1. Introduction
MicroRNAs are a class of small non-coding RNAs (22–23 nucleotides), which regulate gene expression at the post-transcriptional level and play key roles in tumorigenesis and other diseases [Citation1,Citation2]. MiRNAs are dysregulated in almost all solid and hematological malignancies, and specific miRNA expression signatures allow the characterization of different tumors and stages. Thus, miRNAs can also be utilized in cancer patient therapy and diagnosis/prognosis. MiRNAs that are upregulated in cancer cells and contribute to carcinogenesis by inhibiting tumor suppressor genes, are considered oncogenic miRNAs (oncomiRs), while downregulated miRNAs, that normally prevent cancer development by inhibiting the expression of proto-oncogenes, are known as tumor suppressor miRNAs. For example, miR-21 [Citation3], overexpressed in various tumor types, downregulates many tumor suppressor genes regulating cell proliferation, cell death, metastasis and chemoresistance. On the other hand, the miR-34 family is dysregulated in different cancer types including several epithelial tumors, melanomas, neuroblastomas, leukemias and sarcomas and inhibits expression of genes with oncogenic activity, such as MYC and BCL2 [Citation4]. Silencing oncomiRs with miRNA inhibitors or replacing tumor suppressor miRNAs with synthetic miRNA mimics has been demonstrated as a valuable experimental strategy for the treatment of cancer [Citation5].
In cancer, both antagonists and mimics have been developed as miRNA-based therapeutic approaches to achieve tumor relapse. MiRNA antagonists, also known as antimiRs, are single-stranded oligonucleotides which hybridize to the miRNA complementary sequences and interrupt the miRNA activity and/or processing with the resultant increased expression of target tumor suppressor genes. On the other hand, miRNA mimics, have an opposite role by over-expressing the miRNA and thus down-regulating the expression of target genes, such as oncogenes. For example, miRNAs can be restored by miRNA mimics, which act like endogenous miRNAs. MRX34, which was in clinical trials for liver cancer, is a miR-34 mimic [Citation6].
Here, we summarize current insights into the use of miRNA-based therapeutics, and the design of chemically modified miRNA-based drugs. We also review numerous in vivo delivery strategies and show examples of the clinical development of miRNA-targeting therapeutics. By focusing on their role in cancer we show how these therapies can be used and the challenges associated with their clinical application.
2. Challenges
RNA oligonucleotides have features that complicate drug design and efficacy. Challenging characteristics include: (i) degradation by nucleases upon addition into biological systems [Citation7,Citation8] (ii) poor cell membrane penetration [Citation9] (iii) entrappment in the endosome [Citation10] (iv) poor binding affinity for complementary sequences [Citation11] (v) poor delivery to desired target tissues [Citation10] (vi) off-target and unwanted toxicities and (vii) activation of innate immune responses [Citation12].
Since miRNA delivery is a potentially novel therapeutic modality, these challenges are being addressed in many different ways. For example, we show cases where the fast degradation of the naked miRNAs by nucleases can be overcome with chemical modifications to the oligonucleotides, while their hydrophilic characteristics, negative charge and high molecular weight which can block nucleic acids from penetrating the cell membrane. can be overcome with different delivery systems () [Citation13].
Table 1. Delivery vehicles for oligonucleotides.
2.1. Unmodified miRNAs are quickly degraded and cleared in the blood circulation
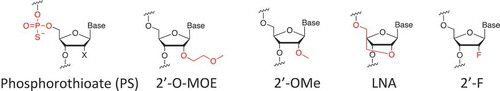
A challenge in miRNA therapeutics is to retain the stability and consistency of miRNAs in circulation. Naked miRNAs with an unmodified 2′ OH in the ribose moiety are degraded within seconds by nucleases such as serum RNase A-type nucleases in the blood [Citation14]. In addition, naked miRNAs are cleared quickly by renal excretion, leading to a short half-life in systemic circulation. The solution for this problem is chemically engineered miRNA modifications on the phosphodiester backbone and the 2ʹ of the ribose that protect the miRNA from degradation and promote the long-lasting potentency of the miRNA [Citation14,Citation15] (). Different chemical modifications have been developed such as: phosphodiester linkages, ribose backbone, 2ʹ-O-(2-Methoxyethyl), 2ʹ-O-Methyl, 2ʹ- locked nucleic acid, and 2ʹ- Fluoro (). These modifications not only improve oligonucleotide stability but also increase binding affinity to the target and help loading into the miRNA-induced silencing complex, both of which improve miRNA performance.
