ABSTRACT
Introduction: There are at the minimum two major, quite different approaches to advance drug discovery. The first being the target-based drug discovery (TBDD) approach that is commonly referred to as the molecular approach. The second approach is the phenotype-based drug discovery (PBDD), also known as physiology-based drug discovery or empirical approach.
Area covered: The authors discuss, herein, the need for developing radiation countermeasure agents for various sub-syndromes of acute radiation syndromes (ARS) following TBDD and PBDD approaches. With time and continuous advances in radiation countermeasure drug development research, the expectation is to have multiple radiation countermeasure agents for each sub-syndrome made available to radiation exposed victims.
Expert opinion: The majority of the countermeasures currently being developed for ARS employ the PBDD approach, while the TBDD approach is clearly under-utilized. In the future, an improved drug development strategy might be a ‘hybrid’ strategy that is more reliant on TBDD for the initial drug discovery via large-scale screening of potential candidate agents, while utilizing PBDD for secondary screening of those candidates, followed by tertiary analytics phase in order to pinpoint efficacious candidates that target the specific sub-syndromes of ARS.
1. Introduction
In today’s modern medicine, subcellular components such as lipids, polysaccharides, nucleic acids, and proteins constitute major categories of pharmaceutical drug targets in the human body. There are other general categories of drug targets as well, including macromolecular complexes (e.g. microbial cell wall constituents), cellular systems or vital networks [Citation1,Citation2]. However, almost all approved drugs today have their mechanisms of action directed at proteins since low specificity and high toxicity are more commonly associated with the other three macromolecules [Citation1,Citation2]. There are about 20,000 proteins reported as being coded for by the human genome of which between 2,000 and 3,000 proteins are identified as suitable for drug interactions and even fewer are considered as appropriate drug targets [Citation1–Citation3]. However, significantly higher estimates of the numbers of proteins have been made due to primarily splicing processes (between 10,000 and several billion different protein species): such processes and estimates are fundamental proteomics issues. Nevertheless, around 600 separate human proteins represent the entire druggable proteome for nearly all pharmaceutical drugs in modern medicine [Citation2]. More than 60% of these druggable human proteins are membrane-associated proteins and are categorized based on function into six protein families: enzymes, transporters, voltage-gated ion channels, G-protein coupled receptors (GPCR), nuclear receptors, and cluster of differentiation (CD) markers [Citation1–Citation3]. A druggable protein could be defined as one that possesses binding pockets favorable to small molecule ligands or peptides and is involved in signal transduction whose dysfunction is linked to the pathophysiological state of interest [Citation3]. The druggable protein is typically membrane bound with long, in vivo half-lives, and its principal domain is usually characterized as possessing the required site-specific affinity and hydrophobic properties [Citation3].
2. Need of countermeasures for acute radiation syndrome (ARS)
The availability of radiation countermeasures for ARS has become a critical US homeland security concern as well as a military concern since the terrorist attacks in 2001 [Citation4,Citation5]. The challenges facing medical countermeasure development are complex [Citation6]. Generally, medical countermeasure development and approval must follow the arduous product review process of the Food and Drug Administration (FDA). Yet, when human efficacy data are not available and the drug or the biologic needs to be developed following the FDA Animal Rule, the scientific and regulatory obstacles may be significantly greater than the challenges faced during the regular drug development and approval process [Citation7]. The FDA Animal Rule is intended to be used in the development of drugs to counter debilitating or lethal conditions resulting from either accidental or deliberate exposure to chemical, biological, radiological, or nuclear agents when human clinical studies for efficacy are neither feasible nor ethical. The FDA may grant marketing approval of a drug or biologic to treat or prevent a serious or life-threatening condition caused by a permanently disabling or lethal toxic agent, if animal efficacy studies adequately establish the likelihood that the drug will provide a clinical benefit in humans [Citation8,Citation9]. Countermeasures being developed should follow the existing requirements for establishing the safety of new agents. The following four criteria must be met to rely on animal efficacy studies:
The pathophysiological mechanism of injury/toxicity by the stimulus/agent is understood, as well as the role of the product in the prevention or significant reduction of said injury or toxicity.
The efficacy of the product is demonstrated in more than one animal model with a response predictive of human response or a single animal species that has been adequately characterized for predicting the human response.
The animal study endpoints clearly related to the desired result in humans, normally reduced mortality or morbidity.
The effective dose can be calculated from kinetic and pharmacodynamics of the product (or relevant data) in both humans and animals.
To date, three radiation countermeasures have been approved by the US FDA for hematopoietic ARS (H-ARS) following the Animal Rule [Citation10–Citation14].
In addition, drug development for FDA approval for human use can be and is often very costly [Citation15,Citation16]. Failure in clinical trials exceeds 90% after drugs are tested in model systems [Citation17]. Based on the R&D costs of 106 randomly selected new drugs/biologics, the average pre-tax cost of new drug development is $2,870 million (2013 dollar value) [Citation18]. In the latter calculation, investment costs in drugs discontinued during development have been added to the costs of agents that finally received full FDA approval. Regardless, the estimated average cost per approved agent was a staggering $1,395 million (2013 dollars). Further, capitalizing costs to the point of marketing yields a total pre-approval cost of $2,558 million (2013 dollars) (over the time it takes from the start of clinical evaluation to marketing approval using 96.8 months, $1,163 million represents the lost opportunity cost of foregoing investments with annual returns of 10.5%). Adding an estimate of the post-approval R&D cost increase, the cost estimates to $2,870 million (2013 dollars). Furthermore, there is not yet a bonafide commercial market for radiation countermeasures for ARS. Although the 2004 Project BioShield funded a government program to procure medical countermeasures, the available funds are small compared to the possible monetary return made by developing and marketing a blockbuster drug intended for other indications [Citation19,Citation20]. The above-mentioned challenges and the absence of a commercial market for radiation countermeasures have left the most successful, the most experienced drug companies at navigating FDA’s complex regulatory drug approval process hesitant to enter into this difficult area of drug development for ARS. Small, start-up biotechnology companies interested in such agents are often technically strong, but have little or no understanding of the complexities of drug development through the FDA approval process; hence, they repeatedly run into problems. To date, no new chemical entity (NCE) has been FDA approved as a radiation countermeasure for ARS. All three of the currently FDA-approved radiation countermeasures (Neupogen, Neulasta, and Leukine) are classified as radiomitigators and represent repurposed agents approved specifically to counter developing H-ARS. These agents were in clinical use for decades for other indications prior to their approval for H-ARS [Citation21–Citation24]. To date, however, no radioprotective agent (i.e. radioprotector) that can be administered prior to exposure has been approved for H-ARS.
The radioprotective agents would be administered to individuals in anticipation of radiation exposure. This means that they would carry a more intrinsic risk, and therefore require additional regulatory scrutiny. Nevertheless, the need to develop such protective agents is very significant in terms of national security. Radiomitigators, by contrast, are given to victims already exposed to radiation and are thus distinguishable from radioprotectors in terms of developmental constraints and regulatory requirements. Furthermore, no single radioprotector or radiomitigator has been approved for gastrointestinal ARS (GI-ARS). Considering the above-mentioned facts, there is a need for a judicious approach for the development of the countermeasures for ARS so that such agents become available sooner rather than later.
2.1. Molecular pathways involved in the cellular response to ionizing radiation
The knowledge of the molecular pathways implicated in the cellular response to ionizing radiation and its correlation with cell-type, radiation quality, and dose specificity continues to evolve. This has given rise to a number of pharmaceutical strategies including a continued focus on preventing cell damage with antioxidant compounds that scavenge free radicals, especially the hydroxyl radical (•OH) from radiolysis [Citation25,Citation26]. Other strategies emanating from the literature involve focusing on compounds that attenuate the expression of a variety of cellular responses; for example, the amelioration of cell damage or cell death pathways involving inflammation or limiting mitotic catastrophe, apoptosis, micronucleation, autophagy, necrosis, or senescence due to irradiation of cells [Citation25–Citation27]. With over half of the human body made up of water (i.e. 50–70%), two-thirds of which is found inside the cell [Citation28–Citation30], the initial chemical effect of irradiation on this water (radiolysis) results in further, but indirect, cellular, and biomolecular damage from the toxic byproducts such as superoxide (HO2) and peroxide (H2O2) of the radiolytic processes [Citation31]. This is particularly true of cellular exposure to low linear energy transfer of ionizing rays or particles; e.g. X-rays, γ-rays, β-particles [Citation25,Citation31,Citation32]. As these primary, energetic species are quickly diminished and attenuated, there is a subsequent and toxic buildup of reactive oxygen species (ROS) and reactive nitrogen species (RNS) via endogenous propagation by cellular systems [Citation31]. These radiation-induced events can quickly activate (i.e. seconds to minutes) the DNA damage response (DDR) system, and they can also trigger cell dysfunctions that ultimately result in cell death for either mitotically active cells (cycling cells) or mitotically inactive cell (non-cycling cells) types [Citation31].
