ABSTRACT
Introduction: The integrin αvβ6 is a promising therapeutic target due to its limited expression in healthy tissue and significant overexpression in cancer and fibrosis. The peptide A20FMDV2, derived from the foot and mouth disease virus, is highly selective for αvβ6, and can be used therapeutically to target αvβ6 expressing cells.
Areas covered: In this review, the authors discuss the logic that led to the discovery of A20FMDV2, the importance of its stereochemistry in receptor-binding, and the strategies employed to use it as a molecular-specific drug delivery system. These strategies include creating A20FMDV2-drug conjugates, genetically modifying oncolytic viruses to express A20FMDV2 and thus redirect their tropism to predominantly αvβ6 expressing cells, creation of A20FMDV2 expressing CAR T-cells, and modifying antibody tropism by inserting A20FMDV2 into the CDR3 loop.
Expert opinion: αvβ6 is one of the most promising therapeutic targets in cancer and fibrosis discovered in the last few decades. The potential use of A20FMDV2 as a molecular-specific αvβ6-targeting agent is extremely promising, particularly when considering the success of the peptide and its variants in clinical imaging.
1. Introduction
Integrins are heterodimeric cell surface receptors capable of bidirectional signaling enabling cells to respond to their immediate microenvironment [Citation1]. αvβ6 is an integrin expressed exclusively on epithelial cells, and is very weak or absent in most healthy, adult tissues [Citation2]. However, αvβ6 expression increases during tissue remodeling including development and wound repair, and also in diseases such as cancer and fibrosis, where its increased expression is associated with disease progression and poorer prognosis [Citation3–5]. In fact, αvβ6 is upregulated on about one-third of all solid cancers and thus represents an excellent differentially expressed cell-surface molecule for detection and treatment of such cancers [Citation6]. For these reasons, αvβ6 has been subject to intensive research toward development of αvβ6-targeting therapies and the characterization of αvβ6 as a biomarker for imaging and diagnosis. Probably the most commonly used agent for targeting αvβ6 is the αvβ6-specific peptide A20FMDV2, derived from a specific serotype of the foot-and-mouth-disease virus. In this review we will discuss the discovery and characterization of A20FMDV2 and describe many of the pre-clinical studies that are enabling development of this peptide as a molecular-specific drug delivery system.
2. The discovery and characterization of A20FMDV2
A20FMDV2 is a 20-mer peptide sequence derived from a protein forming the viral capsid of the O1 BFS serotype of foot-and-mouth-disease virus (FMDV). The structure of FMDV was revealed by x-ray crystallography in 1989 [Citation7], revealing that the viral capsid consists of 4 structural proteins; VP1 to VP4. In 1993, it was discovered that a loop containing an Arg-Gly-Asp sequence was present between the G and H β strands of the VP1 protein, leading to speculation that FMDV could bind to RGD-binding integrins [Citation8]. Subsequent research blocking infection using RGD-peptides revealed that it prevented FMDV infection of cells in vitro suggesting the speculation to be accurate [Citation9,Citation10].
There are eight RGD-binding integrins (αvβ1, αvβ3, αvβ5, αvβ6, αvβ8, αIIbβ3, α5β1 and α8β1) which each binds specific ligands that possess Arg-Gly-Asp as part of their primary sequence. There are over 2600 proteins in humans which have an RGD domain [Citation11]; not all act as adhesive molecules and each has a unique structure, function, and particular tissue expression. Following the discovery of an Arg-Gly-Asp sequence in the GH loop of FMDV, this inspired research to determine whether RGD integrins acted as the means of entry for FMDV. The first RGD integrin shown to act as a receptor for FMDV was αvβ3, however as αvβ3 is not expressed by normal epithelial cells, this was unlikely to be the cause of FMDV infections in animals [Citation12]. In contrast, the integrin αvβ6 is exclusively expressed by epithelial cells.
In 1999 Kraft and colleagues using a phage-library screen discovered that the amino-acid sequence DLXXL appeared to be an αvβ6-specific ligand [Citation13]. This same sequence had been identified previously as an essential component of RGD peptides designed to effectively inhibit FMDV virus infection [Citation10]. With this knowledge, and the fact that leucine is the most common amino acid found immediately adjacent to the RGD motif in FMDV [Citation14], Jackson and colleagues hypothesized that αvβ6 acted as a major receptor for viral entry in to the cell [Citation15]. They showed that it was possible to infect cells previously resistant to FMDV infection by transfecting the cells with the β6 subunit. Furthermore, they demonstrated that blocking αvβ6 with an antibody to αvβ6 prevented viral infection by >99%, suggesting strongly that αvβ6 was the primary integrin responsible for FMDV infection.
The RGD-binding integrins do not all have the same ligand specificity. This is because the regions bordering the RGD sequence regulate the binding specificity and affinity of integrins for their ligands [Citation16,Citation17]. To better understand the dynamics of αvβ6 ligand binding, and to determine which, if any, additional binding motifs regulated ligand specificity, a structure-function analysis of αvβ6 ligand-binding was performed [Citation18]. The authors tested high-affinity ligands for αvβ6 including the latency associated peptide (LAP) from TGFβ1 and two serotypes of FMDV; from each ligand a series of peptides, of different lengths up to 20 amino-acids (aa), with overlapping sequences that each contained the RGD motif, were synthesized and tested in vitro [Citation18].
