ABSTRACT
Introduction
The high attrition rate during drug development remains a challenge that costs a significant amount of time and money. Improving the probabilities of success during the early stages of radiation medical countermeasure (MCM) development for approval by the United States Food and Drug Administration (US FDA) following the Animal Rule will reduce this burden.
Area covered
This article focuses on new technologies involving various organ-on-chip platforms. Of late, there has been rapid development of these technologies, especially in terms of mimicking both normal and abnormal physiological conditions. Here, we suggest possible applications of these novel systems for the discovery and development of radiation MCMs for the acute radiation syndrome (ARS).
Expert opinion
Each organ-on-a-chip system has its own strengths and shortcomings. As such, the system selected for MCM discovery, development, and regulatory approval should be carefully considered and optimized to the fullest extent in order to augment successful drug testing and the minimization of attrition rates of candidate agents. The recent encouraging progress with organ-on-a-chip technology will likely lead to additional radiation MCMs for ARS. The acceptance of organ-on-a-chip technology may be a promising step toward improving the success rate of pharmaceuticals in MCM development.
1. Introduction
During the last two decades, investigators have strived to reconstruct elemental organs by combining primary cells of organs using promising state-of-the-art technologies, with the goal of exploiting these artificial organ-constructs for drug discovery and development while minimizing the high attrition rate for new drugs during the multi-stepped, complex testing processes required for regulatory approval. With continuous improvements, such devices have evolved in both design and function from simple concepts to practical applications for preclinical drug discovery and development [Citation1–3]. Advances in three-dimensional (3D) cell biology and tissue engineering have yielded critical insights in the building of robust systems and devices that often simulate form and function of human tissues [Citation4–6].
Organs-on-chips are microfluidic devices of optically transparent material with high-resolution imaging capability that can be easily monitored for cellular response in real-time. Recent progress in the area of microsystem techniques and cell culture has resulted in select microdevices that might now be considered functional units of specific organs. Organs-on-chips reconstitute tissue-level functionalities in vitro by recapitulating tissue architecture and the mechanical/chemical microenvironment [Citation3]. These microdevices attempt to mimic the biomechanical forces observed inside the body and serve, in part, by regulating cellular functions and cell outcome. Lately, induced pluripotent stem cells (iPSCs) have been leveraged, via their extraordinary biodevelopmental capacities, to develop in a confined, controlled manner, highly organized cellular complexes of differentially mixed cell systems that resemble distinct tissues of select organs of the body, i.e. ‘organs-on-chips’; thus, allowing for several additional types of organs-on-chip systems not currently possible with cell lines or primary cells. Organs-on-chips attempt to replicate tissue-level responses with human cells, enabling predictions of human responses to drugs under investigation and disease/injury models. The organ-on-a-chip platform has been shown to have critical roles in various preclinical stages of drug development [Citation7]. Since the cost of drug development and regulatory approval is significantly increasing with time due to the limited predictability of 2D monolayer tissue culture and animal models, organ-on-chip technology appears to have great potential for drug discovery and development, and may also reduce the use of experimental animals in such endeavors. Pharmacokinetic and pharmacodynamic analyses are usually conducted in animal models; however, such analysis might be possible by microfluidically operated organs-on-chips [Citation8].
In brief, this technology has enormous potential to advance drug development, as well as in modeling human disease and physiology. There are a large number of reports for various disease models and drug testing using organs-on-chips [Citation7,Citation8]. However, there are only a limited number of reports using organ-on-chip models for radiation biology, particularly for the development of radiation medical countermeasures (MCMs) for acute radiation syndrome (ARS), a disease complex that encompasses a large number of organs [Citation9–11]. Studies of radiation injury within select organ systems often employ various animal models; accordingly, it is expected that organ-on-chip models for a large number of organs will be developed and used supplementally for studying radiation injury and radiation MCM discovery and development in due course of time [Citation12–15].
Nuclear accidents and terrorist activities can cause a devastating impact worldwide [Citation16,Citation17]. During the last few years, several US government agencies have been cognizant of radiological or nuclear threats that could jeopardize public health and national security.