2.2. Ionizing radiation oxidative stress: effects on proteins and cellular adaptive responses
Amplification of oxidative stress through ROS, RNS, and/or reactive carbonyl species (RCS) that overload the cellular system as a result of irradiation can modify or oxidize carbohydrates, nucleic acids, lipids, and proteins [Citation33,Citation34]. However, about 70% of these oxidative insults are absorbed primarily by proteins. Because of their high abundance in cells, these protein modifications can result in a wide variety of structural and functional impairments including changes in the expression of proteins [Citation33,Citation34]. Under normal physiological conditions, antioxidant defenses, including non-enzymatic (e.g. glutathione, ascorbate), direct enzymatic (e.g. superoxide dismutases, glutathione peroxidases), and ancillary enzymes (e.g. peroxiredoxins, disulfide reductase) serve as scavengers or buffers to minimize oxidative and/or nitrosative damages to proteins [Citation35–Citation38]. They also help to protect redox-sensitive amino acid residues such as methionine, cysteine, tyrosine, tryptophan, etc. from irreversible oxidation [Citation33–Citation38]. However, in a pathophysiological state characterized by overproduction of ROS/RNS/RCS, including the loss or weakening of the DDR system and antioxidant defenses, cellular proteome can be negatively impacted by oxidative stress [Citation33–Citation38]. These pathophysiological conditions can impact most, if not all, of the cellular biochemical processes because the majority of cellular functions and structure components are mediated or supplied by proteins and because protein function is transduced through molecular interactions involving amino acid side-chains. Carbohydrates and lipids under oxidative stress conditions can disrupt the cellular membrane integrity to form reactive carbonyl species that can modify cysteine, lysine, and histidine residues in proteins [Citation33–Citation38].
Because oxidative modification of proteins can ultimately result in the degradation of cellular function, proteins are deemed fundamental to the understanding of pathophysiological conditions under radiation-induced oxidative stress and to discovering novel biological targets for radiation countermeasure development. In this context, identification and characterization of proteins and the specific amino acid side-chains within those proteins that are susceptible to oxidative/nitrosative post-translational modification (PTM) as a result of radiation-induced oxidative stress can provide a better understanding of target proteins that can serve as promising molecular targets for therapeutic and/or diagnostic purposes. The application, therefore, of the redox proteomics approach by mass spectrometry and/or chemoselective (i.e. biochemical and immunological techniques) methods can provide evidence of modified proteins such as protein-bound carbonyls, including the modification sites and the relative quantification of total oxidized PTM in the cellular proteome as a result of irradiation [Citation33–Citation38].
2.3. Causative role of protein oxidation in the cellular pathophysiology of ionizing radiation
In vivo evidence of altered protein signaling at radiation exposures of <1 Gy along with evidence of increased levels of oxidative PTMs that includes, but not limited to protein carbonylation and s-nitration at <10 Gy, have been reported in the literature and serve as examples of the effect of radiation on cellular proteins [Citation31]. Studies into the molecular mechanisms of cellular resistance and survival under acute, intense irradiation in a wide range of extremely radiation resistant organisms (e.g. Deinococcus radiodurans select strains of Escherichia coli, Thermus thermophilus) suggest that the lethal effects of radiation might be governed to a large extent by oxidative stress-mediated protein damage [Citation39,Citation40]. These findings show that double-strand DNA breaks (DSBs) between highly sensitive and extremely resistant microbial cell types are relatively constant, but the yield of protein oxidation is highly variable and quantitatively related to cell survival [Citation39,Citation40]. These experimental and observational findings postulate progressive accumulation of oxidative damage to the proteome and dysregulation of protein homeostasis as major determinants of cell survival after irradiation. The inferences drawn from all of these findings point to the critical role and prospects of redox active proteins in host cells, as well as the insights gained through application of redox proteomics techniques, provide a promising basis for new strategies in drug discovery and development to combat radiation toxicity in humans. A number of oxidized protein residues commonly detected under oxidative stress and their detection methods have been reported and covered extensively by others [Citation31,Citation33,Citation34,Citation38].
Direct or indirect radiation-induced damage, via ROS/RNS/RCS production, has been reported in the literature for protein complexes such as c-Abl (cytoplasmic Abelson tyrosine kinase), c-Jun N-terminal kinases (JNK), tumor suppressor protein p53, nuclear factor-κB (NF-κB), heat shock transcription factor 1 (HSF1), phosphoinositide 3-kinase (PI3K)/protein kinase B or Akt (PKB/Akt), extracellular signal-regulated kinases (ERK), phospholipase C-γ (PLC-G), cytoplasmic Janus protein tyrosine kinases (JAKs)/signal transducers, and activators of transcription (STAT) [Citation41]. Oxidative stress damage to proteins has been implicated in other complex human diseases such as Amyotrophic lateral sclerosis, Parkinson, and Alzheimer which involve another redox-sensitive protein [Citation42]. In these pathophysiological conditions, the monomeric globular form of cellular prion protein (PrPC) and aggregation of its oxidized methionine residues have been implicated in causing the normal form of the protein (i.e. PrPC) to be misfolded into the abnormal isoform (PrPSC) in the disease state [Citation43–Citation46]. This copper-binding protein PrPC that undergoes oxidative modification or inactivation in a prion-like disease model has been shown to have a direct correlation to decrease activity and expression of superoxide dismutases [Citation44–Citation46]. It has also been shown to have a correlation with changes in cellular vascular responses as a result of oxidative stress from irradiation [Citation44–Citation46]. Since the majority of severe protein oxidation damage results in carbonylation or inactivation of active site residues that cannot be repaired, these damaged proteins ultimately aggregate and can lead to overall cellular dysfunction or ultimately to cell death [Citation37]. Therefore, understanding the oxidative modification of redox proteins under radiation-induced oxidative stress conditions may be critical for new discovery and development of molecular protein targets for next-generation radiation countermeasures.
3. Approaches for drug discovery
Though biomedical science for drug development has moved forward by leaps and bounds, there are apprehensions regarding the cost and efficiency of translating scientific advances into non-toxic, safe, and efficacious drugs for various indications. In the case of drug development for the protection and treatments of injuries from unwanted exposures to ionizing radiation, only a limited number of successes have been achieved. The reason for this is largely due to the sheer number of obstacles arising from not only technical, ethical, and funding issues, but also the biological complexity of the clinical entity (radiation injury). Despite the significant efforts and resources devoted to drug discovery, there are limited data that are publicly accessible as to how these drugs under study work. The drug discovery strategies include both molecular and empirical approaches. The molecular approaches are usually hypothesis-driven and are referred to as target-based drug discovery (TBDD) while empirical approaches are known as phenotype-based drug discovery (PBDD) because they rely on phenotypic measures of response. Recombinant technology and other omics approaches for engineering screening assays with defined molecular targets have enabled target-based drug discovery. In the phenotypic approach, drugs are identified without the need for prior knowledge of their specific target or site of action by first identifying their mode of action in cells, tissues, or animals in an unbiased manner. Based on the recent literature, the PBDD appears to be a more successful strategy for small-molecule, first-in-class drugs. This finding is somewhat unexpected since medical research is based on the notion that a better understanding of the molecular mechanism of disease, followed by genetic and molecular advances, would lead to an increase in new medicines. The reason for this success of the phenotype-based strategy may be due to the unbiased identification of the molecular mechanism of action [Citation47]. Both approaches have their strengths, weaknesses, advocates, and critics. Understanding the strengths and limitations of TBDD and PBDD is essential to develop novel pharmacological therapeutics [Citation48].
3.1. Target-based drug discovery (TBDD)
Omics technology has enabled target-based drug discovery and has allowed for possible testing against large libraries of compounds through high-throughput screening [Citation49]. Human genome sequencing has increased tremendously the number of available molecular targets for drug development. When a gene is linked to a disease, there is a strong possibility that an agent targeting that specific gene or gene product has a good probability for successful development. Several target classes have been shown to be amenable to the target-based approach [Citation50]. As the molecular understanding of a disease increases, the use of a target-based approach improves. The TBDD approach is superior in its screening capacity, and it can effectively develop novel drugs for a validated target, but the process of target validation is complicated and associated with a high degree of uncertainty. However, the application of high throughput screening technologies has not been used in any meaningful fashion in terms of discovering and developing new drugs to counter unwanted radiation injuries.