Many of the peptides were able to inhibit αvβ6-dependent adhesion, but those with longer sequences C-terminal to the aspartate of RGD were more potent. Three peptides were subsequently designed for further investigation; A20FMDV1 (YTASARGDLAHLTTTHARHL), A20FMDV2 (NAVPNLRGDLQVLAQKVART), and A20LAP (GFTTGRRGDLATIHGMNRPF; note the conserved isoleucine rather than leucine in the DLXXI of LAP). In assays of αvβ6-specific inhibition of cell binding to LAP, or the binding of biotinylated variants of the three peptides to immobilized recombinant αvβ6, the potency always followed the same sequence: A20FMDV2> A20LAP>A20FMDV1, with A20FMDV2 showing very high affinity for αvβ6 since A20FMDV2 inhibited αvβ6 binding to LAP by 50% at just 0.5 nM. However, there were no obvious clues in the sequence of these linear peptides as to why A20FMDV2 was the better peptide.
Earlier studies of the crystal structure of the serotype from which A20FMDV2 was derived reported that the sequence C-terminal to the RGD could form a helix [Citation5]. The helix-predictive software Agadir (http//:agadir.crg.es) predicted that indeed all three peptides should form α-helices after the RGD motif [Citation18]. This prediction was confirmed by nuclear magnetic resonance (NMR) imaging of the peptides in the helix-promoting buffer trifluoroethanol. The data revealed that each peptide formed a 3D structure with the RGD-binding domain at the tip of a loop/hairpin, followed by an adjacent C-terminal α-helix (). The length of the α-helix varied across the three peptides, with the longest helix observed in A20FMDV2 and shortest in A20FMDV1. The length and stability of the helix were shown to correlate with affinity of the peptides for αvβ6 [Citation18].
Figure 1. 3D Structure of A20 peptides. A20FMDV2 and A20LAP STD NMR transfer maps onto the averaged helical structures of these peptides illustrate a defined high definition contact face on each peptide. The ability of these peptides to easily form helices is important for enhanced efficacy toward αvβ6 as helix formation proves a structural definition of a face of amino acids that includes the side chains of the extended LXXL motif as well as residues further toward the C-terminus of these sequences. In contrast the A20FMDV1 structure does not present a clear RGDLXXL interface, presumed to be because of the poor helix forming C-terminus. (Data generated and analyzed by Dr Mark Howard, University of Leeds)
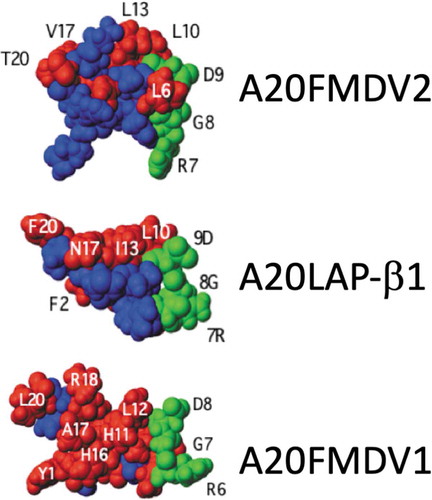
The importance of the helix was confirmed by generating peptides of A20FMDV2 with D-Valines in the C-terminus that destroyed helix formation and simultaneously also the biological activity. While NMR in PBS showed the three linear peptides were unstructured, Saturation Transfer Determined-NMR (STD-NMR) in PBS confirmed that when the peptides were bound to recombinant αvβ6 they adopted the RGD-helix shape and indicated that the two leucines (in A20FMDV2) were adjacent in space and located very close to the integrin primary sequence, suggesting a binding face. Since EDTA, which chelates the cations essential for integrin function, could completely inhibit binding of the three peptides to αvβ6 it seems that the high affinity of the αvβ6-binding peptides, especially A20FMDV2, was because the 20mer peptide had two integrin-binding sites: the cation-dependent RGD which probably bound initially, and the two leucines (presumably leucine and isoleucine in LAPβ1 and LAPβ3) that were presented as a single hydrophobic binding face due to their being brought into juxtaposition by the α-helix. shows the predicted STD-NMR 3D structures when in association with αvβ6. Note the close relationship between the RGD and the adjacent Leu:Leu/Ile pair in A20FMDV2 and A20LAP compared with A20FMDV1.
Since its discovery in 2007, A20FMDV2 has been used widely for an array of applications to specifically target αvβ6 for imaging and therapy and also to develop small molecule inhibitors of αvβ6. The most common use for A20FMDV2 and variants is for improved radio-imaging of fibrosis and cancer in mice [Citation6,Citation19,Citation20] which has now translated to humans [Citation21–24]. We will not discuss the value of imaging studies in this review except where modifications of A20FMDV2 have improved its activity in vitro and/or in vivo and thus would be expected to improve its value as a molecular-specific vector for delivery of therapy.
3. Therapeutic targeting of αvβ6 with A20FMDV2
3.1. αvβ6-specific drug delivery
The ideal anti-cancer drug should be selective for neoplastic cells, leaving normal cells unaffected and minimizing side effects. By targeting receptors or proteins expressed at selectively higher levels on cancer versus normal cells, drugs and toxins could potentially be targeted with effective toxic doses to neoplastic cells only. Two of the leading approaches for targeted drug delivery include conjugating the drug/toxin directly to the targeting ligand or through using a carrier system such as a nanoparticle or virus. The carrier system is engineered so that the selective ligand is expressed on the surface of the particle to provide cancer-selective targeting, while the drug, toxin, or even nucleic acid delivering a cytotoxic effect, is not effective until the particle is internalized by the cell.
Liposomes are synthetic nanoparticles used for drug delivery [Citation25] that have been used to assist in the delivery of alendronate (ALD) to tumor cells [Citation26]. Delivery of ALD to tumor cells sensitizes them to killing by γδ T cells, however the therapeutic efficacy of the ALD could be improved. Toward this, Hodgins and colleagues designed a liposomal ALD (L-ALD) with A20FMDV2 conjugated to the surface of the liposomes, with the aim of increasing delivery to αvβ6 expressing cells [Citation27]. A significant improvement in sensitization of tumor cells was observed using the A20FMDV2 containing L-ALD, evident by increased efficacy of γδ T cell killing in vitro. The A20FMDV2 targeting liposomes were labeled with [111In] for SPECT imaging, and injected into mice bearing αvβ6-positive and -negative tumors, but unfortunately there was not a selective accumulation of radioactivity in the αvβ6-positive tumors, the reasons for which remain unclear.
