1. Background
After China’s reform and opening up, notably since the implementation of ‘the national significant new drug innovation program,’ China’s biopharma has achieved rapid development, Chinese scientists are transforming from the imitation of the generic drugs to the pursuit of the best-in-class drugs and first-in-class drugs [Citation1–Citation4].
The discovery of new drugs is one of the sophisticated intellectual activity. It required scientists to keep continuous track of new medicinal chemistry strategies. For a long time, drug discovery in China has mainly relied on traditional medicinal chemical approaches such as bioisosterism, scaffold hopping, etc., which lead to substantial time delays and inefficient usage of data in the complex drug design. In recent years, interdisciplinary teamwork at the interface between medicinal chemistry, informatics, biology, and multiple techniques working in concert provides us more purposeful drug design approaches, such as structural biology-guided optimization, multiparameter optimization, and biological system-mediated drug delivery, etc [Citation5–Citation7]. Flexible application of these strategies is making the drug discovery process more efficient by reducing the number and duration of design cycles required to optimize lead compounds into safe and efficient drug candidates. Herein, we aim to present a brief introduction to the medicinal chemistry strategies, applied for the discovery of representative drugs (candidates) in China.
2. Medicinal chemistry approaches in the discovery of new drugs and candidates in China
2.1. Discovery of imrecoxib via the balanced inhibition strategy
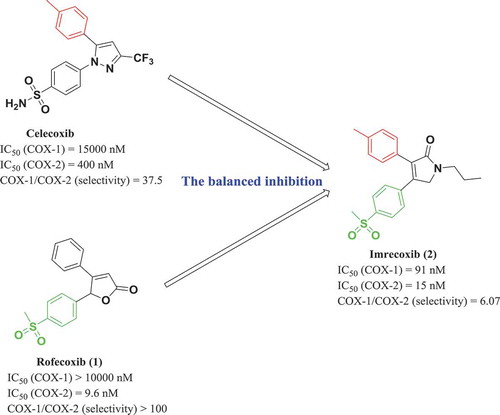
In the human body, each enzyme plays a unique and essential physiological activity. Although its over-expression may cause some diseases, over-inhibition leads to serious side effects. For example, initially, highly selective inhibition of cyclooxygenase-2 (COX-2) was believed to eliminate inflammation without gastrointestinal damage caused by inhibition of COX-1, such as Rofecoxib (1). However, clinical studies have shown that Rofecoxib can cause severe cardiovascular problems after prolonged use due to the highly selective inhibition of COX-2. To avoid the risk of cardiovascular disorders caused by highly selective COX-2 inhibitors, and to prevent gastrointestinal damage caused by significant inhibition of COX-1, Guo Zongru proposed the balanced inhibition strategy of COX-1/COX-2. Based on the known pharmacophore of a COX-2 inhibitor, his research group reported the design and synthesis of a series of compounds with pyrrolidone as core moiety. Among them, Imrecoxib (2), also known as BAP-909, was approved as a balanced COX-1 and COX-2 inhibiting drug with an IC50 value of 91 nM and 15 nM, respectively (). In 2005, Imrecoxib was approved by China’s State Food and Drug Administration for the symptomatic treatment of osteoarthritis [Citation8,Citation9].
2.2. Discovery of albuvirtide via HSA drug delivery strategy
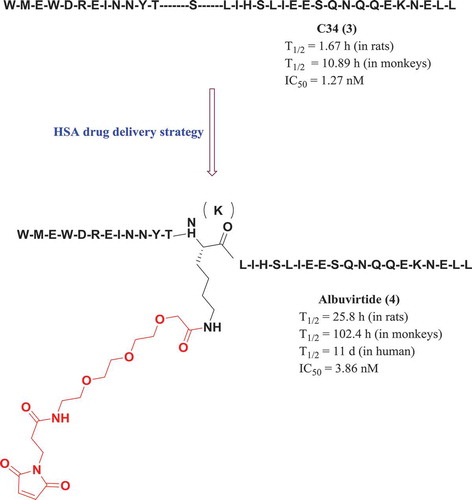
Human serum albumin (HSA) is the most abundant protein in human serum, and mainly it serves as a natural carrier for a myriad of molecules. It has a circulatory half-life of about 19 days and exhibits no antigenicity. Therefore, HSA is an ideal carrier for improving the pharmacokinetic profile (half-life extension) and for the targeted delivery of drugs. C34 (3) is a peptide inhibitor of HIV-1 membrane fusion with a short half-time. Using the HAS drug delivery strategy, compound 3 was further modified by using 3-maleimimidopropionic acid to form a prodrug (Albuvirtide, 4), which can covalently bind to HSA in vivo. The combined complex can be efficiently and intensively transported in the blood to optimize the pharmacokinetic properties and to reduce the cytotoxicity. As shown in , the half-life period of Albuvirtide is significantly longer than that of 3. At present, Albuvirtide was developed by China’s Frontier Company, has been successfully approved by China’s State Food and Drug Administration as the world’s first long-acting HIV fusion inhibitor and the first indigenously developed anti-HIV drug in China in 2018 [Citation10,Citation11].