3.1.1. Strengths of TBDD
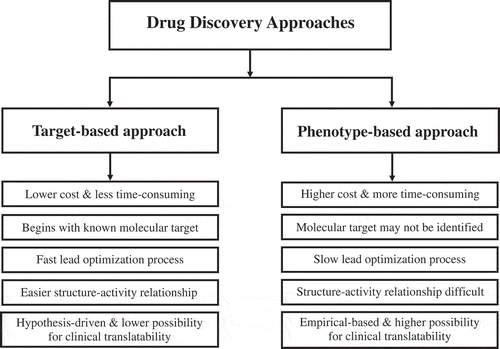
Overall, TBDD has been considered successful for drug discovery within restricted clinical areas of concern; further, the approach is not limited to small molecules. Biologics such as antibody and other proteins, gene therapy, and nucleic acid-based agents are examples of agents discovered through the target-based approach. This approach is relatively simple to execute, based on the molecular mechanism of the agent, fast, easy, and cost-effective (). With known molecular targets, drug development efforts can use binding kinetics, mutational analysis, molecular pharmacology, genomics, biochemistry, computational modeling, and crystallography to understand how an agent interacts with its target. This strategy allows for an easy to understand structure–activity relationship, the identification of biomarkers, and the development of additional agents acting on the same target. With a greater understanding of the molecular basis of a disease, the utility of TBDD increases. Human genetics is very helpful in the identification of potential drug targets. If a gene is linked to a disease, it is comparatively easy to be successful in developing a drug for that indication by targeting that specific gene or its gene product. Sometimes, rare genetic conditions direct us to identify drug targets for common clinical conditions. There are selected genotypes that promote radiosensitivity and are associated with various clinical conditions. The migration and invasion inhibitory protein gene expression is associated with radiosensitivity in human nasopharyngeal carcinoma cells [Citation51]. Melanoma cells have been reported to demonstrate a heterogeneous range of radiosensitivity and are radiosensitized by inhibition of proto-oncogene B-RAF [Citation52]. Similarly, there are genotypes that promote radioresistance. Notch is known to promote radioresistance of glioma stem cells [Citation53].
Figure 1. Comparison of target-based and phenotype-based approaches for drug development. There are various positive and negative attributes for each drug discovery approach.
GPCRs are the most widely studied drug targets, mostly due to their high rate of clinical success for therapeutic target selection in many human disease indications. There are about 475 GPCR drugs and agents that have been developed and are known to act at 108 unique GCPRs. This number represents about 34% of all drugs that have been approved up-to-date by the US FDA [Citation54]. Before the advent of cloned molecular targets, GPCRs were helpful for PBDD based on unbiased observations of associated pharmacological responses; however, modern advances in recombinant and genomic technologies have brought GPCRs to prominence in the TBDD approach.
3.1.2. Challenges of TBDD
In the recent past, the pharmaceutical industry has experienced a gradual decline in productivity and this decline coincides with the introduction of TBDD strategy. An over-reliance on omics and TBDD strategy and underscoring PBDD approach may be the potential reason for decreased success in the discovery of first-in-class drugs. The TBDD approach can effectively develop novel drugs for validated targets, but target validation is complex and coupled with uncertainty [Citation55]. The recombinant systems can help investigators accelerate a drug discovery effort, but the technology does not always translate into the desired clinical results. Biological systems are very complex and simplified recombinant technology in lower vertebrate animal models or in vitro systems may fail to capture the complex biology of human patients or their clinical conditions. Targets in cell-based assays do not always behave in a manner that is exactly comparable to the complex systems of whole organisms. Results obtained from gene engineering in model systems may not always exactly translate to patients. Even when a drug target is known and the mechanism is well understood, this basic information can still fail to capture the entire story about the pharmacotherapeutic consequences. For example, the agent may act at more than one target and the efficacy observed may not be due to the mechanism initially anticipated.
3.2. Phenotype-based drug discovery (PBDD)
In the phenotypic strategy, drugs or biologics are identified without knowledge of specific molecular targets. In this approach, the pharmacological actions are first identified in cells, tissues or animal models. An important example of the utility of using such an approach can be seen as the current array of clinically useful antibiotics which have been identified by their ability to kill bacteria or slowdown their growth without the knowledge of the molecular targets involved for such actions. High throughput screening assays have been generally applied quite successfully to identify promising agents from large pools of candidates [Citation56,Citation57]. In earlier phenotypic high-throughput assays, cells or organisms were largely manually manipulated using microtiter plates along with the use of fluorescent or luminescent reagents, automated plate readers, and advanced data processing. This system has significantly progressed and now robotic liquid handling devices are commonly used for high-throughput phenotypic screening. Phenotypic assays test drugs in intact biological systems such as cells, tissues, or animals, in an attempt to improve the translation of drug discovery for clinical use. It has been suggested that the less a biological system is perturbed, the more accurately it will predict how a drug will intervene in the clinic within the human patient [Citation49]. Phenotypic assays come in different forms; namely the types and status of investigating cells (in vitro, ex-vivo), tissues, or animal models to study the response related to human disease conditions. Induced pluripotent stem cells (iPSC) and differentiated cells to appropriate phenotype, 3D tissue culture, 3D printed tissues, organ on a chip, organoids, and co-cultures of various cell types are all valuable technologies and resources for the phenotypic approach for the discovery and development of drugs for clinical use. Although various animal models used in the phenotypic strategy may not precisely mimic the human disease condition, they might provide the opportunity to efficiently screen large numbers of potential agents of interest. Further, select aspects of the agent’s pharmacology may be revealed within these simplified models of intact organisms or their composite networks. In brief, the application of these model systems that are amendable to high throughput screening might offer an invaluable new set of analytic tools to both identify and to translate more judiciously radioprotective or radiomitigative agents for ARS [Citation58]. Despite the reality that phenotypic approaches often use semi-empirical methods that lack a full understanding of the complete array of molecular targets and associated molecular mechanisms, they are in fact a rich resource of information concerning pathogenic processes. As such, these approaches have served to identify important biomarkers but not necessarily biomarkers that translate to human disease [Citation47].
3.2.1. Strengths of PBDD
In TBDD, the starting point is a well-defined molecular target that is expected to play an important role in disease development. During the last few decades, it has been the dominant approach for the drug discovery driven by omics approach. Because of TBDD’s limited number of successes, there has been a renewed interest in using PBDD approaches that do not depend on the identity of a specific target. Recent data from FDA-approved first-in-class small-molecule drugs suggest that the PBDD approach has been more productive than the TBDD strategy in an era when the majority of efforts were focused on the molecular approach of TBDD [Citation59,Citation60]. Very often, drug targets are not well known and a fresh approach using PBDD is needed. Since TBDD is focused on identifying small molecules interacting with a specific target, very often the TBDD-derived positive ‘hits’ and ‘leads’ yield poor, clinically relevant responses, as highlighted by subsequent evaluations using more biologically complex systems, such as 3D culture, organ culture, and animal models. By using the PBDD approach one can largely avoid such negative surprises, since such screening starts in a complex system and move forward to identify the target, opposite to TBDD approach in this respect. Phenotypic screening is devoid of bias for specific targets and may capture the uncertainty as well as the complexity of biological systems; i.e. it lets ‘biology’ itself identify targets and mechanisms. Overall, the translatability of such approach is higher compared to that of TBDD (). When recombinant systems fail to capture the biological complexity, the PBDD assay system becomes important. With the use of new technologies that are now currently available for PBDD, it becomes easier to establish and validate assays that translate effectively to human diseases. Though PBDD is a challenging strategy on multiple levels of drug discovery, it has a successful track record of providing first-in-class drugs. It is a powerful method to provide a route to enhance innovation in the drug industry and to deliver truly novel therapeutic agents for unmet needs. Phenotypic screening and mechanistic studies should increase the rate of success in drug discovery.
3.2.2. Challenges of PBDD
There is ample evidence to support the notion that phenotypic screening can be highly effective for the identification of active drug candidates that lead to first-in-class drugs. The gap between screening an active candidate (i.e. a positive ‘hit’) and developing an efficacious drug is immense and more challenging than for a positive hit with a known molecular target. Optimizing drugs in the absence of known molecular targets can be difficult. As expected, PBDD approaches have considerable challenges, such as hit validation and target deconvolution. The risk of advancing a drug with incomplete knowledge of its mechanism of action or without characterizing its molecular target brings uncertainty and concerns. In the absence of a well-defined target, the mechanistic information from a research model or clinical hypothesis may be helpful to alleviate the concerns. The most convincing reason for pursuing a PBDD strategy is the advantage of investigating the agent in a relevant in vitro assay and in vivo animal model systems, minimally perturbed, but just enough, to generate the right physiopathological conditions. As target identification is not always achievable, one must decide whether to advance a candidate drug in the absence of target knowledge [Citation61]. The exceptional promise of PBDD is its ability to utilize a disease phenotype to discover novel treatments for diseases for which the actual cause is either unknown or the scientific knowledge is insufficient to afford compelling molecular targets to be clearly identified. However, PBDD should not be regarded just as an alternative screening technology.