Pancreatic ductal adenocarcinoma (PDAC) has a 5 year survival rate of less than 5% [Citation28], with current treatment options limited and relative failure to improve mortality rates over the last 50 years [Citation29]. Reports show αvβ6 is overexpressed in most PDAC tissues [Citation30,Citation31], but is absent or weak in normal pancreatic cells, making αvβ6 a promising therapeutic target. A20FMDV2 conjugated to tesirine, a toxic DNA-binding pyrrolobenzodiazepine, was selectively delivered to αvβ6 expressing cells of PDAC xenografts in mice [Citation32]. The results showed effective and significant suppression of growth rates of established tumors. In fact, in some experiments the A20FMDV2-drug conjugate eliminated established xenografts completely and therefore significantly extended the lifespan of the mice [Citation32].
Some groups have attempted to modify A20FMDV2 in order to improve its in vivo biodistribution and tumor targeting abilities. The Sutcliffe group pegylated A20FMDV2 and reported that a bi-pegylated variant demonstrated an increased affinity for αvβ6 in vitro and an improved clearance of nonspecifically bound peptide [Citation33]. In separate studies the Brimble team replaced key residues in the A20FMDV2 sequence to improve its stability in serum and reported that an N-terminal d-Asn or C-terminal d-Thr resulted in peptides with improved relative activity and better serum stability [Citation34]. Thus while the naturally occurring A20FMDV2 sequence has developed over millennia a remarkable specificity for αvβ6, allowing viruses to infect mammalian cells, for pre-clinical and clinical use we can still provide valuable modifications (see for examples).
Table 1. Chemical modifications of A20FMDV2 to improve its biodistribution
3.2. Oncolytic viruses
The idea of using viruses to selectively kill cancer cells has existed for decades, however in recent years there has been a surge of interest in re-targeting viral tropism to selectively target neoplastic cells. The purpose of an oncolytic virus is to selectively replicate only in cancer cells leading to cell death [Citation36]. This also leads to a secondary response as the cells burst and the virus is released in to the tumor microenvironment, triggering an immune response. Adenoviruses are a well characterized family of viruses which are excellent candidates for use as an oncolytic virus as they are easily manipulated and genetically modified [Citation36]. summarizes the Ad5 variants that have incorporated A20FMDV2 to target αvβ6 as a component of their design.
Table 2. Oncolytic viruses incorporating A20FMDV2
The biology of Ad5 infection is well understood. The principal binding receptor for adenovirus is the Coxsackie and Adenovirus Receptor (CAR) which is widely expressed [Citation38]. Subsequent internalization requires the target cells to express integrins αvβ3 or αvβ5 which bind to the RGD motif in the penton base of the adenovirus [Citation39]. Major issues with tumor-targeting using Ad5 viruses include that erythrocytes express CAR and the complement receptor 1 which also binds to Ad5 [Citation40] leading to their clearance by the liver and resulting in hepatic toxicity. In addition, an inflammatory cytokine response also limits the dose of virus administered.
Thus, potentially therapeutic Ad5 viruses are commonly genetically manipulated to minimize interaction of the virus with normal cells via CAR. Following identification of the regions responsible for CAR recognition on the fiber proteins of the viral capsid [Citation40], they have subsequently been mutated to decrease Ad5 – CAR binding. For example; the S408E-P409A mutation, known as KO1, causes a reduction in transduction efficiency [Citation31]. Additionally, a deletion in the FG loop of the knob domain (residues 489 to 492, known as TAYT), significantly reduced Ad5 – CAR binding [Citation41].
To further direct the binding specificity of Ad5 viruses several groups have genetically modified the fiber-knob to incorporate peptide motifs that should change the tropism of Ad5 [Citation42–44]. Coughlan et al. (2009) modified the fiber-knob of an oncolytic Adenovirus (Ad) 5-EGFP by incorporating A20FMDV2, generating the Ad5-EGFP-A20 to target αvβ6-expressing cancers [Citation44]. Studies in vitro showed a 50-fold CAR-independent increase in transduction of αvβ6 positive cells, and up to a 480-fold increase in cytotoxicity, compared with the wild-type virus (Ad5-EGFP-WT). In vivo, uptake of the Ad5-EGFP-A20 virus by the liver was reduced five-fold and selective retention in αvβ6-positive xenografts was increased two-fold.
In subsequent studies Coughlan and colleagues (2012) modified the Ad5-EGFP-A20 by incorporating a TAYT mutation to reduce interaction of the virus with the CAR [Citation45], creating Ad5-477dlTAYT(A20). After intravenous injection in experimental animals, the improved virus design eliminated erythrocyte coagulation and exhibited both reduced cytokine response and toxicity, allowing for an increased dose of the virus to be used in vivo. However, despite the increased dose, the virus failed to show improved tumor therapy, thought likely to be due to the limited permeation of the tumor mass by the virions.