2.3. Discovery of thioraviroc via structural biology-guided and multiparameter optimization
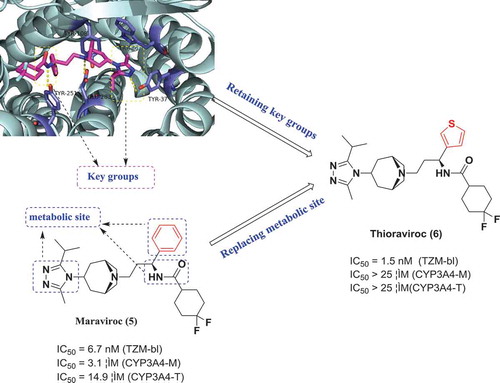
Structural biology-guided optimization is a fundamental strategy for finding and optimizing lead compounds of therapeutic importance since structural biology most directly illustrates the binding mode of drug-protein complexes. Also, drug discovery is a multiparameter optimization (MPO) process, because a successful drug should possess a satisfying balance of potency against its therapeutic targets, physicochemical characteristics, pharmacokinetics profiles (absorption, distribution, metabolism, and elimination) and safety [Citation12]. Maraviroc (5) is the first small molecule CCR5 antagonist to be approved for marketing. However, it has found that 5 has a strong inhibition ability to CYP450 (CYP3A4: IC50 = 3.1 uM), which may cause drug interactions and lead to adverse reactions and further, poor metabolic stability results in a low bioavailability of 5. After analyzing the crystal structure of CCR5 in complex with 5, Liu Hong’s research group found that the amide moiety and triazole moiety are essential for the CCR5-binding activity (). Also, metabolic pathways of 5 revealed that the phenyl group, amide moiety, and triazole moiety are the primary metabolic sites. Based on the observed findings, they have designed and synthesized a series of new CCR5 inhibitors that retained the amino and triazole groups and replaced the benzene ring. Among these series, compound 6 (Thioraviroc) displayed enhanced CCR5 inhibitory activity and pharmacokinetic characteristics but didn’t show significant inhibition to CYP450. Thioraviroc is currently in phase I clinical trials [Citation13].
2.4. Discovery of K5a2 via the structural optimization based on crystallographic studies
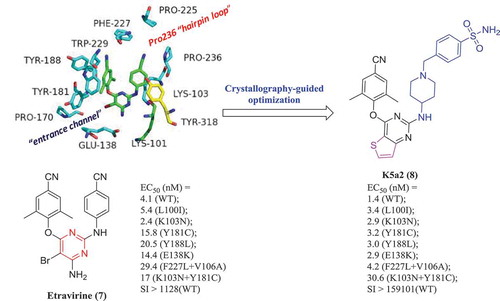
Based on the traditional drug design concept, a potent drug should occupy the binding pocket, interacting efficiently with the residues around the binding site. Structure-based design is typically focused on the optimization of a chemical series to obtain additional binding contacts and to increase the binding affinity for the target protein. Etravirine (7) is a new generation HIV-1 non-nucleoside reverse transcriptase inhibitor (NNRTI) marketed in the United States in 2008. However, with the extensive clinical application of 7, a variety of resistant mutants have emerged. By analyzing the crystallographic studies of 7 with NNRTIs binding pocket (NNIBP) (PDB: 3M8Q), our research group has found that NNIBP contains solvent-accessible regions that provide broad chemical space to accommodate novel substituents, such as tolerant region I (the Pro236 hairpin loop) and tolerant region II (the entrance channel). Based on the structure of these tolerant regions, we have designed and synthesized a series of compounds targeting the tolerant region I and II through rational drug design. Among them, compound K-5a2 (8) is superior to Etravirine in potency against HIV-1 wild-type and most clinically common mutants (). Currently, K5a2 exists as a potent anti-HIV-1 candidate and is in the pre-clinical research stage [Citation14].
Figure 5. Structural and essential information of some other representative best-in-class drugs (candidates) discovered by outstanding medicinal chemists in China [Citation15–Citation19].