2.2. Limited penetration of miRNAs in vivo and in vitro
Hundreds of miRNAs have been found to be involved in disease development and progression, especially in cancer. Although major developments have been made to improve the delivery of miRNAs, the leading challenge of miRNA therapeutics is successful delivery to the target tissue with efficient penetration of payload to a specific site [Citation18]. In cancer, the leaky structure of tumor blood vessels causes poor blood perfusion, which reduces the delivery efficacy of naked miRNAs. To overcome this limitation different delivery vehicles have been developed (): (1) Nanocarrier (Liposomes) based methods capitalizing on leaky tumor vessels (the EPR effect) [Citation19] (2) Polymers used as a nanoparticles for the EPR effect [Citation20] (3) conjugation-based methods where a sugar, peptide or lipid is covalently conjugated to 3ʹ-end of the passenger strand, or peptides, aptamers, antibody-conjugation for tissue specificity [Citation21,Citation22] (4) Exosomes [Citation23] and (5)Viral vectors [Citation20]. Viral vectors have been used for in-vivo delivery. The spectrum of viral vectors is very broad including both delivery vehicles developed for transient short-term and permanent long-term expression. The most applied viral vectors are based on adenoviruses. A substantial number of clinical trials have been conducted or are currently in progress applying viral vectors [Citation24]. In some cases nanoparticles are used to deliver vectors expressing miRNAs. The plasmid vector expressing the miRNA were bound to a cationic polymer by electroststic interactions and then loaded on NPs [Citation25].
Lipid based nanoparticles are a general group that includes other liposomes, solid nano particles (SLNS), and nanostructured lipid carriers (NLCS) [Citation26]. Both Liposomes and Polymers are subtypes of NPs which are very useful for overcoming delivery challenges, due to their biocompatibility and biodegradability.
In addition, single/double-stranded oligonucleotides such as miRNA are negatively charged which results in low tissue permeability. The current delivery solution is to construct nanoparticles with size and properties that are changeable due to different microenvironments, conditions or time series (). Nanoparticles consist of components leading to controlled release and efficient diffusion of the therapeutic cargoes in diseased tissues. The delivery of miRNA with nanocarriers for downregulated miRNA or inhibition of overexpressed miRNA has shown a good response in overcoming diseased conditions in some clinical trials [Citation27]. The nanocarriers increase the stability of the miRNA and the transfection efficiency into the cells many fold as compared with naked miRNA.
2.3. Endosome escape is challenging
Whether miRNAs are delivered by cationic lipids, nanoparticles or cell-type-specific delivery vehicles, the intracellular trafficking of miRNA often begins in the early endosome compartment. Afterward, the early endosomes merge with late endosomes and transfer their contents. Late endosomal vesicles have an acidic environment (pH 5–6). The endosomal content then moves to the lysosomes, which are further acidified (pH ~4.5) and have various nucleases that stimulate the degradation of oligonucleotides/miRNA mimics. To bypass lysosomal degradation, miRNA mimics have to escape from the endosome into the cytoplasm, where they can engage with the RNAi machinery. Endosomal escape is a major challenge for efficient miRNA delivery. There are different strategies to promote endosomal release: (1) pH-sensitive lipoplexes [Citation10] (2) pH-sensitive polyplexes [Citation28] (3) Photosensitive molecules [Citation29] (4) Cationic nanoparticles [Citation30].
2.4. Extrahepatic delivery faces several challenges
For liver delivery, the known trivalent N-acetylgalactosamine (GalNAc) conjugate binds with high specificity and affinity to the asialoglycoprotein receptor on hepatocytes, resulting in specific oligonucleotide delivery and gene silencing in hepatocytes. The success of the GalNAc platform demonstrates that functional tissue delivery of therapeutic oligonucleotides is the basis for any clinical exploration. Another type of conjugation used for improving miRNA delivery is lipids [Citation31]. The majority of studied lipidconjugated miRNAs, such as cholesterol-conjugated miRNAs, accumulate in the liver (∼60–80%). However, cholesterol-modified miRNAs also exhibit accumulation and productive silencing in extrahepatic tissues. Moreover, local injection of cholesterol modified siRNA leads to functional gene silencing in brain, vagina and skin [Citation31].