Due to the dose-limiting side effects of Ad5 viruses, A20FMDV2 was subsequently incorporated into the fiber knob of Ad48, a rare and poorly characterized adenovirus [Citation46]. This was used to create a virus pseudotype; an Ad5 virus with Ad48-A20 fiber knob proteins (Ad5/kn48.DG.A20) [Citation47]. Compared with the parent vector, a new Ad5.KO1.HI.A20 variant, and the Ad5/kn48.DG.A20 showed increased transduction of αvβ6 positive BT-20 breast cancer cells (270 and 180 fold, respectively). Similarly, increased transduction was also observed using primary epithelial ovarian cancer (EOC) cells, which provides a good model for in vitro virotherapy (70-old for Ad5.KO1.HI.A20, and 60-fold using Ad5/kn48.DG.A20),
Over the years, Ad5 has been subject to much manipulation in order to make it more suitable for use as an oncolytic virus. For example, the AdΔΔ virus was successfully genetically modified to improve potency and selectivity in cancer versus normal cells [Citation48]. Using the AdΔΔ virus, Man et al. (2018) incorporated A20FMDV2 into the fiber-knob and introduced the TAYT mutation, creating Ad5-3Δ-A20T [Citation49].
Using pancreatic cancer cells, Ad5-3Δ-A20T showed selectivity for αvβ6 positive cells, and interestingly also demonstrated infection of the stellate cell population, a non-epithelial, αvβ6-negative cell type, that are the principal pancreatic mesenchymal cells. Using 3D cocultures of αvβ6 pancreatic cancer cells and pancreatic stromal cells, Ad5-3Δ-A20T inhibited cell growth and invasion at lower doses than its AdΔΔ precursor. Furthermore, using pancreatic cancer xenografts in mice, tumor progression was significantly reduced using either adenoviral treatments, from 11 days in the untreated animals to a median of 42 days in the animals treated with Ad5-3Δ-A20T. Ad5-3Δ-A20T was subsequently radiolabelled with Iodine-125 to determine the biodistribution of the virus [Citation50]. They demonstrated that uptake of the virus was rapid, with Ad5-3Δ-A20T replication in tumor cells significantly higher than the wild-type virus 48 hours post-administration in tumor cells. A summary of each of the αvβ6 targeting oncolytic viruses using A20FMDV2 can be found in .
3.3. Chimeric antigen receptor T-cells (CAR T-cells)
CAR T-cells are a type of immunotherapy where host T cells are removed from patients and genetically engineered to express a chimeric antigen receptor directed against a specific antigen, expressed on their cancer. The immunotherapy has shown favorable results in treating hematological malignancies [Citation51,Citation52], however a CAR T-cell therapy for the treatment of solid tumors has yet to be approved for clinical use. Given the contrast in αvβ6 expression in normal and malignant cells, it is an appealing candidate for a CAR T-cell-based therapy.
The first CAR targeting αvβ6 was engineered using A20FMDV2 [Citation53]. Using PDAC, breast and ovarian xenograft models expressing αvβ6, the αvβ6-targeting CAR caused tumor xenograft regression, extending survival of mice from 63 days in the control group to 82 days in the αvβ6-targeting group. One of the main challenges in using CAR T-cells against solid tumors is ensuring they reach and survive in the tumor microenvironment (TME). Thus, a second study by the Maher group sought to improve the therapeutic activity of the first A20FMDV2-CAR [Citation54].
For this, they engineered the A20FMDV2-CAR to co-express an IL-8 receptor; cysteine-amino acid-cysteine receptor (CXCR). As IL-8 is overexpressed in the TME, it was hypothesized that expression of these chemokine receptors on the CAR T-cells would improve their migration into the TME. In vitro migration assays showed that the A20FMDV2-CXCR-CAR increased the number of T cells migrating toward IL-8 from 8% using A20FMDV2-CAR to 30%. These CARs had a more favorable toxicity profile and superior anti-tumor activity; as tumor burden was significantly lower in mice treated with the A20FMDV2-CXCR2-CAR compared to the original A20FMDV2-CAR. To date no clinical studies with CAR T-cells have been undertaken where αvβ6 is the target.
3.4. Modifying antibody tropism
Antibody-based cancer therapies are becoming increasingly available and offer huge potential for the basis of new therapies. One method of engineering recombinant antibodies is to insert a target-specific sequence in to a single-chain Fv (scFv) antibody fragment. One example is modification of MFE-23, a well-studied scFv which binds to Carcino-Embryonic Antigen (CEA) and which has proven to be successful in clinical trials [Citation55,Citation56]. Using MFE-23 as a scaffold, a 17-mer derivative of A20FMDV2 was inserted into the CDR3 variable-heavy chain loop at a position predicted through structural mapping of MFE-23 to be at the surface of the molecule [Citation57]. The variant antibody bound specifically to αvβ6 cells and was able to block its biological activity. However, as the antibody was of murine origin, the authors wished to create a human variant for potential therapeutic use. By using the previously established humanized shMFE, the peptide was again inserted in to the same heavy chain loop. The resulting scFv antibody proved to be a better inhibitor of αvβ6-dependent cell binding than the mouse scFv version, and that of the free A20FMDV2 peptide, the latter in part likely to be due to the bivalent nature of antibodies.
The importance of αvβ6 as a potential therapeutic target led to the development of a novel structurally designed scFv human phage antibody library based on A20FMDV2. The original NMR studies of A20FMDV2 [Citation18] indicated which elements of the A20FMDV2 sequence and structure were critical for the affinity of the peptide for αvβ6. Thus, Man et al. [Citation58] designed a DNA sequence that encoded a 30mer amino-acid sequence that retained all the essential residues of A20FMDV2, replaced non-essential residues with random residues and flanked the resulting sequence with residues from other proteins known to be strong N- and C-terminal helix-capping (stabilizing) sequences. The scFv library was generated by inserting this 90bp fragment into the CDR3 loop of the variable heavy region and screened on recombinant αvβ6 and αvβ6-expressing cell lines. Numerous scFv antibodies were identified that were αvβ6-specific and most inhibited αvβ6-dependent functions to a greater or lesser degree. Peptides from the CDR3 regions of the two lead scFv, D25 and D34, were synthesized, shown to be αvβ6-specific, function-inhibiting peptides. When these peptides were analyzed by NMR, the 3D structure again showed a pronounced α-helix adjacent to the RGD site in both αvβ6-specific peptides [Citation58].