![Figure 5. Structural and essential information of some other representative best-in-class drugs (candidates) discovered by outstanding medicinal chemists in China [Citation15–Citation19].](/cms/asset/ab58c2b2-ec26-4ff1-93d8-dce8f7cc9fe6/iedc_a_1775577_f0005_b.gif)
3. Conclusion
This article briefly summarized some highlighted drugs (candidates) discovery process in China, which we believe is of great significance to increase the global influence of Chinese innovation drug research. We aimed to suggest the potential generality of these strategies and inspire medicinal chemists to apply the essential strategy and related insights to other drug discovery campaigns. In addition, there are also some other representative best-in-class drugs (candidates) discovered by outstanding medicinal chemists in China, including Icotinib, ASK-120,067, GDZ824 and Quinoloxin (). It should be noted that the criteria for selecting cases in this review are not comprehensive but typical. All in all, introducing novel strategies and technologies that speed up drug discovery and the drug development cycle is of great importance both in the highly competitive pharmaceutical industry as well as in academia [Citation5,Citation21].
4. Expert opinion
The implementation of ‘the national significant new drug innovation program’ is promoting the transformation of biopharma in China from the imitation of the generic drugs to original innovation. Meanwhile, many outstanding medicinal chemists have emerged in China. However, the most remarkable achievements of new drug discovery in China remain within the realm of follow-on-based innovation, and the original drugs are still rare. Thus, we should strengthen basic research and learn cutting-edge technologies to increase our capacity for original innovation.
In 2015, Tu Youyou was awarded the Nobel Prize in medicine for the significant research on artemisinin, an antimalarial drug derived from the sweet wormwood plant, called Artemisia annua. By applying modern techniques to a heritage provided by 5000 years of Chinese traditional practitioners, Tu Youyou has delivered its riches into the 21st century. It is a great encouragement for the in-depth investigation of traditional Chinese medicine. Also encouragingly, during the preparation of this paper, China’s first original anti-Alzheimer’s drug GV-971 was launched in China, ending a 17-year history of no new medicine for this disease. The listing of GV-971 opens up a new path for the research and development of drugs for Alzheimer’s disease, which is of far-reaching significance for the promotion of China’s international status in the field of innovative drug research [Citation22]. Almost at the same time, Zanubrutinib, an oral Bruton tyrosine kinase small molecule inhibitor, was approved by the Food and Drug Administration (FDA) for marketing in the United States. Zanubrutinib is the first Chinese innovative anti-tumor drug approved by the US FDA, which marks the increasing internationalization of China’s bio-pharma [Citation23]. Also, on 20 November 2019, Chinese scientists found that selective activation of TWIK-related acid-sensitive K+3 (TASK-3) subunit-containing channels is analgesic in rodent models, and the potential of TASK-3 as a new target was systematically evaluated based on CHET3, as a first TASK-3 selective agonist. It marks China’s significant progress in target discovery and confirmation [Citation24]. At present, COVID-19 is a massive challenge for people around the world. Based on the three-dimensional structure of the 2019-nCoV main protease, Liu Hong’s group and his collaborators designed and synthesized two compounds 11a and 11b, which exhibited a satisfactory antiviral potency (11a: EC50 = 0.53 µM, 11b: EC50 = 0.72 µM). The research result has been published in Science and is considered to be of great significance for the treatment of COVID-19 [Citation25]. By considering all the above facts, although the starting point of biopharma in China is not as high as developed countries, it has grown rapidly in recent years, and it is playing an increasingly important role globally.
Last but not least, research integrity and student training should never be overlooked. Firstly, academic misconduct is a global problem, and of course, it also exists in China. For cultivating research integrity, Li Tang proposed some crucial and practical suggestions [Citation26]. Also, Ph.D. in China faces more pressure than the other parts of the world, and the government is working to address these problems by raising salaries and providing psychological counseling [Citation27]. The solution to social problems requires the joint efforts of the country, the government, and the people, which is also crucial to improving China’s reputation and influence in the world.
Declaration of interest
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Moku B, Ravindar L, Rakesh KP, et al. The significance of N-methylpicolinamides in the development of anticancer therapeutics: synthesis and structure-activity relationship (SAR) studies[J]. Bioorg Chem. 2019;86:513–537.
- Rakesh KP, Marichannegowda MH, Srivastava S, et al. Combating a master manipulator: staphylococcus aureus immunomodulatory molecules as targets for combinatorial drug discovery. ACS Comb Sci. 2018;20(12):681–693.
- Ravindar L, Bukhari S, Rakesh KP, et al. Aryl fluorosulfate analogues as potent antimicrobial agents: SAR, cytotoxicity and docking studies. Bioorg Chem. 2018;81:107–118.
- Sun Z A total of 139 new drug certificates have been issued under the “major new drug innovation and production” program. [cited 2019 Jul 31]. https://baijiahao.baidu.com/s?id=1640561605505866307&wfr=spider&for=pc
- Wu G, Zhao T, Kang D, et al. Overview of recent strategic advances in medicinal chemistry. J Med Chem. 2019;62(21):9375–9414.
- Zhan P, Pannecouque C, De Clercq E, et al. Anti-HIV drug discovery and development: current innovations and future trends. J Med Chem. 2016;59(7):2849–2878.