ARS is a condition that is triggered by excessive exposures to unwanted radiation [Citation13,Citation18,Citation19]. ARS includes three sub-syndromes: hematopoietic (H-ARS), gastrointestinal (GI-ARS), and central nervous system or neurovascular system (CNS- or NV-ARS). CNS/NV-ARS is the major cause of death within a few days following exposure to high doses of radiation [Citation12,Citation20–23]. Total- or partial-body radiation exposures of ≥2 Gy are associated with H-ARS and gastrointestinal injury [Citation24]. Ionizing radiation exposure does not discriminate: every organ and system of the body is susceptible to injury following sufficiently intense irradiation. However, the sensitivity of different organs/systems differs and sometimes dramatically so; accordingly, select organs/systems have been investigated more in depth than others for radiation injury and for MCM efficacy. In this regard, the hematopoietic, gastrointestinal, cardiovascular, and central nervous systems have been the principal organ systems evaluated relative to the acute radiation syndrome. Organ systems such as the hematopoietic and gastrointestinal systems owe their high radiosensitivity due to high mitotic indices and associated high self-renewal, especially within progenitorial compartments tasked to produce large numbers of daughters that upon maturation, gain specific and vital functions (e.g. crypt cells of gastrointestinal systems giving rise to maturing epithelial lining cells of the gut villi). In brief, following acute, intense radiation exposures, these vital progenitorial compartments are damaged and disrupted at the base of the cellular ‘supply chain’ of essential functional end cells. By contrast, the cardiovascular and central nervous sub-syndromes generally manifest only following more acute and intense radiation exposures, and are driven largely by endothelial injury and vascular leakage that bring on edematous and immune responses [Citation24–26]. Some organs are well-studied for the delayed effects, such as the lung and kidney. A limited number of organ-on-chip models currently available might be pertinent to the study of ARS and the associated MCM research and development; hence, the focus of these models in this report. To date, the US Food and Drug Administration (US FDA) has approved Neupogen, Neulasta, Leukine, and Nplate for the treatment of H-ARS and these require parenteral administration shortly after radiation exposure (24–48 h) [Citation18–29]. Although there are limited studies using organ-on-a-chip platforms for studying radiation injury and for investigations of radiation MCMs, this is a growing area of research interest and there will undoubtedly be significant progress during the next few years. There are, however, a few initial but elegant reports by a group at Harvard using organ-on-chip platforms for understanding radiation injury and countermeasures [Citation11,Citation27]. We believe that radiation biology will be greatly benefited from the building of specific human organ/tissues in vitro using various organ-on-a-chip platforms.
2. Development of 3D in vitro models: spheroids to microfluidics
2.1. Spheroids
Spheroids are 3D cell culture techniques that are currently being used in high-throughput drug discovery [Citation28]. Spheroid formation of cells in culture depends on the self-aggregation capacity of those cells cultured without having attachment surfaces; as such, cultured spheroids have been shown to be particularly useful for investigating molecular and cellular mechanisms of cell–cell interactions (). Out of various techniques used to form spheroids, the hanging drop culture system is moving toward commercialization for high-throughput drug discovery [Citation29]. Besides hanging drop cultures, spheroid microplates with round, tapered, or v-shaped low attachment bottoms also promote spheroid formation. Another novel procedure to create spheroids is through the use of magnetic levitation; spheres generated through this method have been used successfully as well in high-throughput assays for drug screening, again suggesting that this type of culture system holds promise in the realm of drug discovery [Citation30].
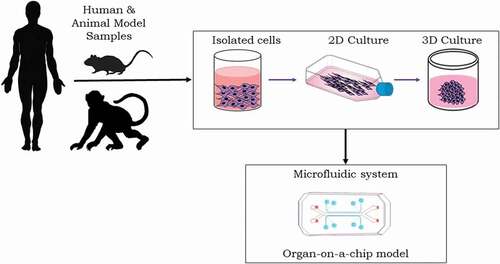
Figure 1. Microfluidic ‘organ-on-a-chip’ platform. Preclinical studies rely on various culture systems (e.g. 2D or 3D in vitro cell cultures), and in vivo animal models play important role for studying radiation injury and MCM development. Such in vitro systems lack the 3D physiological tissue environment. The microfluidic organ-on-a-chip platforms enable controllable cell culture within an organotypic microarchitectural environment.