4. Summary
A20FMDV2 was identified and characterized as an αvβ6-specific peptide with very high affinity for this integrin. In a comprehensive analysis of integrin-targeted peptides using a single ELISA methodology Kapp et al., (2017) [Citation59] recently compared A20FMDV2 to many αvβ6-specific peptides. Their data independently confirmed that A20FMDV2 was highly specific for αvβ6 and possessed an IC50 of less than 1 nM, far better than most of the tested peptides and an extremely promising starting point as an imaging and therapeutic agent. Thus, since its discovery in 2007 multiple groups have used A20FMDV2 to develop or modify their imaging and therapeutic agents to target αvβ6 in fibrosis and cancer. Following on from the numerous pre-clinical murine studies showing effectiveness of αvβ6-targeted peptide radioimaging [Citation6,Citation33,Citation60] imaging of αvβ6 in humans has now become a reality [Citation21–24]. Even more exciting, is that now peptide-drug therapy given into the circulation has been shown to also be effective, peptide-drug targeting of αvβ6 could provide a novel and effective additional component of therapy for up to one-third of all solid cancers. However, it should be noted that 3 of 15 patients included in a GSK imaging study of [18F]FB-A20FMDV2 had low, but detectable titers of antibodies to A20FMDV2 before imaging took place, suggesting pre-exposure to the serotype of FMDV virus from which A20FMDV2 is derived [Citation22]. Encouragingly, the antibody titers remained low even after imaging. While these patients did not exhibit adverse effects to the tracer, we cannot exclude that such antibodies may result in resistance to A20FMDV2-targeted therapy.
5. Expert opinion
The integrin αvβ6 must be considered one of the most promising therapeutic targets for fibrosis and cancer that has been identified in the last several decades. Expressed at low or undetectable levels (by immunochemistry) on most tissues, αvβ6 is upregulated on multiple different cancers and several types of fibrosis, and provides us with a window of opportunity for selective delivery of effective therapies for these life threatening conditions where formerly few, if any, existed. Even though the evidence that expression of integrin αvβ6 appeared to be a prognostic marker in cancer and fibrosis has been available for over two decades there have been relatively few clinical trials that have investigated targeting this integrin. In 2014 Merck published the results of the POSEIDON trial [NCT01008475] a Phase I/II trial in colon cancer patients where the pan-αv abituzumab was added in combination with standard of care (cetuximab and irinotecan) [Citation61]. The primary endpoint for this trial of progression-free survival was not met and this treatment strategy was not developed further. However, a retrospective subgroup analysis discovered that patients with high expression of αvβ6 on their left-sided tumors responded well to the addition of Abituzumab to standard of care with improved PFS and OS compared with those tumors of low αvβ6 expression or right-sided tumors (6.4 vs 4.2 months and 25 vs 10 months respectively). As the correlation between high αvβ6 expression on colon cancer and poor OS was published in 2005 it is perhaps surprising that the POSEIDON trial did not stratify their patients for therapy, as the primary endpoint may have been achieved. Biogen-Idec produced the αvβ6-blocking humanized antibody STX-100 and have tested it for treatment of Idiopathic Pulmonary Fibrosis [NCT03573505). Unfortunately it was stopped in 2019 for safety reasons, details of which remain unclear. This limited number of studies just highlights the need for more clinical trials and deeper analysis to select which patient groups are most likely to respond positively to αvβ6-targeted therapies.
The use of antibodies for therapy can be hugely successful but they are also enormously expensive to produce in bulk to GMP standards. By comparison, small molecules and peptides are relatively cheap to make compared with specific antibodies, and can offer effective alternatives for therapy [Citation62,Citation63]. Additionally their small size can allow deeper penetration of tumors than 150kDa-sized immunoglobulins [Citation64]. Small molecule inhibitors of receptor tyrosine kinases are well-established therapeutics but recently biotechs (e.g. Pliant [https://pliantrx.com/]and Morphic [https://morphictx.com/]) have invested heavily in small molecule inhibitors of the ligand-binding site of αv integrins.
By comparison few companies are developing therapeutic peptides. Their relatively poor pharmacokinetics (i.e. their small size means they are excreted rapidly), their potential for immune rejection or susceptibility to serum proteases offers several likely reasons why many biotechs have not invested in developing peptides for therapy. But peptides can be modified to improve their pharmacokinetics and serum stability, may be small and unstructured and thus not necessarily immunogenic. They therefore offer enormous potential in illuminating the science underlying diseases. This is particularly true of A20FMDV2 and some of its variants when considering its success in locating and being retained by αvβ6-expressing tissues in humans [Citation21–23] and its success in pre-clinical therapy studies [Citation32].
Most interactions of cells with their environment, be it with extracellular matrix, other cells or pathogens are usually through protein-protein interactions. This means for every receptor there is a definable amino-acid sequence arranged in a specific stereochemistry that provides a specific binding site on a ligand. A20FMDV2 is special example of one of these defined amino-acid sequences in that within just 20 amino-acids it provides two separate receptor-binding sites (RGD and hydrophobic LXXL/I sites) and a shape stabilizing α-helix. Its ability as an unstructured linear peptide to assume a 3D shape when bound to αvβ6 that closely matches the FMDV virus protein from which it was derived is critical in its specificity and activity. Its remarkable activity (<1 nM) and specificity for αvβ6 are rare among peptide inhibitors of integrins and almost certainly unique as a linear integrin-inhibiting peptide.