4. Development of radiation countermeasures for ARS
To date, only three radiation countermeasures have been approved by the US FDA and all three agents were in clinical use for decades for other indications () [Citation6,Citation62]. Recently, these agents were repurposed for H-ARS indication following the FDA Animal Rule [Citation13,Citation22–Citation24,Citation63]. While these agents are FDA-approved for the treatment of H-ARS, demonstrated efficacy in the NHP model does not ensure that these agents will be beneficial to victims in the wake of a nuclear detonation. Given the important implications and complexity of systemic injury leading to either multiple organ involvement (MOI) or with multiple organ failure (MOF), additional research is needed [Citation64]. These countermeasures were developed following the PBDD approach. These agents were well known for improving blood cytopenias (particularly neutropenia) under various clinical conditions. Their approval for H-ARS was based on such attributes. As stated above, these agents were approved by the FDA following Animal Rule [Citation7]. Furthermore, the majority of the radiation countermeasures for ARS under advanced stages of development are being developed using the PBDD approach. There are nine radiation countermeasures under advanced development which have received FDA investigational new drug (IND) status () [Citation65–Citation81]. Out of these nine agents, only one agent, CBLB502, was identified based on well-characterized receptor as a target (Toll-like receptor-5, TLR5) for a ligand (truncated flagellin, TLR5 ligand) [Citation67]. Several additional agents, such as cytokines and growth factors [Citation62,Citation82], indralin [Citation83,Citation84], tocols [Citation85,Citation86] metformin [Citation87–Citation90], lipopeptides [Citation91–Citation93], anti-ceramide antibody [Citation94–Citation96], cellular therapeutic agents [Citation97–Citation100], TP508 [Citation101–Citation103], inhibitors of various pathways [Citation104–Citation106], Oltipraz [Citation107], and R-spondin1 [Citation108–Citation110] are under development as radiation countermeasures for various sub-syndromes of ARS () and most of these agents are being developed following PBDD strategy. There are several agents under advanced development that show considerable promise and, accordingly, may very well receive FDA approval for human use in the near future.
Table 1. Drug discovery approaches used for US FDA-approved radiation countermeasures for ARS.
Table 2. Radiation countermeasures for ARS with US FDA investigational new drug status.
Table 3. Additional radiation countermeasures under development for ARS using animal models.
In our opinion, the best strategy for discovering the next generation radiation countermeasures that would target various sub-syndromes of ARS would be to use a hybrid of both, the TBDD and the PBDD strategies [Citation48]. Both strategies would be coupled to initial phases of advanced, high throughput screening in order to identify potential drug candidates of interest. Entolimod (TLR-5 ligand/CBLB502) development as a radiation countermeasure for ARS is an example of such a combined approach for TBDD and PBDD [Citation67,Citation68]. There is consensus that the effect of acute, high levels of ionizing radiation from total-body exposure (i.e. those doses in excess of the accepted dose that results in 50% lethality within 30 days for mice (LD50/30) and 60 days for canine and nonhuman primates (NHP) of exposure (LD50/60)), promote the complex symptomatology of radiation-induced MOI and MOF as major pathogenic contributors of the most severe forms of ARS [Citation111]. As a result, the pathophysiologies of the ARS-associated MOI and MOF pose additional and unique challenges to ‘one drug-one target’ approach typically associated with TBDD because of the biological complexity associated with radiation-induced damage. The acute phase of the disease manifests as vital cell populations of critical organ systems are depleted and/or there is a loss of rapidly turning-over cell renewal systems; e.g, in case of pronounced, radiation-mediated cytocidal effects that serve in part to define the classical sub-syndromes observed in the hematopoietic and gastrointestinal systems. However, the disease progression can manifest in subsequent perturbations of ‘latently resting’ cell systems such as in the lung, kidney, and liver that can show damage in different ways and at different times through indirect (bystander) and functional effects of the systemic mechanisms that have been associated with ionizing radiation [Citation112,Citation113]. This delayed effect of ionizing radiation will often manifest as well within tissues and organ systems that are generally considered less sensitive to acute toxicity of ionizing radiation. A number of different pathophysiologic processes appear to be responsible for this ‘delayed effect’: these include, but not limited to secretion of pro-inflammatory cytokines, stimulation of inflammatory cascade, and induction of endothelial dysfunction. All of the latter processes appear to be linked together to underpin MOI/MOF and are consistent with the concept for the late damage observed in organ systems such as the respiratory, kidney, liver and cardiovascular [Citation112,Citation113]. At least in theory and in terms of ‘new drug discovery for ARS’, a better understanding of the radiobiology may be by unifying TBDD and PBDD approaches and coupling these approaches to initial, high throughput screenings of potential drug candidates. This would allow development and use of a combination of multiple pharmacological agents aimed at different druggable targets to adequately address the biological complexity of the underlying mechanisms of the ARS syndrome in its entirety.
4.1. Lessons learned while developing radiation countermeasures
Hematopoietic stem cell and progenitor cell injuries usually underpin hematopoietic tissue dysfunctions and ultimately bone marrow failure, and in turn lead to death after exposure to a lethal dose of ionizing radiation: as such, protecting such vital stem cell and progenitor populations should be a primary goal in the development of radiation countermeasures against radiation injuries [Citation114]. Generally, the primary cause of death is an infection due to a compromised innate immune system that is dictated largely by the post-irradiation viability and recoverability of the progenitorial cell populations. Apoptotic cell death, a primary mode of cell death of hematopoietic progenitors, is mostly determined by the activation of the p53 (cellular tumor antigen p53) signaling pathway [Citation115–Citation119].
Radiation-induced apoptosis in some radiosensitive tissues is mediated by the activation of p53. Therefore, it suggests that the inhibition of p53 might provide a radioprotective pathway [Citation120]. This was validated by investigating pifithrin-α, an inhibitor of p53, which was able to protect mice from exposure with lethal doses of γ-radiation [Citation121]. It was noted however that suppression of p53 for radioprotection has its limitations. Activation of p53 induces massive apoptosis in the hematopoietic system, but it triggers growth arrest that contributes to tissue recovery in other tissues, e.g. epithelial cells. It is well known that p53-deficient mice are resistant to radiation-induced H-ARS but sensitive to GI-ARS due to the lack of growth arrest in crypt epithelial cells which leads to mitotic catastrophe [Citation122]. In brief, the inhibition of p53 has not proven to be an entirely useful strategy to develop radiation countermeasures.
4.2. Radioprotection by modulation of NF-kB pathway
NF-κB is a protein complex that controls transcription of DNA, cytokine production, and cell survival in general: for some agents under development as ARS-countering medicinals, NF-kB seems to play an important role in radioprotection or in radiomitigation. The protective role of NF-kB plays out three ways and is mediated via transcriptional activation of multiple genes coding for: (1) anti-apoptotic proteins inhibiting apoptotic pathways [Citation123]; (2) growth factors and cytokines which induce cell proliferation and are responsible for survival of hematopoietic progenitors and other stem cells; and (3) potent reactive oxygen species-scavenging antioxidant proteins, such as manganese superoxide dismutase (MnSOD, also known as superoxide dismutase 2 (SOD-2)) [Citation124]. There are several Toll-like receptor (TLR) agonists such as CBLB502, CBLB612, CBLB613, and α-1-antitrypsin, which bind with different TLRs leading to NF-kB signaling and radioprotection [Citation67,Citation68,Citation91,Citation92,Citation125,Citation126]. Recombinant human interleukine-12 (rhuIL-12, HemaMax/NMIL12-1) is also known to modulate NF-kB activation. Unlike the above-mentioned agents, BIO 300 (nanosuspension)/Genistein is a promising radiation countermeasure which has been shown to inhibit NF-κB activation [Citation69,Citation70].
5. Conclusion
During the last decade, we observed a decline in the number of new drugs entering advanced phases of clinical evaluation, as well as an apparent decline in the pharmaceutical industry’s overall productivity, in terms of bringing new, clinically useful medicinals into the market. Such declines might suggest that the introduction of the TBDD strategy and its heavy reliance during this period may very well be a contributing factor. This does not suggest or mean that we should discard the TBDD approach since it has several advantages over the PBDD strategy in respect of its high throughput screening strategy and its ability to have a well-defined drug discovery approach. The primary step in the discovery of new biologically active small molecules is screening. Clearly, phenotypic or physiologic screening has utility in the initial phases of drug-discovery; specifically, in terms of identifying new molecules with bioactivity directed at higher levels of biologic organization (as compared to the TBDD approach that has an exclusively molecular focus). Accordingly, phenotypic screening should be considered as a primary method to discover a new pool of bioactive molecules [Citation127].