2.2. Organoids
Organoids are an emerging technology in drug discovery that serve to bridge monolayer cell culture and animal research, and are valuable in vitro cell systems for biomedical use for tissue homeostasis, injury, and disease modeling, drug testing, and regenerative medicine [Citation31]. The 3D tissue constructs appear similar to natural tissue architecture in vivo and composite cellular subtypes, thus mimicking major features of native human tissues and organ systems of experimental animals [Citation32,Citation33]. In many respects, this is a more realistic and cost-effective in vitro model than patient-derived xenograft models in mice. Though organoids can be derived from cell lines, these are generated with embryonic stem cells, iPSC, or with ex vivo propagation of patient cells [Citation34]. With time, organoids generated from iPSC cells are being utilized increasingly for both drug discovery and precision medicine [Citation35]. However, there are also challenges associated with this new technology such as production-complexities, long growth times, and difficulty in producing enough reproducible organoids needed for drug discovery.
2.3. Microfluidics
Microfluidics is a technology that processes and maneuvers microscale fluid systems; i.e. the technology is used to precisely control fluids (10−9 to 10−18 L) with the help of channels the size of microns [Citation36]. Although these channels are extremely small by definition, they have large surface areas that provide for high mass transfers that promote its application in various types of microfluidic technology via its control of small volumes, low use of reagents, fast mixing speeds, and rapid responses [Citation37]. A microfluidic system integrates sample preparation, separation, reactions, detection, and basic operating units (e.g. cell culture, cell lysis, and sorting).
3. ‘Organ-on-a-chip’ systems
Organ-on-a-chip cell culture systems incorporate a wide range of basic biological, chemical, and material science processes and technologies. This technology was selected as one of the ‘Top Ten Emerging Technologies’ in the World Economic Forum [Citation38].
Various organs-on-chips have been used in a large number of laboratories, with the majority of cultured materials being physically supported by a polydimethylsiloxane (PDMS) type plastic that is permeable, biocompatible, flexible, optically clear, and, in general, easy to use. However, there is a critical flaw in that small hydrophobic molecules are absorbed into PDMS leading to the absorption of drugs [Citation3]. As the latter presents a problem for the drug industry, especially in terms of commercialization, it has become necessary to find appropriate substitutes for supporting these culture systems. There are reports to fabricate microfluidic devices utilizing a fluoroelastomer, which is resistant to the absorption of small hydrophobic drugs, and may be a useful platform to build organs-on-chips for commercial use in the pharmaceutical industry [Citation39].
3.1. Single organ-on-a-chip
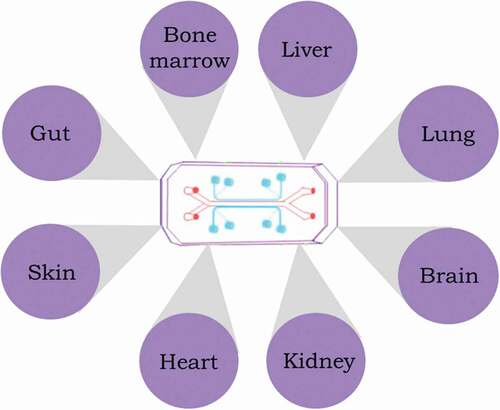
A large number of single-organ chips have been developed for evaluating disease models and drug investigation. Some of these organ-on-chip platforms for single organs are briefly discussed below. A majority of these organ tissues are sensitive to radiation injury and may play an important role in studying radiation injury and for developing radiation countermeasures destined for regulatory approval for human use. Furthermore, it is important to note that the same organ model might be used in different chips for investigating different parameters, e.g. toxicity, physiology, inflammation, etc. ().
Figure 2. Representation of the organ-on-a-chip concept device for studying radiation injury and developing MCMs.