What can we learn from this? Firstly that evolution can generate patterns in our proteins that we can exploit scientifically and potentially therapeutically. Secondly that there must be other discrete peptide sequences that serve as ligands to therapeutically important receptors that could be developed as drugs. Additionally, the knowledge that two binding sites enhances the activity of A20FMDV2 should suggest that suitable chemical linkage of two weak inhibitors to a target, together could be sufficient to generate an effective inhibitor.
The future will see the continued use of A20FMDV2 and its variants for pre-clinical and clinical imaging and therapy. More importantly, the clinical imaging data for A20FMDV2 suggest strongly that its capacity to mimic its parent virus and be internalized by cells in an αvβ6-specific manner is retained in humans. This bodes well for development of multiple different A20FMDV2-based drugs carrying different therapeutic payloads designed to kill specific cancers. As with all drugs we must consider whether we can predict the source of potential resistance. We have already mentioned that some people have been identified who possessed antibodies to A20FMDV2 [Citation22] and others have identified that the integrin αvβ6 on cancer cell-derived extracellular vesicles [Citation65,Citation66]. So when targeting αvβ6 on cancer cells with A20FMDV2-conjugates, it is possible that some of the drugs will be adsorbed by αvβ6-expressing exosomes.
Article highlights
A20FMDV2 is a 20mer peptide derived from the foot and mouth disease virus, and has high affinity and selectivity for the integrin αvβ6 due to the presence of a post-RGD domain α-helix
A20FMDV2 can be utilized as a means of molecular-specific drug delivery by decorating the surface of drug-loaded liposomes or by directly conjugating to cytotoxic compounds
Adenoviruses can be genetically modified to express A20FMDV2, thus increasing delivery of the virus to αvβ6-expressing cancer cells.
CAR T-cells have been developed that express A20FMDV2 in their CAR to target αvβ6-expressing cancer cells, including a CAR T-cell with A20FMDV2/IL-8 receptor co-expression in order to improve delivery to the tumor microenvironment.
Inserting the A20FMDV2 sequence in to a single-chain Fv antibody fragment, the variant antibody selectively bound to αvβ6 at its ligand-binding site, thus giving the scFv the ability to block αvβ6 activity.
This box summarizes key points contained in the article.
Declaration of interest
A Meecham is supported by a DTP 2017 LIDO Studentship from the BBSRC while JF Marshall is supported by the BBRSC Industrial CASE scheme, Cancer Research UK, Pancreatic Cancer UK, and the Pancreatic Cancer Research Fund. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
We thank Dr Mark Howard (University of Leeds) for generating the STD-NMR data that defined the 3D structure of integrin-bound A20FMDV2.
Additional information
Funding
References
- Hynes RO. Integrins: a family of cell surface receptors. Cell. 1987;48(4):549–554.
- Breuss JM, Gallo J, DeLisser HM, et al. Expression of the beta 6 integrin subunit in development, neoplasia and tissue repair suggests a role in epithelial remodeling. J Cell Sci. 1995 Jun;108(Pt 6):2241–2251.
- Saini G, Porte J, Weinreb PH, et al. αvβ6 integrin may be a potential prognostic biomarker in interstitial lung disease. Eur Respir J. 2015;46(2):486–494.
- Niu J, Li Z. The roles of integrin alphavbeta6 in cancer. Cancer Lett. 2017 Sep 10;403:128–137.
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002 Sep 20;110(6):673–687.
- Saha A, Ellison D, Thomas GJ, et al. High-resolution in vivo imaging of breast cancer by targeting the pro-invasive integrin alphavbeta6. J Pathol. 2010 Sep;222(1):52–63.
- Acharya R, Fry E, Stuart D, et al. The three-dimensional structure of foot-and-mouth disease virus at 2.9 a resolution. Nature. 1989;337(6209):709–716.
- Logan D, Abu-Ghazaleh R, Blakemore W, et al. Structure of a major immunogenic site on foot-and-mouth disease virus. Nature. 1993;362(6420):566–568.
- Fox G, Parry NR, Barnett PV, et al. The cell attachment site on foot-and-mouth disease virus includes the amino acid sequence RGD (arginine-glycine-aspartic acid). J Gen Virol. 1989;70(Pt 3):625–637.
- Liebermann H, Dölling R, Schmidt D, et al. RGD-containing peptides of VP1 of foot-and-mouth disease virus (FMDV) prevent virus infection in vitro. Acta Virol. 1991;35(1):90–93.
- Ruoslahti E. RGD and other recognition sequences for integrins. Annu Rev Cell Dev Biol. 1996;12(1):697–715.
- Berinstein A, Roivainen M, Hovi T, et al. Antibodies to the vitronectin receptor (integrin alpha v beta 3) inhibit binding and infection of foot-and-mouth disease virus to cultured cells. J Virol. 1995;69(4):2664–2666.
- Kraft S, Diefenbach B, Mehta R, et al. Definition of an unexpected ligand recognition motif for alphav beta6 integrin. J Biol Chem. 1999;274(4):1979–1985.
- Mateu MG, Valero ML, Andreu D, et al. Systematic replacement of amino acid residues within an arg-gly-asp-containing loop of foot-and-mouth disease virus and effect on cell recognition. J Biol Chem. 1996;271(22):12814–12819.
- Jackson T, Sheppard D, Denyer M, et al. The epithelial integrin alphavbeta6 is a receptor for foot-and-mouth disease virus. J Virol. 2000 Jun;74(11):4949–4956.
- Pierschbacher MD, Ruoslahti E. Influence of stereochemistry of the sequence Arg-Gly-Asp-Xaa on binding specificity in cell adhesion. J Biol Chem. 1987;262(36):17294–17298.