It is difficult to say, which drug discovery strategy is optimal. Sometimes a drug target is well defined and the advantages of a TBDD approach are compelling, while in other situations, suitable targets are not distinct and phenotypic assays are important. Furthermore, each unique drug discovery challenge requires its own resolution and using the best of both TBDD and PBDD approaches for identification and the development of safe and efficacious new radiation countermeasures for their successful translation into clinical use appears to be a reasonable strategy. Nevertheless, in this general drug development area, i.e. ‘radiation countermeasures’ there continues to be a significant underutilization of cutting edge high throughput screening of potential agents, regardless of the basic TBDD or PBDD strategy employed. Insufficient funding for such analytic processes is partly responsible for the latter situation and not necessarily the radiation biology research community per se. A rethinking of research funding priorities might be useful to resolve this pressing issue. Though TBDD approaches have obvious utility; however, when the omics approach fails to accurately capture all biological complexity, searching for phenotypic assays that better translate into the clinic appears useful. In brief, PBDD strategies have an important role in modern drug discovery, aided by a novel and emerging target identification or deconvolution techniques that are increasingly being used in modern phenotypic screening approaches.
The collaborative efforts combining TBDD and PBDD, along with system biology and bioinformatics, will be necessary to harness the technological breakthroughs to identify and develop the novel therapeutics for human use.
6. Expert opinion
Though an optimal drug discovery strategy is difficult to achieve, synergy of different strategies such as multiple-target drug design, a translational approach, molecular imaging, drug repositioning, and regenerative medicine may be better for achieving the goal. A multiple target-based drug approach may overcome the complexity of single target identification. Repurposing failed drugs can provide alternative mechanisms to combat different indications [Citation128]. It is obvious that no single preclinical model system can fully reflect all the complexities and intricacies found in the human subject. By striving for a better system and greater understanding of these strategies, we can definitely make headway in the discovery and development of new therapeutic agents for various human diseases. The enabling technologies are becoming more sophisticated and better at handling some of the ongoing and remaining challenges for both TBDD and PBDD approaches.
Generation of ROS/RNS, cellular oxidative stress, tissue inflammation, and cell death are the downstream consequences of intense radiation exposures that can lead to MOI and MOF and ultimately to death. In brief, unlike other diseases which are organ or system specific, radiation injury often involves multiple organs of various systems. As a result, finding optimal treatment for different sub-syndromes involving various organs/systems (hematopoietic, GI, pulmonary, cutaneous, neurovascular) is not an easy task. One would likely need to screen a large number of agents for each sub-syndrome and then select the agents for various sub-syndromes using poly-pharmacy approaches to achieve an optimal remedy for radiation injury. The widespread acceptance of automated high-throughput screening (HTS) and lately, ultra-high throughput screening (uHTS) represents optimal choices, presenting skilled, drug development investigators with unique opportunities to screen huge libraries against an increasing range of targets for lead discovery. Usually, HTS is designated as assessing between 10,000 and 100,000 agents per day with uHTS ability to handle more than 100,000 agents per day. Drug discovery has rapidly come to rely on this screening capability. There are additional, recently developed techniques that need to be used for drug development for ARS following TBDD and PBDD approaches.
At one time, phenotypic screening was the only drug discovery strategy employed and majority of the first-in-class agents were discovered as a result of this strategy. With the introduction of high throughput assays in the genomic era and new generation molecular technologies, TBDD became the predominant approach. Unfortunately, such strategy shift did not fulfill the expectation of the pharmaceutical industry and led to high attrition rate for new agents compared to the status before the shift. With such realization, leading pharmaceutical companies returned to a balanced strategy utilizing both PBDD and TBDD approaches. High throughput technologies were crucial to the shift from PBDD to TBDD and are again important in enabling a balanced strategy utilizing both PBDD and TBDD. With time, high throughput technology will be extensively used in the PBDD strategy [Citation56].
CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats: regions in the bacterial genome that help defend against invading viruses) techniques allow to modify specific genes while sparing all others [Citation129]. This approach elucidates the association between a specific gene and its impact on the organism. One important application of such technology is to facilitate making animal models with precise genetic changes to study the progression and treatment of human diseases. The use of newer, organ-on-a-chip technologies that are amenable for high volume analytics might facilitate the ARS drug development process(es): these assay systems attempt to recapitulate on microchips, the microarchitecture, and functions of living human tissues and organs, such as the bone marrow, intestine, lung, kidney, skin, and blood-brain barrier [Citation130]. These microchips offer a potential alternative to traditional animal testing. Each individual organ-on-chip is composed of a flexible polymer of the size of a computer pen drive. It contains hollow microfluidic channels lined with living human cells interfaced with a human endothelial cell vasculature, and mechanical forces can be applied to mimic the physical microenvironment of living organs. It could lay the foundations for the future of personalized medicine with the integration of patient-derived cells. Another innovative approach is the 3D cell culture model that is better than the traditional 2D monolayer culture due to improved cell–cell interactions and structures resembling in vivo architecture. During the last 10 years, significant 3D cell culture systems have been created [Citation131]. Three dimensional culture systems hold great promise for applications in drug discovery by bridging the gap between traditional 2D monolayer cell culture and animal models. Recent progress suggests that the transition from 2D to 3D cell cultures is promising, but the technology maturity and the cost are the main concerns. Significant effort is needed to substantiate reproducibility and high throughput analysis to establish validated 3D cell culture models. In brief, phenotypic high-throughput screens have identified a large number of agents that are effective in various cell-based in-vitro systems and animal models for various indications, but follow-up investigations to test the efficacy of such agents in large animals are needed.
In our opinion, the TBDD and the PBDD approaches are not antagonistic but rather complementary and essential for the development of next-generation therapeutic agents with unique attributes. For optimal success, the best strategy for developing the next generation radiation countermeasures that would target the various sub-syndromes of ARS would be to use a hybrid of both, the TBDD and the PBDD strategies [Citation48]. Following the discovery and development of specific therapeutics via the use of the TBDD/PBDD strategies for given ARS sub-syndromes, a combination of therapeutics, i.e. polypharmacy approach, would be used to clinically manage ARS in its entirety. Irrespective of the strategy we use, our goal should be to translate preclinical scientific knowledge into meaningful development of therapeutic agents. Further, additional emphasis should be placed on incorporating into any future drug development strategies advanced, HTS technologies of potential agents, as these processes have been significantly, underutilized in efforts to develop new types and classes of radiation countermeasures. Supporting government agencies and private organizations need to reprioritize their funding stream in order to better foster this research area and to correct this deficit. In the near future, the tools and methods for the identification of molecular targets will improve to help the integration of target-based and phenotypic approaches to achieve the goal of developing novel countermeasures. Translational tools should be employed early to test key hypotheses sooner rather than later in order to reduce the later risks encountered in the drug discovery and development process. Humanized mouse models with human cells or tissue transplants are useful in human disease research. Immunodeficient mice, which do not reject xenografts are indispensable for generating additional appropriate models. Since 2000, a large number of immunodeficient mice suitable for generating humanized mice have been developed [Citation132].
In a real-life scenario, only patients within a very narrow acute dose range (perhaps whole body doses between 2 Gy and 6 Gy) would be expected to benefit from such drugs. Below 2 Gy, exposed victims would likely survive without drug treatment, while victims exposed to a dose above 6 or 8 Gy would most likely die despite treatment. In a real-life radiation terrorism situation, a limited number of victims would fall into the dose range where they might be expected to benefit from the drug (in terms of enhanced survival). Furthermore, without very good individual dosimetry, it would be hard to identify those who would likely to benefit. Additionally, if the drug were to have adverse side effects, its overuse in people minimally exposed to radiation might produce harm in addition to benefits.
In the future, drug-design approaches should take into account the benefit of multi-target drugs and combination therapeutics for next-generation systemic drugs with multiple fitting and low affinity with their protein or molecular target. Instead of depending on a single target, one should focus on multiple targets that are perturbed by irradiation within complex molecular and cellular networks that are linked specifically to given ARS-specific disease states in order to develop more efficient radiation countermeasures. We anticipate that additional radiation countermeasures for various sub-syndromes of ARS, with better attributes based on TBDD and PBDD development strategies, will receive FDA approval under its Animal Rule in the near future. This would allow multiple options available for the prophylaxis and treatment of radiation exposed victims who have received high doses of radiation.
Article highlights
There are two approaches to advance drug discovery; target-based drug discovery (TBDD; molecular approach) and phenotype-based drug discovery (PBDD; empirical approach)
The TBDD and the PBDD are not antagonistic but these two approaches of drug discovery are complementary to each other.