Targeting miRNAs to extra-hepatic tissues remains an obstacle, limiting the use of miRNA-based therapies. Lipid and polymer nanoparticles can be formulated for efficient cellular uptake and endosomal escape but they still preferentially accumulate in the liver, kidney and spleen. To overcome this challenge extra hepatic scaffold like lipids, peptides, aptamers or antibodies conjugated on the particles result in targeting via recognition of specific receptors [Citation21,Citation32].
2.5. Off-target effects and unwanted on-target effects
Once miRNAs are delivered into the cytoplasm and released from the endosome, one of the major issues related to miRNA therapy is the off-target effect of miRNAs. MiRNAs are produced to target various pathways by imperfect hybridization with 3′ UTRs, thus they might cause undesirable gene silencing of other genes. Such off-target gene silencing can cause potential toxicities and reduced therapeutic effects. The evidence that a single miRNA might target numerous mRNAs requires special attention as it implies the possibility of unpredictable side effects. Even if a specific miRNA is effectively targeted, one might also find unwanted on-target effects [Citation33]. To overcome this obstacle, one approach is to use low doses of combined miRNAs that synergistically regulate the expression of the same target gene [Citation34]. One example of multiple miRNAs effects in cancer is co-transfection of miR-34a and miR-15a/16 which led to increased cell cycle arrest in NSCLC due to the fact that miR-15a and miR-16 specifically downregulate CCNE1 and CCND3. Such gene regulation brings a complementary effect to cell-cycle regulation by miR-34 [Citation35].
2.6. miRNAs have the potential to activate the immune system
Double-stranded RNAs can be considered pathogens by the host system and the innate immune system can recognize them and become activated. For example, systemic miRNA delivery, like other types of nucleic acid, can activate the innate immune system leading to toxicities and significant unwanted side effects. Systemic administration of miRNA duplexes can trigger secretion of inflammatory cytokines and type I interferons (IFNs) through Toll-like receptors (TLRs). TLRs 3, 7 and 8 are activated by single or double-stranded RNAs (dsRNAs) to drive innate and adaptive immune responses. These TLRs sense dsRNA molecules in cellular endosomal and lysosomal compartments to trigger the type I interferon (IFN) pathway and also cytokine production [Citation36]. Scientist are still studying the immune responses related to miRNAs, but it seems clear that the chemical modifications shown in also reduce recognition by the immune system [Citation8].
Another interest is the immune system reaction to the delivery vehicle, which is highly positive charged and might cause toxicity and lead to the immune system activation. When NPs enter the body, interactions with the immune system are inescapable. NPs size, shape, hydrophobicity and surface modifications are the major components that impact the interactions between nanoparticles and the immune system [Citation37]. Thus, both the miRNA- and the delivery vehicle have to be explored. Targeting of the carrier to the specific tissues will allow for decreased dosing and thus likely reduce immune responses.
3. Conclusions
Further understanding of the biological and functional mechanisms, and chemical and bioengineering of miRNAs will continue to improve this new therapeutic modality. What is obvious, however, is that the more we understand the roles of miRNAs in diseases, the more likely it is that these basic studies will translate into novel clinical applications. Once these potential obstacles discussed here are resolved, miRNA therapeutics should show continuing promise as therapeutic molecules for various diseases.
4. Expert opinion
An increasing number of research studies point to the future use of miRNAs as biomarkers or drugs for pathogenic conditions, especially cancer. The first FDA approved small RNA drugs (RNAi-based) have recently entered clinical medicine. Since then there has been a growing number of studies for miRNAs in preclinical and clinical research applications [Citation38]. For example, mimics to miR-16 are in clinical trial for mesothelioma, a form of lung cancer. Another example involves mimics to miR-29b to minimize fibrous scar formation, while another clinical trial utilizes an antimiR to miR-155, which plays a key role in differentiation, function, and proliferation of blood and lymphoid cells in lymphoma [Citation39]. In another example, antimiRs to miR-10b have been proposed to treat glioblastoma, one of the most aggressive forms of brain cancer [Citation40].