- Majken D, Richardson J, Vukelić B, et al. The disulfide bond of an RGD4C motif inserted within the Hi loop of the adenovirus type 5 fiber protein is critical for retargeting to αv -integrins. J Gene Med. 2012;14(12):788-797.
- DiCara D, Rapisarda C, Sutcliffe JL, et al. Structure-function analysis of Arg-Gly-Asp helix motifs in alpha v beta 6 integrin ligands. J Biol Chem. 2007 Mar 30;282(13):9657–9665.
- Hausner SH, Abbey CK, Bold RJ, et al. Targeted in vivo imaging of integrin αvβ6 with an improved radiotracer and its relevance in a pancreatic tumor model. Cancer Res. 2009 Jul 15;69(14):5843–5850.
- Hausner SH, Bauer N, Hu LY, et al. The effect of bi-terminal PEGylation of an integrin αvβ6–targeted 18f peptide on pharmacokinetics and tumor uptake. J Nucl Med. 2015 May;56(5):784–790.
- Hausner SH, Bold RJ, Cheuy LY, et al. Preclinical development and first-in-human imaging of the integrin αvβ6 with [18F]αvβ6-binding peptide in metastatic carcinoma. Clin Cancer Res. 2019 Feb 15;25(4):1206–1215.
- Lukey PT, Coello C, Gunn R, et al. Clinical quantification of the integrin αvβ6 by [18F]FB-A20FMDV2 positron emission tomography in healthy and fibrotic human lung (PETAL study). Eur J Nucl Med Mol Imaging. 2020;47(4):967–979.
- Maher TM, Simpson JK, Porter JC, et al. A positron emission tomography imaging study to confirm target engagement in the lungs of patients with idiopathic pulmonary fibrosis following a single dose of a novel inhaled αvβ6 integrin inhibitor. Respir Res. 2020;21(1). 10.1186/s12931-020-01339-7.
- Saleem A, Helo Y, Win Z, et al. Integrin αvβ6 positron emission tomography imaging in lung cancer patients treated with pulmonary radiation therapy. Int J Radiat Oncol Biol Phys. 2020;107(2):370–376.
- Allen TM, Cullis PR. Liposomal drug delivery systems: from concept to clinical applications. Adv Drug Deliv Rev. 2013;65(1):36–48.
- Parente-Pereira AC, Shmeeda H, Whilding LM, et al. Adoptive immunotherapy of epithelial ovarian cancer with Vγ9Vδ2 T cells, potentiated by liposomal alendronic acid. J Iimmunol. 2014;193(11):5557–5566.
- Hodgins NO, Al-Jamal WT, Wang JTW, et al. Investigating in vitro and in vivo αvβ6 integrin receptor-targeting liposomal alendronate for combinatory γδ T cell immunotherapy. J Control Release. 2017;256:141–152.
- Thomas JK, Kim MS, Balakrishnan L, et al. Pancreatic cancer database: an integrative resource for pancreatic cancer. Cancer Biol Ther. 2014;15(8). 10.4161/cbt.29188.
- UK CR. Pancreatic cancer mortality statistics; 2015. [cited 2020 Sep]. Available from: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/pancreatic-cancer/mortality
- Reader CS, Vallath S, Steele CW, et al. The integrin αvβ6 drives pancreatic cancer through diverse mechanisms and represents an effective target for therapy. J Pathol. 2019;249(3):332–342.
- Steiger K, Schlitter AM, Weichert W, et al. Perspective of α v β6-integrin imaging for clinical management of pancreatic carcinoma and its precursor lesions. Mol Imaging. 2017;16:153601211770938.
- Moore KM, Desai A, Delgado Bde L, et al. Integrin αvβ6-specific therapy for pancreatic cancer developed from foot-and-mouth-disease virus. Theranostics. 2020;10(7):2930–2942.
- Hausner SH, Kukis DL, Gagnon MKJ, et al. Evaluation of [64Cu]Cu-DOTA and [64Cu]Cu-CB-TE2A chelates for targeted positron emission tomography with an αvβ6-specific peptide. Mol Imaging. 2009 Mar-Apr;8(2):111–121.
- Hung KY, Harris PWR, Desai A, et al. Structure-activity relationship study of the tumour-targeting peptide A20FMDV2 via modification of Lys16, Leu13, and N- and/or C-terminal functionality. Eur J Med Chem. 2017;136:154–164.
- Hausner SH, Carpenter RD, Bauer N, et al. Evaluation of an integrin αvβ6-specific peptide labeled with [18F]fluorine by copper-free, strain-promoted click chemistry. Nucl Med Biol. 2013;40(2):233–239.
- Baker AT, Aguirre-Hernández C, Halldén G, et al. Designer oncolytic adenovirus: coming of age. Cancers (Basel). 2018;10(6):201.
- Roelvink PW, MiLee G, Einfeld DA, et al. Identification of a conserved receptor-binding site on the fiber proteins of CAR-recognizing adenoviridae. Science (New York, NY). 1999;286(5444):1568–1571.
- Uhlén M, Fagerberg L, Hallström BM, et al. Proteomics. Tissue-based map of the human proteome. Science (New York, NY). 2015;347(6220):1260419.
- Wickham TJ, Filardo EJ, Cheresh DA, et al. Integrin alpha v beta 5 selectively promotes adenovirus mediated cell membrane permeabilization. J Cell Biol. 1994;127(1):257–264.
- Carlisle R, Di Y, Cerny A, et al. Human erythrocytes bind and inactivate type 5 adenovirus by presenting Coxsackie virus-adenovirus receptor and complement receptor 1. Blood. 2009;113(9):1909–1918.
- Shayakhmetov DM, Gaggar A, Ni S, et al. Adenovirus binding to blood factors results in liver cell infection and hepatotoxicity. J Virol. 2005;79(12):7478–7491.