There is a need to incorporate advanced, high throughput screening of potentially useful agents. Additional agency/organizational funding to support this is critical in order to correct this major deficit in ongoing radiation countermeasure drug development work effort.
No single preclinical system can completely reflect all the complexities and intricacies of ARS or any given human disease in general.
The best strategy for developing the countermeasures for the acute radiation syndrome (ARS) would be to use a combination of both, the TBDD and the PBDD approaches.
Only three radiation countermeasures (Neupogen, Neulasta, and Leukine) for hematopoietic ARS have received Food and Drug Administration (FDA) approval following Animal Rule. They are based on PBDD and all three are repurposed drugs.
There are nine agents having FDA investigational new drug status for ARS which are being developed following PBDD and/or TBDD approaches.
This box summarizes key points contained in the article.
Declaration of interest
TM Seed is an employee of Tech Micro Services. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
The opinions or assertions contained herein are the professional views of the authors and do not necessarily represent the Armed Forces Radiobiology Research Institute, the Uniformed Services University of the Health Sciences, or the Department of Defense, USA. Mention of specific therapeutic agents does not constitute endorsement by the U.S. Department of Defense, and trade names are used only for the purpose of clarification.
Additional information
Funding
References
- Overington JP, Al-Lazikani B, Hopkins AL. How many drug targets are there? Nat Rev Drug Discov. 2006;5:993–996.
- Uhlen M, Fagerberg L, Hallstrom BM, et al. Proteomics. Tissue-based map of the human proteome. Science. 2015;347:1260419.
- Bull SC, Doig AJ. Properties of protein drug target classes. PLoS One. 2015;10:e0117955.
- Russell PK. Project BioShield: what it is, why it is needed, and its accomplishments so far. Clin Infect Dis. 2007;45(Suppl 1):S68–S72.
- Seed TM. Radiation protectants: current status and future prospects. Health Phys. 2005;89:531–545.
- Singh VK, Seed TM. A review of radiation countermeasures focusing on injury-specific medicinals and regulatory approval status: part I. Radiation sub-syndromes, animal models and FDA-approved countermeasures. Int J Radiat Biol. 2017;93:851–869.
- U.S. Food and Drug Administration. Guidance for industry: product development under the Animal Rule. 2015 [cited 2018 Jul 5]. Available from: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM399217.pdf
- Allio T. Product development under FDA's Animal Rule: Understanding FDA's expectations and potential implication for traditional development programs. Therap Inno Regul Sci 2016:50:660–670.
- Aebersold P. FDA experience with medical countermeasures under the Animal Rule. Adv Prev Med. 2012;2012:507571.
- Singh VK, Seed TM. An update on sargramostim for treatment of acute radiation syndrome. Drugs Today (Barc). 2018;54:679–693.
- U.S. Food and Drug Administration. FDA approves Leukine for acute radiation syndrome. 2018 [cited 2018 Apr 1]. Available from: https://www.fda.gov/downloads/EmergencyPreparedness/Counterterrorism/MedicalCountermeasures/AboutMCMi/UCM603226.pdf
- National Institute of Allergic and Infectious Diseases. Pegfilgrastim approved for treating acute radiation syndrome. 2015 [cited 2016 Aug 18]. Available from: https://www.niaid.nih.gov/topics/radnuc/Pages/pegfilgrastim.aspx
- Farese AM, MacVittie TJ. Filgrastim for the treatment of hematopoietic acute radiation syndrome. Drugs Today (Barc). 2015;51:537–548.
- U.S. Food and Drug Administration. FDA approves Neupogen for treatment of patients with radiation-induced myelosuppression following a radiological/nuclear incident. 2015 [cited 2016 Jul 6]. Available from: http://www.fda.gov/EmergencyPreparedness/Counterterrorism/MedicalCountermeasures/AboutMCMi/ucm443245.htm
- Maher C, Hu-Primmer J, MacGill T, et al. Meeting the challenges of medical countermeasure development. Microb Biotechnol. 2012;5:588–593.
- Matheny J, Mair M, Smith B. Cost/success projections for US biodefense countermeasure development. Nat Biotechnol. 2008;26:981–983.
- Zhavoronkov A. Artificial intelligence for drug discovery, biomarker development, and generation of novel chemistry. Mol Pharm. 2018;15:4311–4313.
- DiMasi JA, Grabowski HG, Hansen RW. Innovation in the pharmaceutical industry: new estimates of R&D costs. J Health Econ. 2016;47:20–33.
- Cohen J. Biodefense: 10 years after reinventing Project BioShield. Science. 2011;333:1216–1218.
- Carroll J. Traversing the landscape of Project BioShield. Biotechnol Healthc. 2007;4:7–8.
- Singh VK, Newman VL, Seed TM. Colony-stimulating factors for the treatment of the hematopoietic component of the acute radiation syndrome (H-ARS): a review. Cytokine. 2015;71:22–37.
- Amgen Inc. Neupogen (filgrastim) injection for subcutaneous or intravenous use. 2015 [cited 2015 Apr 2]. Available from: http://pi.amgen.com/united_states/neupogen/neupogen_pi_hcp_english.pdf
- Amgen Inc. Neulasta (pegfilgrastim) injection for subcutaneous use. 2015 [cited 2015 Nov 19]. Available from: http://pi.amgen.com/united_states/neulasta/neulasta_pi_hcp_english.pdf
- Sanofi-Aventis U.S. LLC. LEUKINE® (sargramostim) for injection, for subcutaneous or intravenous use. 2018 [cited 2018 Apr 1]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/103362s5240lbl.pdf?utm_campaign=20180329%20MCMi&utm_medium=email&utm_source=Eloqua
- Johnke RM, Sattler JA, Allison RR. Radioprotective agents for radiation therapy: future trends. Future Oncol. 2014;10:2345–2357.
- Maier P, Wenz F, Herskind C. Radioprotection of normal tissue cells. Strahlenther Onkol. 2014;190:745–752.
- Wouters BG. Cell death after irradiation: how, when and why cells die. In: Joiner M, van der Kogel A, editors. Basic clinical radiobiology. 4th ed. London: Edward Arnold; 2009. p. 27–40.
- Mitchell HH, Hamilton TS, Steggerda FR, et al. The chemical composition of the adult human body and its bearing on the biochemistry of growth. J Biol Chem. 1945;158:625–637.
- Ellis KJ. Human body composition: in vivo methods. Physiol Rev. 2000;80:649–680.
- Wang Z, Deurenberg P, Wang W, et al. Hydration of fat-free body mass: review and critique of a classic body-composition constant. Am J Clin Nutr. 1999;69:833–841.
- Reisz JA, Bansal N, Qian J, et al. Effects of ionizing radiation on biological molecules–mechanisms of damage and emerging methods of detection. Antioxid Redox Signal. 2014;21:260–292.
- Allen AO. Radiation chemistry of aqueous solutions. J Phys Colloid Chem. 1948;52:479–490.
- Villamena FA. Molecular basis of oxidative stress: chemistry, mchanisms, and disease pathogenesis. Hoboken (NJ): Wiley; 2013.
- Dalle-Donne I, Scaloni A, Butterfield DA. Redox proteomics: from protein modifications to cellular dysfunctions and diseases. Hoboken (NJ): Wiley; 2006.
- Sies H. Strategies of antioxidant defense. Eur J Biochem. 1993;215:213–219.
- England K, Cotter TG. Direct oxidative modifications of signalling proteins in mammalian cells and their effects on apoptosis. Redox Rep. 2005;10:237–245.
- Cecarini V, Gee J, Fioretti E, et al. Protein oxidation and cellular homeostasis: emphasis on metabolism. Biochim Biophys Acta. 2007;1773:93–104.
- Kohen R, Nyska A. Oxidation of biological systems: oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol Pathol. 2002;30:620–650.
- Daly MJ. Death by protein damage in irradiated cells. DNA Repair (Amst). 2012;11:12–21.
- Krisko A, Radman M. Biology of extreme radiation resistance: the way of Deinococcus radiodurans. Cold Spring Harb Perspect Biol. 2013;5.
- Martindale JL, Holbrook NJ. Cellular response to oxidative stress: signaling for suicide and survival. J Cell Physiol. 2002;192:1–15.
- Manoharan S, Guillemin GJ, Abiramasundari RS, et al. The role of reactive oxygen species in the pathogenesis of Alzheimer’s disease, Parkinson’s disease, and Huntington’s disease: a mini review. Oxid Med Cell Longev. 2016;2016:8590578.
- Hartl FU. Protein misfolding diseases. Annu Rev Biochem. 2017;86:21–26.