3.1.1. Bone marrow-on-a-chip
Bone marrow is an integral component of the immune system; all immune cell progenitors originate in the bone marrow, and some mature there as well. These immune cells perform their defensive functions by moving into the blood and circulating through the lymphoid organs, including the lymph nodes. Infection or disease affecting the bone marrow can compromise to a significant extent the immune response leading to serious diseases or death. The bone marrow contains hematopoietic as well as non-hematopoietic cells that are enmeshed within a vascularized trabeculated stromal network [Citation40]. Hematopoietic stem cells give rise to blood cells that reside in specific microenvironments in the bone marrow, known as niches. These niches provide stem cells with the regulatory signals needed for their maintenance and for the generation of mature blood cells throughout life [Citation41]. As such, bone marrow tissue is defined by its self-renewing attributes and thus, is very sensitive to radiation injury. Acute radiation injury of the hematopoietic system manifests as a distinct clinical entity that is known as H-ARS. As mentioned above, ionizing radiation exposures of 2 Gy or more generally leads to low blood leukocyte counts, with resulting degrees of immunosuppression and susceptibilities to consequential infections. Further, such exposures may increase the likelihood of not only uncontrolled bleeding and hemorrhage but also bone marrow failure. Without proper treatment, serious illness or death are likely outcomes. Thus, developing the ways and means to effectively protect and mitigate acute radiation injuries of blood forming tissues becomes a critically important clinical issue [Citation19].
Various niches of bone marrow have specific components and functions during hematopoiesis, therefore making it difficult to replicate this microenvironment using simple in vitro systems [Citation42]. Early attempts to recreate hematopoietic tissues in vitro relied on conventional cell culture systems that were planar in nature (two dimensional) and comprised mainly of two cell layers: the under growth of stromal cells and pocketed over growths of hematopoietic cells of various blood cell lineages [Citation43,Citation44]. Although these hematopoietic cultures lack all of the architecture and functionalities of in situ bone marrow, they have the decided advantage of allowing for the direct microscopic analyses and scoring of numbers of the major types of hematopoietic stem cells, lineage-committed progenitors, and early maturing daughters, either under steady-state conditions or following insult with given physiochemical toxicants [Citation45]. Further, it should be noted that the longevity of these cultures are quite variable and dependent largely on the species (e.g. murine, canine, primate, etc.) from which the hematopoietic tissue originated.
Torisawa and colleagues combined in vivo tissue engineering and in vitro microfluidics in the design of a bone marrow-on-a-chip platform in an attempt to replicate more closely the basic architecture and physiology of the hematopoietic system [Citation10]. These researchers demonstrated the ability of the bone marrow-on-a-chip platform to produce both red and white blood cells [Citation11]. This platform, however promising, was only able to maintain cultured murine hematopoietic stem and progenitor cells for a limited time in culture, i.e. approximately 2 weeks. Nevertheless, interesting radiobiological findings were obtained in preliminary studies using this system. For example, when this bone marrow-on-chip platform was exposed to gamma radiation, the production of leukocytes declined. Furthermore, when this chip was treated with two radiation MCMs previously shown to have radioprotective ability (granulocyte-colony stimulating factor or bactericidal/permeability-increasing protein), the hematopoietic stem cells and myeloid cells increased in number. These results were encouraging, suggesting the value of the platform not only for basic investigations of hematopoiesis but also as a screening platform for investigating radiation MCMs for efficacy. In particular, the utility of this novel MCM testing platform has been demonstrated by the positive tests of the radiomitigative efficacy of granulocyte colony-stimulating factor, a well-known and US FDA-approved radiation biologic. Still in another report, further improvements in the development of the bone marrow-on-a-chip system have been described that allow for sustained culture and reproduction of hematopoietic stem and progenitor cells for 28 days in a microfluidic environment with intact bone marrow niche [Citation46]. To show the utility of such a model for drug discovery, Bruce et al. developed a bone marrow-on-a-chip platform to study acute lymphoblastic leukemia and investigated the antimetabolite chemotherapeutic agent, cytarabine [Citation47]. A vascularized human bone marrow-on-a-chip has been shown to support the differentiation and maturation of blood cell lineages and to restore numbers of CD34+ populations after exposures to chemotherapeutic agent, 5-fluorouracil, and gamma-radiation [Citation48]. They were able to maintain human hematopoietic stem and progenitor cell (HSPC) function on chip for 1 month, and also demonstrated human-relevant dose sensitivity to ionizing radiation.