- Zhan P, Itoh Y, Suzuki T, et al. Strategies for the discovery of target-specific or isoform-selective modulators. J Med Chem. 2015;58(19):7611–7633.
- Penning TD, Talley JJ, Bertenshaw SR, et al. Synthesis and biological evaluation of the 1,5-diarylpyrazole class of cyclooxygenase-2 inhibitors: identification of 4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl]benze nesulfonamide (SC-58635, celecoxib). J Med Chem. 1997;40(9):1347–1365.
- Chen X, Bai J, Shen F, et al. Imrecoxib: a novel and selective cyclooxygenase 2 inhibitor with anti-inflammatory effect. Acta Pharmacol Sin. 2004;25(7):927–931.
- Chong H, Yao X, Zhang C, et al. Biophysical property and broad anti-HIV activity of albuvirtide, a 3-maleimimidopropionic acid-modified peptide fusion inhibitor. PLoS One. 2012;7:3.
- Zhang H, Jin R, Yao C, et al. Combination of long-acting HIV fusion inhibitor albuvirtide and LPV/r showed potent efficacy in HIV-1 patients. AIDS Res Ther. 2016;10(13):8.
- Liu H, Long S, Rakesh KP, et al. Structure-activity relationships (SAR) of triazine derivatives: promising antimicrobial agents[J]. Eur J Med Chem. 2000;185:111804-111818.
- Peng P, Chen H, Zhu Y, et al. Structure-based design of 1-heteroaryl-1,3-propanediamine derivatives as a novel series of cc-chemokine receptor 5 antagonists. J Med Chem. 2018;61(21):9621–9636.
- Kang D, Fang Z, Li Z, et al. Design, synthesis, and evaluation of thiophene[3,2-d]pyrimidine derivatives as HIV-1 non-nucleoside reverse transcriptase inhibitors with significantly improved drug resistance profiles. J Med Chem. 2016;59(17):7991–8007.
- Hu S, Xie G, Zhang D, et al. Synthesis and biological evaluation of crown ether fused quinazoline analogues as potent EGFR inhibitors. Bioorg Med Chem Lett. 2012;22(19):6301–6305.
- Ren X, Pan X, Zhang Z, et al. Identification of GZD824 as an orally bioavailable inhibitor that targets phosphorylated and nonphosphorylated breakpoint cluster region-Abelson (Bcr-Abl) kinase and overcomes clinically acquired mutation-induced resistance against imatinib. J Med Chem. 2013;56(3):879–894.
- Zhang C, Zheng M, Li Y, et al. Design, synthesis, and structure-activity relationship studies of 3-(phenylethynyl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine derivatives as a new class of src inhibitors with potent activities in models of triple negative breast cancer. J Med Chem. 2015;58(9):3957–3974.
- Guo D, Li J, Lin H, et al. Design, synthesis, and biological evaluation of novel tetrahydroprotoberberine derivatives (THPBs) as selective α1A-adrenoceptor antagonists. J Med Chem. 2016;59(20):9489–9502.
- Zhao F, Li J, Chen Y, et al. Design, synthesis, and biological evaluation of indoline and indole derivatives as potent and selective α1A-Adrenoceptor antagonists. J Med Chem. 2016;59(8):3826–3839.
- Xu T, Zhang L, Xu S, et al. Pyrimido[4,5-d]pyrimidin-4(1H)-one derivatives as selective inhibitors of EGFR threonine790 to methionine790 (T790M) mutants. Angew Chem Int Ed Engl. 2013;52(32):8387–8390.
- Du J, Guo J, Kang D, et al. New techniques and strategies in drug discovery. Chinese Chem Lett. 2020. DOI:10.1016/j.cclet.2020.03.028.
- Song S There had been no new drug in the field for 17 years before the domestic alzheimer’s drug GV-971 came to market. https://www.sohu.com/a/351270239_115362. 2011 Nov 2.
- Huang Z Historical breakthrough! China’s cancer drug has been approved for sale in the United States. [cited 2019 Nov 15]. http://news.cctv.com/2019/11/15/ARTIqoxLUAX6W8bDAgGS7YvB191115.shtml
- Liao P, Qiu Y, Mo Y, et al. Selective activation of TWIK-related acid-sensitive K+3 subunit-containing channels is analgesic in rodent models. Sci Transl Med. 2019;11(519):eaaw8434.
- Dai W, Zhang B, Su H, et al. Structure-based design of antiviral drug candidates targeting the SARS-CoV-2 main protease [published online ahead of print, 2020 Apr 22]. Science. 2020 Apr 22;eabb4489. doi: 10.1126/science.abb4489.
- Li T. Five ways China must cultivate research integrity. Nature. 2019;575(7784):589–591.
- Woolston C, O’Meara S. PhD students in China report misery and hope. Nature. 2019;575(7784):711–713.