- Zeng L, Zou W, Wang G. Cellular prion protein (PrP(C)) and its role in stress responses. Int J Clin Exp Med. 2015;8:8042–8050.
- Zocche Soprana H, Canes Souza L, Debbas V, et al. Cellular prion protein (PrP(C)) and superoxide dismutase (SOD) in vascular cells under oxidative stress. Exp Toxicol Pathol. 2011;63:229–236.
- Brown DR, Besinger A. Prion protein expression and superoxide dismutase activity. Biochem J. 1998;334(Pt 2):423–429.
- Swinney DC. Phenotypic vs. target-based drug discovery for first-in-class medicines. Clin Pharmacol Ther. 2013;93:299–301.
- Gintant GA, George CH. Introduction to biological complexity as a missing link in drug discovery. Expert Opin Drug Discov. 2018;13:753–763.
- Croston GE. The utility of target-based discovery. Expert Opin Drug Discov. 2017;12:427–429.
- Santos R, Ursu O, Gaulton A, et al. A comprehensive map of molecular drug targets. Nat Rev Drug Discov. 2017;16:19–34.
- Zhou HP, Qian LX, Zhang N, et al. MIIP gene expression is associated with radiosensitivity in human nasopharyngeal carcinoma cells. Oncol Lett. 2018;15:9471–9479.
- Sambade MJ, Peters EC, Thomas NE, et al. Melanoma cells show a heterogeneous range of sensitivity to ionizing radiation and are radiosensitized by inhibition of B-RAF with PLX-4032. Radiother Oncol. 2011;98:394–399.
- Wang J, Wakeman TP, Lathia JD, et al. Notch promotes radioresistance of glioma stem cells. Stem Cells. 2010;28:17–28.
- Hauser AS, Attwood MM, Rask-Andersen M, et al. Trends in GPCR drug discovery: new agents, targets and indications. Nat Rev Drug Discov. 2017;16:829–842.
- Sams-Dodd F. Target-based drug discovery: is something wrong? Drug Discov Today. 2005;10:139–147.
- Wagner BK. The resurgence of phenotypic screening in drug discovery and development. Expert Opin Drug Discov. 2016;11:121–125.
- Black CB, Duensing TD, Trinkle LS, et al. Cell-based screening using high-throughput flow cytometry. Assay Drug Dev Technol. 2011;9:13–20.
- Matsusaki M, Case CP, Akashi M. Three-dimensional cell culture technique and pathophysiology. Adv Drug Deliv Rev. 2014;74:95–103.
- Swinney DC, Anthony J. How were new medicines discovered? Nat Rev Drug Discov. 2011;10:507–519.
- Caskey CT. The drug development crisis: efficiency and safety. Annu Rev Med. 2007;58:1–16.
- Moffat JG, Vincent F, Lee JA, et al. Opportunities and challenges in phenotypic drug discovery: an industry perspective. Nat Rev Drug Discov. 2017;16:531–543.
- Singh VK, Hanlon BK, Santiago PT, et al. A review of radiation countermeasures focusing on injury-specific medicinals and regulatory approval status: part III. countermeasures under early stages of development along with ‘standard of care’ medicinal and procedures not requiring regulatory approval for use. Int J Radiat Biol. 2017;93:885–906.
- Singh VK, Seed TM. An update on sargramostim for treatment of acute radiation syndrome. Drugs Today (Barc). 2018;54:679–693.
- Yeddanapudi N, Clay MA, Durham DP, et al. Informing CONOPS and medical countermeasure deployment strategies after an improvised nuclear device detonation: the importance of delayed treatment efficacy data. Int J Radiat Biol. 2018. In press. doi:10.1080/09553002.2018.1532618 .
- Whitnall MH, Elliott TB, Harding RA, et al. Androstenediol stimulates myelopoiesis and enhances resistance to infection in gamma-irradiated mice. Int J Immunopharmacol. 2000;22:1–14.
- Yeh S, Kang HY, Miyamoto H, et al. Differential induction of androgen receptor transactivation by different androgen receptor coactivators in human prostate cancer DU145 cells. Endocrine. 1999;11:195–202.
- Burdelya LG, Krivokrysenko VI, Tallant TC, et al. An agonist of Toll-like receptor 5 has radioprotective activity in mouse and primate models. Science. 2008;320:226–230.
- Krivokrysenko VI, Shakhov AN, Singh VK, et al. Identification of granulocyte colony-stimulating factor and interleukin-6 as candidate biomarkers of CBLB502 efficacy as a medical radiation countermeasure. J Pharmacol Exp Ther. 2012;343:497–508.
- Akimoto T, Nonaka T, Ishikawa H, et al. Genistein, a tyrosine kinase inhibitor, enhanced radiosensitivity in human esophageal cancer cell lines in vitro: possible involvement of inhibition of survival signal transduction pathways. Int J Radiat Oncol Biol Phys. 2001;50:195–201.
- Landauer MR, Srinivasan V, Seed TM. Genistein treatment protects mice from ionizing radiation injury. J Appl Toxicol. 2003;23:379–385.
- Ghosh SP, Kulkarni S, Perkins MW, et al. Amelioration of radiation-induced hematopoietic and gastrointestinal damage by Ex-RAD(R) in mice. J Radiat Res. 2012;53:526–536.
- Kang AD, Cosenza SC, Bonagura M, et al. ON01210.Na (Ex-RAD(R)) mitigates radiation damage through activation of the AKT pathway. PLoS One. 2013;8:e58355.
- Basile LA, Ellefson D, Gluzman-Poltorak Z, et al. HemaMax, a recombinant human interleukin-12, is a potent mitigator of acute radiation injury in mice and non-human primates. PLoS One. 2012;7:e30434.
- Neumedicines. HemaMax™ (rHuIL-12) for acute radiation syndrome. 2014 [cited 2014 Oct 27] Available from: http://www.neumedicines.com/
- Singh VK, Elliott TB, Mandalam R, et al. Myeloid progenitors mitigate radiation injury and improve intestinal integrity after whole-body irradiation. In NATO RTO HFM-223 Symposium “Biological Effects of Ionizing Radiation Exposure and Countermeasures: Current Status and Future Perspectives”; 2012 Oct 8–10; Ljubljana, Slovenia; 2012. p. 1–13.
- Singh VK, Christensen J, Fatanmi OO, et al. Myeloid progenitors: a radiation countermeasure that is effective when initiated days after irradiation. Radiat Res. 2012;177:781–791.
- Garofalo MC, Ward AA, Farese AM, et al. A pilot study in rhesus macaques to assess the treatment efficacy of a small molecular weight catalytic metalloporphyrin antioxidant (AEOL 10150) in mitigating radiation-induced lung damage. Health Phys. 2014;106:73–83.
- MacVittie TJ, Gibbs A, Farese AM, et al. AEOL 10150 mitigates radiation-induced lung injury in the nonhuman primate: morbidity and mortality are administration schedule-dependent. Radiat Res. 2017;187:298–318.
- Soligenix Inc. OrbeShield™ for gastrointestinal acute radiation syndrome (GI ARS). 2016 [cited 2016 Jul 10]. Available from: http://www.soligenix.com/pipeline/vaccinesbiodefense/orbeshield-for-gastrointestinal-acute-radiation-syndrome-gi-ars/
- Pluristem Therapeutics Inc. U. S. FDA clears Pluristem’s IND to treat victims exposed to acute radiation. 2018 [cited 2018 May 1]. Available from: http://www.pluristem.com/wp-content/uploads/2018/04/ARS_IND_final_isa.pdf
- Pluristem Therapeutics Inc. Studies of PLX-R18 in ARS. 2017 [cited 2017 Dec 28]. Available from: http://www.pluristem.com/acute-radiation-syndrome-ars/
- Singh VK, Romaine PL, Seed TM. Medical countermeasures for radiation exposure and related injuries: characterization of medicines, FDA-approval status and inclusion into the Strategic National Stockpile. Health Phys. 2015;108:607–630.
- Vasin MV, Antipov VV, Chernov GA, et al. Studies of the radiation-protective effects of indralin on the hematopoietic system of different species of animals. Radiat Biol Radioecol. 1996;36:168–189.
- Vasin MV, Semenov LF, Suvorov NN, et al. Protective effect and the therapeutic index of indralin in juvenile rhesus monkeys. J Radiat Res. 2014;55:1048–1055.
- Singh VK, Beattie LA, Seed TM. Vitamin E: tocopherols and tocotrienols as potential radiation countermeasures. J Radiat Res. 2013;54:973–988.
- Singh VK, Hauer-Jensen M. Gamma-tocotrienol as a promising countermeasure for acute radiation syndrome: current status. Int J Mol Sci. 2016;17:e663.