- Dmitriev I, Krasnykh V, Miller CR, et al. An adenovirus vector with genetically modified fibers demonstrates expanded tropism via utilization of a coxsackievirus and adenovirus receptor-independent cell entry mechanism. J Virol. 1998;72(12):9706–9713.
- Kurachi S, Tashiro K, Sakurai F, et al. Fiber-modified adenovirus vectors containing the TAT peptide derived from HIV-1 in the fiber knob have efficient gene transfer activity. Gene Ther. 2007;14(15):1160–1165.
- Coughlan L, Vallath S, Saha A, et al. In vivo retargeting of adenovirus type 5 to alphavbeta6 integrin results in reduced hepatotoxicity and improved tumor uptake following systemic delivery. J Virol. 2009 Jul;83(13):6416–6428.
- Coughlan L, Vallath S, Gros A, et al. Combined fiber modifications both to target αvβ6 and detarget the coxsackievirus–adenovirus receptor improve virus toxicity profiles in vivo but fail to improve antitumoral efficacy relative to adenovirus serotype 5. Hum Gene Ther. 2012;23(9):960–979.
- Coughlan L, Uusi-Kerttula H, Ma J, et al. Retargeting adenovirus serotype 48 fiber knob domain by peptide incorporation. Hum Gene Ther. 2014;25(4):385–394.
- Uusi-Kerttula H, Coughlan JD, Coughlan L. Pseudotyped αvβ6 integrin-targeted adenovirus vectors for ovarian cancer therapies. Oncotarget. 2016;7(19):27926–27937.
- Oberg D, Yanover E, Adam V, et al. Improved potency and selectivity of an oncolytic E1ACR2 and E1B19K deleted adenoviral mutant in prostate and pancreatic cancers. Clin Cancer Res off J Am Assoc Cancer Res. 2010;16(2):541–553.
- Man YKS, Davies JA, Coughlan L, et al. The novel oncolytic adenoviral mutant Ad5-3Δ-A20T retargeted to αvβ6 integrins efficiently eliminates pancreatic cancer cells. Mol Cancer Ther. 2018;17(2):575–587.
- Stella MYK, Foster J, Carapuça E, et al. Systemic delivery and SPECT/CT in vivo imaging of 125 I-labelled oncolytic adenoviral mutants in models of pancreatic cancer. Sci Rep. 2019;9(1):1-12.
- Schuster SJ, Svoboda J, Chong EA, et al. Chimeric antigen receptor T cells in refractory B-cell lymphomas. N Engl J Med. 2017;377(26):2545–2554.
- Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377(26):2531–2544.
- Whilding LM, Parente-Pereira AC, Zabinski T, et al. Targeting of aberrant αvβ6 integrin expression in solid tumors using chimeric antigen receptor-engineered T cells. Mol Ther. 2017;25(1):259–273.
- Whilding LM, Halim L, Draper B, et al. CAR T-cells targeting the integrin αvβ6 and co-expressing the chemokine receptor CXCR2 demonstrate enhanced homing and efficacy against several solid malignancies. Cancers (Basel). 2019;11(5):674.
- Boehm MK, Corper AL, Wan T, et al. Crystal structure of the anti-(carcinoembryonic antigen) single-chain fv antibody MFE-23 and a model for antigen binding based on intermolecular contacts. Biochem J. 2000;346(2):519-528.
- Chester KA, Mayer A, Bhatia J, et al. Recombinant anti-carcinoembryonic antigen antibodies for targeting cancer. Cancer Chemother Pharmacol. 2000;46(Suppl):S8-S12.
- Kogelberg H, Tolner B, Thomas GJ, et al. Engineering a single chain fv antibody to αvβ6 integrin using the specificity-determining loop of a foot-and-mouth disease virus. J Mol Biol. 2008 Oct 3;382(2):385–401.
- Man YKS, DiCara D, Chan N, et al. Structural guided scaffold phage display libraries as a source of bio-therapeutics. PLoS One. 2013;8(8):e70452.
- Kapp TG, Rechenmacher F, Neubauer S, et al. A comprehensive evaluation of the activity and selectivity profile of ligands for RGD-binding integrins. Sci Rep. 2017;7(1). 10.1038/srep39805.
- Hausner SH, DiCara D, Marik J, et al. Use of a peptide derived from foot-and-mouth disease virus for the noninvasive imaging of human cancer: generation and evaluation of 4-[18F]fluorobenzoyl A20FMDV2 for in vivo imaging of integrin alphavbeta6 expression with positron emission tomography. Cancer Res. 2007 Aug 15;67(16):7833–7840.
- Élez E, Kocáková I, Höhler T, et al. Abituzumab combined with cetuximab plus irinotecan versus cetuximab plus irinotecan alone for patients with KRAS wild-type metastatic colorectal cancer: the randomised phase I/II POSEIDON trial. Ann Oncol. 2015;26(1):132–140.
- Imai KTA, Takaoka A. Comparing antibody and small-molecule therapies for cancer. Nat Rev Cancer. 2006;6(9):714–727.
- Lavanya V, Adil M, Ahmed N, et al. Small molecule inhibitors as emerging cancer therapeutics. Integr Cancer Sci Ther. 2014;1(3):39–46.
- Scodeller PaA P, Asciutto EK. Targeting tumors using peptides. Molecules. 2020;25(4):808.
- Fedele C, Singh A, Zerlanko BJ, et al. The αvβ6 integrin is transferred intercellularly via exosomes. J Biol Chem. 2015;290(8):4545–4551.
- Lu H, Bowler N, Harshyne LA, et al. Exosomal αvβ6 integrin is required for monocyte M2 polarization in prostate cancer. Matrix Biol. 2018;70:20–35.