To date, there has been various studies and major improvements in understanding the mechanisms and efficiency of miRNA therapeutics, but particular obstacles to maximium efficiency are still unsolved. These challenges include: targeted delivery, specificity, stability, immune activation and toxicity in vivo and in vitro.
We believe that miRNA therapeutics will have a major part in cancer therapy in the future. Specifically, personalized cancer medicine can be accomplished by constructing the unique miRNA mimic or antagonist for patients disease based on the patient’s miRNA expression profiles. We expect that new strategies and developments will overcome the remaining biological challenges for miRNA delivery and expedite the great therapeutic potential of miRNAs in the cancer field.
Declaration of interest
F Slack discloses financial interests and Scientific Advisory Board roles with Mira DX and MiRNA Therapeutics. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
The authors thank M Kovaliov for her help in preparing the figures for this manuscript.
Additional information
Funding
References
- Hayes J, Peruzzi PP, Lawler S. MicroRNAs in cancer: biomarkers, functions and therapy. Trends Mol Med. 2014;20(8):460–469.
- Li Y, Kowdley KV. MicroRNAs in common human diseases. Genomics Proteomics Bioinformatics. 2012;10(5):246–253.
- Gilles M-E, Hao L, Huang L, et al. Personalized RNA-medicine for pancreatic cancer. Clin Cancer Res. 2018;24(7):1734–1747.
- Kasinski AL, Kelnar K, Stahlhut C, et al. A combinatorial microRNA therapeutics approach to suppressing non-small cell lung cancer. Oncogene. 2015;34(27):3547–3555.
- Zhang B, Pan X, Cobb GP, et al. microRNAs as oncogenes and tumor suppressors. Dev Biol. 2007;302(1):1–12.
- Beg MS, Brenner AJ, Sachdev J, et al. Phase I study of MRX34, a liposomal miR-34a mimic, administered twice weekly in patients with advanced solid tumors. Invest New Drugs. 2017;35(2):180–188.
- Zhang Z, Qin Y-W, Brewer G. et al. MicroRNA degradation and turnover: regulating the regulators. Wiley Interdiscip Rev RNA. 2012;3(4):593–600.
- Stepanov G, Zhuravlev E, Shender V, et al. Nucleotide modifications decrease innate immune response induced by synthetic analogs of snRNAs and snoRNAs. Genes (Basel). 2018;9(11):531.
- Zhao C, Sun X, Li L. Biogenesis and function of extracellular miRNAs. ExRNA. 2019;1(1):38.
- Paliwal SR, Paliwal R, Vyas SP. A review of mechanistic insight and application of pH-sensitive liposomes in drug delivery. Drug Deliv. 2015;22(3):231–242.
- Denzler R, McGeary SE, Title AC, et al. Impact of MicroRNA levels, target-site complementarity, and cooperativity on competing endogenous RNA-regulated gene expression. Mol Cell. 2016;64(3):565–579.
- Meng Z, Lu M. RNA interference-induced innate immunity, off-target effect, or immune adjuvant? Front Immunol. 2017;8:331.
- Chen Y, Gao D-Y, Huang L. In vivo delivery of miRNAs for cancer therapy: challenges and strategies. Adv Drug Deliv Rev. 2015;81:128–141.
- Segal M, Biscans A, Gilles M-E, et al. Hydrophobically modified let-7b miRNA enhances biodistribution to NSCLC and downregulates HMGA2 in vivo. Mol Ther Nucleic Acids. 2020;19:267–277.
- Lennox KA, Behlke MA. Chemical modification and design of anti-miRNA oligonucleotides. Gene Ther. 2011;18(12):1111–1120.
- Akbarzadeh A, Rezaei-Sadabady R, Davaran S, et al. Liposome: classification, preparation, and applications. Nanoscale Res Lett. 2013;8(1):102.
- Bolhassani A, Javanzad S, Saleh T, et al. Polymeric nanoparticles: potent vectors for vaccine delivery targeting cancer and infectious diseases. Hum Vaccin Immunother. 2014;10(2):321–332.
- Gasparello J, Manicardi A, Casnati A, et al. Efficient cell penetration and delivery of peptide nucleic acids by an argininocalix[4]arene. Sci Rep. 2019;9(1):3036.
