nicotinic natural products, notably cytisine. While the cytisine structure did not lead to a viable drug candidate, a bicyclic benzazepine template led to drug-like nAChR partial agonists, including varenicline [38,110,111].
References
References 110 and 111 were added
110. Coe JW, Rollema H, O’Neill BT. Case history: ChantixTM/ChampixTM (varenicline tartrate), a nicotinic acetylcholine receptor partial agonist as a smoking cessation aid. Annual Rep Med Chem 2009; 44:71-101.
111. Nides M, Oncken C, Gonzales D, Rennard S, Watsky EJ, Anziano R, Reeves KR. Smoking cessation with varenicline, a selective alpha4beta2 nicotinic receptor partial agonist: results from a 7-week, randomized, placebo- and bupropion-controlled trial with 1-year follow-up. Arch Intern Med 2006; 166(15):1561-1568.
Table Note. BID: Twice daily. QD: Once daily. #Continuous quit rates were higher for 1.0 mg varenlicine QD (37.3%) and 1.0 mg varenicline BID (48%) compared to placebo (17.1%). Overall varenicline was well tolerated. $Although Nides et al. was published in 2008, the Chantix Prescribing information indicates this study was among the six trials demonstrating the efficacy of varenicline for smoking cessation. *69% of subjects titrated to maximum dose. ^CAR at weeks 13-24 was higher for varenicline-treated subjects (70.5%) than those switching to placebo (49.6%). Varenicline’s efficacy over placebo was maintained at week 52 (43.6% CAR vs. 36.9% CAR, respectively) 68. ‡ Varenicline and placebo groups with stable cardiovascular disease showed no significant differences in cardiovascular mortality or adverse cardiovascular events 89. † In participants with a history of psychiatric illness 9-12 weeks and 9-24 weeks CARs with varenicline (38%, 25.5%) were higher than that in participants without psychiatric illness (29.2%, 18.3%). Neither varenicline nor bupropion increased moderate-to-severe neuropsychiatric events. At weeks 9-12 follow-up CAR was higher in participants receiving varenicline compared to all other treatment groups (odds ratio vs. placebo: 3.61; vs. NRT 1.68; and vs. bupropion 1.75) 84 .