- Xu G, Wu H, Wang Y, et al. Amelioration of ionizing irradiation-induced hematopoietic injury by metformin in mice. 58th Annual Meeting of the Radiation Research Society; San Juan, Puerto Rico; 2012.
- Miller RC, Murley JS, Grdina DJ. Metformin exhibits radiation countermeasures efficacy when used alone or in combination with sulfhydryl containing drugs. Radiat Res. 2014;181:464–470.
- Mujica-Mota MA, Salehi P, Devic S, et al. Safety and otoprotection of metformin in radiation-induced sensorineural hearing loss in the guinea pig. Otolaryngol Head Neck Surg. 2014;150:859–865.
- Xu G, Wu H, Zhang J, et al. Metformin ameliorates ionizing irradiation-induced long-term hematopoietic stem cell injury in mice. Free Radic Biol Med. 2015;87:15–25.
- Shakhov AN, Singh VK, Bone F, et al. Prevention and mitigation of acute radiation syndrome in mice by synthetic lipopeptide agonists of Toll-like receptor 2 (TLR2). PLoS One. 2012;7:e33044.
- Singh VK, Ducey EJ, Fatanmi OO, et al. CBLB613: A TLR 2/6 agonist, natural lipopeptide of Mycoplasma arginini, as a novel radiation countermeasure. Radiat Res. 2012;177:628–642.
- Ciorba MA, Riehl TE, Rao MS, et al. Lactobacillus probiotic protects intestinal epithelium from radiation injury in a TLR-2/cyclo-oxygenase-2-dependent manner. Gut. 2012;61:829–838.
- Kolesnick R, Fuks Z. Radiation and ceramide-induced apoptosis. Oncogene. 2003;22:5897–5906.
- Rotolo J, Stancevic B, Zhang J, et al. Anti-ceramide antibody prevents the radiation gastrointestinal syndrome in mice. J Clin Invest. 2012;122:1786–1790.
- Paris F, Fuks Z, Kang A, et al. Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science. 2001;293:293–297.
- Singh VK, Wise SY, Singh PK, et al. Alpha-tocopherol succinate- and AMD3100-mobilized progenitors mitigate radiation-induced gastrointestinal injury in mice. Exp Hematol. 2012;40:407–417.
- Singh VK, Wise SY, Singh PK, et al. Alpha-tocopherol succinate-mobilized progenitors improve intestinal integrity after whole body irradiation. Int J Radiat Biol. 2013;89:334–345.
- Saha S, Bhanja P, Kabarriti R, et al. Bone marrow stromal cell transplantation mitigates radiation-induced gastrointestinal syndrome in mice. PLoS One. 2011;6:e24072.
- Singh VK, Wise SY, Fatanmi OO, et al. Progenitors mobilized by gamma-tocotrienol as an effective radiation countermeasure. PLoS One. 2014;9:e114078.
- Ryaby JT, Sheller MR, Levine BP, et al. Thrombin peptide TP508 stimulates cellular events leading to angiogenesis, revascularization, and repair of dermal and musculoskeletal tissues. J Bone Joint Surg Am. 2006;88(Suppl 3):132–139.
- Olszewska-Pazdrak B, Loucas BD, Carney DH Post-exposure treatment with Chrysalin (TP508) enhances bone marrow stem cell recovery and intestinal crypt cell proliferation in CD-1 mice. 59th Annual meeting of the Radiation research Society; New Orleans, LA; 2012
- McVicar SD, Rayavara K, Carney DH. Radiomitigation and tissue repair activity of systemically administered therapeutic peptide TP508 is enhanced by PEGylation. AAPS J. 2017;19:743–753.
- Medhora M, Gao F, Jacobs ER, et al. Radiation damage to the lung: mitigation by angiotensin-converting enzyme (ACE) inhibitors. Respirology. 2012;17:66–71.
- Greenberger J, Lazo JS, Sharlow ER, et al. PI3 kinase inhibitor LY294002 is both a radioprotector and radiation mitigator. 14th International Congress of Radiation Research; Warsaw, Poland; 2011
- Paoluzzi L, Figg WD. Histone deacetylase inhibitors are potent radiation protectants. Cancer Biol Ther. 2004;3:612–613.
- Kim SG, Nam SY, Kim CW. In vivo radioprotective effects of oltipraz in gamma-irradiated mice. Biochem Pharmacol. 1998;55:1585–1590.
- Bhanja P, Saha S, Kabarriti R, et al. Protective role of R-spondin1, an intestinal stem cell growth factor, against radiation-induced gastrointestinal syndrome in mice. PLoS One. 2009;4:e8014.
- Zhao J, Kim KA, De Vera J, et al. R-spondin1 protects mice from chemotherapy or radiation-induced oral mucositis through the canonical Wnt/beta-catenin pathway. Proc Natl Acad Sci USA. 2009;106:2331–2336.
- Gu X, Wang X, Xiao H, et al. Silencing of R-spondin1 increases radiosensitivity of glioma cells. Oncotarget. 2015;6:9756–9765.
- Williams JP, McBride WH. After the bomb drops: a new look at radiation-induced multiple organ dysfunction syndrome (MODS). Int J Radiat Biol. 2011;87:851–868.
- Macia IGM, Lucas Calduch A, Lopez EC. Radiobiology of the acute radiation syndrome. Rep Pract Oncol Radiother. 2011;16:123–130.
- Fliedner TM, Dorr DH, Meineke V. Multi-organ involvement as a pathogenetic principle of the radiation syndromes: a study involving 110 case histories documented in SEARCH and classified as the bases of haematopoietic indicators of effect. Br J Radiol Suppl. 2005;27:1–8.
- Shao L, Luo Y, Zhou D. Hematopoietic stem cell injury induced by ionizing radiation. Antioxid Redox Signal. 2014;20:1447–1462.
- Gudkov AV, Komarova EA. The role of p53 in determining sensitivity to radiotherapy. Nat Rev Cancer. 2003;3:117–129.
- Selivanova G. p53: fighting cancer. Curr Cancer Drug Targets. 2004;4:385–402.
- Fisher DE. The p53 tumor suppressor: critical regulator of life & death in cancer. Apoptosis. 2001;6:7–15.
- Lin A, Karin M. NF-kappaB in cancer: a marked target. Semin Cancer Biol. 2003;13:107–114.
- Orlowski RZ, Baldwin AS Jr. NF-kappaB as a therapeutic target in cancer. Trends Mol Med. 2002;8:385–389.
- Komarova EA, Gudkov AV. Could p53 be a target for therapeutic suppression? Semin Cancer Biol. 1998;8:389–400.
- Komarov PG, Komarova EA, Kondratov RV, et al. A chemical inhibitor of p53 that protects mice from the side effects of cancer therapy. Science. 1999;285:1733–1737.
- Komarova EA, Kondratov RV, Wang K, et al. Dual effect of p53 on radiation sensitivity in vivo: p53 promotes hematopoietic injury, but protects from gastro-intestinal syndrome in mice. Oncogene. 2004;23:3265–3271.
- Karin M, Lin A. NF-kappaB at the crossroads of life and death. Nat Immun. 2002;3:221–227.
- Xu Y, Kiningham KK, Devalaraja MN, et al. An intronic NF-kappaB element is essential for induction of the human manganese superoxide dismutase gene by tumor necrosis factor-alpha and interleukin-1beta. DNA Cell Biol. 1999;18:709–722.
- Singh VK, Pollard HB. Patents for Toll-like receptor ligands as radiation countermeasures for acute radiation syndrome. Expert Opin Ther Pat. 2015;25:1085–1092.
- Krivokrysenko VI, Toshkov IA, Gleiberman AS, et al. The Toll-like receptor 5 agonist entolimod mitigates lethal acute radiation syndrome in non-human primates. PLoS One. 2015;10:e0135388.
- Cho YS, Kwon HJ. Identification and validation of bioactive small molecule target through phenotypic screening. Bioorg Med Chem. 2012;20:1922–1928.
- Vasaikar S, Bhatia P, Bhatia PG, et al. Complementary approaches to existing target sased drug discovery for identifying novel drug targets. Biomedicines. 2016;4:27.
- Rentmeister A. CRISPR craze conquers the RNA world: precise manipulation of DNA and RNA based on a bacterial defense system. Angew Chem Int Ed Engl. 2015;54:4710–4712.
- Zhang B, Radisic M. Organ-on-a-chip devices advance to market. Lab Chip. 2017;17:2395–2420.
- Yuan H, Xing K, Hsu HY. Trinity of three-dimensional (3D) scaffold, vibration, and 3D printing on cell culture application: a systematic review and indicating future direction. Bioengineering (Basel). 2018;5:57.
- Gootenberg DB, Turnbaugh PJ. Companion animals symposium: humanized animal models of the microbiome. J Anim Sci. 2011;89:1531–1537.