- Karlsen TA, Brinchmann JE. Liposome delivery of MicroRNA-145 to mesenchymal stem cells leads to immunological off-target effects mediated by RIG-I. Mol Ther. 2013;21(6):1169–1181.
- Li J, Liang H, Liu J, et al. Poly (amidoamine) (PAMAM) dendrimer mediated delivery of drug and pDNA/siRNA for cancer therapy. Int J Pharm. 2018;546(1):215–225.
- Biscans A, Coles A, Haraszti R, et al. Diverse lipid conjugates for functional extra-hepatic siRNA delivery in vivo. Nucleic Acids Res. 2018;47(3):1082–1096.
- Daei P, Ramezanpour M, Khanaki K, et al. Aptamer-based targeted delivery of miRNA let-7d to gastric cancer cells as a novel anti-tumor therapeutic agent. Iran J Pharm Res. 2018;17(4):1537–1549.
- Li X, Corbett AL, Taatizadeh E, et al. Challenges and opportunities in exosome research-perspectives from biology, engineering, and cancer therapy. APL Bioeng. 2019;3(1):011503.
- Lundstrom K. Viral vectors in gene therapy. Diseases. 2018 May 21;6:2.
- Salah Z, Abd El Azeem EM, Youssef HF. et al. Effect of tumor suppressor MiR-34a loaded on ZSM-5 nanozeolite in hepatocellular carcinoma: in vitro and in vivo approach. Curr Gene Ther. 2019;19(5):342–354.
- Ghasemiyeh P, Mohammadi-Samani S. Solid lipid nanoparticles and nanostructured lipid carriers as novel drug delivery systems: applications, advantages and disadvantages [Review Article]. Res Pharm Sci. 2018 July 1;13(4):288–303.
- Slack FJ, Chinnaiyan AM. The role of non-coding RNAs in oncology. Cell. 2019;179(5):1033–1055.
- Christie RJ, Nishiyama N, Kataoka K. Minireview:delivering the code: polyplex carriers for deoxyribonucleic acid and ribonucleic acid interference therapies. Endocrinology. 2010;151(2):466–473.
- Linsley CS, Wu BM. Recent advances in light-responsive on-demand drug-delivery systems. Ther Deliv. 2017;8(2):89–107.
- Bilensoy E. Cationic nanoparticles for cancer therapy. Expert Opin Drug Deliv. 2010;7(7):795–809.
- Biscans A, Coles A, Haraszti R, et al. Diverse lipid conjugates for functional extra-hepatic siRNA delivery in vivo. bioRxiv. 2018;47(3):1082–1096.
- Esposito CL, Cerchia L, Catuogno S, et al. Multifunctional Aptamer-miRNA conjugates for targeted cancer therapy. Mol Ther. 2014;22(6):1151–1163.
- Suter SR, Ball-Jones A, Mumbleau MM, et al. Controlling miRNA-like off-target effects of an siRNA with nucleobase modifications. Org Biomol Chem. 2017;15(47):10029–10036.
- Lai X, Eberhardt M, Schmitz U, et al. Systems biology-based investigation of cooperating microRNAs as monotherapy or adjuvant therapy in cancer. Nucleic Acids Res. 2019;47(15):7753–7766.
- Bandi N, Vassella E. miR-34a and miR-15a/16 are co-regulated in non-small cell lung cancer and control cell cycle progression in a synergistic and Rb-dependent manner. Mol Cancer. 2011;10(1):55.
- Yu H-R, Huang L-H, Li S-C. Roles of microRNA in the immature immune system of neonates. Cancer Lett. 2018;433:99–106.
- Liu Y, Hardie J, Zhang X, et al. Effects of engineered nanoparticles on the innate immune system. Semin Immunol. 2017;34:25–32.
- Rupaimoole R, Slack FJ. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov. 2017;16(3):203–222.
- Foss FM, Querfeld C, Kim YH, et al. Ph 1 study of MRG-106, an inhibitor of miR-155, in CTCL. J clin oncol. 2018;36(15_suppl):2511.
- Teplyuk NM, Uhlmann EJ, Gabriely G, et al. Therapeutic potential of targeting microRNA-10b in established intracranial glioblastoma: first steps toward the clinic. EMBO Mol Med. 2016;8(3):268–287.