3.1.2. Gut-on-a-chip
The gut-on-a-chip platform is most likely useful for studying the effect of acute, high-dose radiation exposures and for the development of MCMs for related injuries. The GI tract provides a natural barrier to various external environment insults, much like the skin. In this regard, the GI tissue has significant structures that contain immune cells that maintain immune homeostasis for such insults, especially for invading pathogens [Citation49]. Epithelial cells are responsible for physical partition between GI tract lumen and the underlying tissues, while mucous cells maintain a luminal mucosal layer that limits contact between epithelial cells and the microbial content of the GI tract itself. Under the epithelial cells, a vasculature provides a ready source of immunocytes (leukocytes) that respond to various types and degrees of physical/biologic/toxic insults and serve to modulate the subsequent tissue inflammation. Peyer’s patches, which are rich in lymphocytes, are located throughout the ileum and function to monitor gut microbes and to prevent the outgrowth of potentially pathogenic flora. The component of the GI tract’s microbiome is intimately associated with lymphoid tissues of the gut and imparts an immune-regulatory function [Citation50]. These features make the GI tract a vital part of immunity. As such, this platform would provide a much-needed tool for drug discovery and development, especially for MCM drugs that are administered orally. The optimal gut-on-chip platform should include the mucus layer, epithelial barrier, immune cells, microbial interface, peristalsis motion, and must have the capability to transport nutrients through the epithelium. A majority of these attributes have been researched, developed, and folded into existing gut-specific chip platforms [Citation50,Citation51].
Gut-on-chip models developed over the last several years differ in complexity and function. Earlier platforms were simple, and with time these platforms improved gradually and significantly and became increasingly more complex [Citation52–54]. These platforms have proven valuable for investigating basic elements of gut physiology and the associated alterations in response to outside microbial stimuli or drugs. Such gut-on-chip models can be used to evaluate the relationship between immune components and drugs under investigation. In this regard, a human gut-on-a-chip platform with human intestinal epithelial and vascular endothelial cells has been used to study radiation injury in the GI tract and to evaluate the efficacy of radiation countermeasures. The microvascular endothelium is the early target of radiation injury, and endothelial apoptosis is upstream of injury to the intestinal epithelium. It has been shown that radiation-induced epithelial cell injury and DNA damage are mediated via endothelial apoptosis and reactive oxygen species generation, which lead to cytoskeletal changes and structural modifications within the endothelial cells. Gamma radiation exposure to the gut-on-a-chip demonstrated the following measurable, radiobiologically relevant endpoints: generation of reactive oxygen species, DNA fragmentation, cytotoxicity, apoptosis, villus blunting, tight junction disruption, and loss of intestinal barrier integrity [Citation27]. It was shown that pretreatment with dimethyloxaloylglycine (a radioprotector) suppressed expression of all of these endpoints, thus highlighting the potential of this gut-on-a-chip platform to serve as an in vitro model system for studying GI-ARS and for the screening of new and more effective radiation MCMs. As such, chips for various regions of the GI tract are being developed for investigation, e.g. colon intestine-on-a-chip, duodenum intestine-on-a-chip, etc. Microbiome disruption is a key feature of radiation-associated GI injuries. As such, detailed analyses of the disruption provide a unique opportunity for the development and assessment of MCMs [Citation55]. Several studies have demonstrated successful intestine-on-a-chip co-culture with intestinal microbiome utility in dissecting how microbiome metabolites of the microbial flora can modulate intestinal pathology [Citation54,Citation56–59].
3.1.3. Skin-on-a-chip
The skin is the largest organ of the body, and it is also the first line of defense of the human body to protect against invading pathogens. It is essentially an immune organ with components of both the innate and adaptive immune systems with comparable response capacities. Most importantly however, this vital organ serves as a physical barrier to separate the internal organs from the external environment. The skin is made up of two layers: the epidermis with keratinocytes and Langerhans cells, and the dermis with mast cells and dendritic cells T-cells. All of these cells participate in mounting an immune response to microbes. Irradiation of human skin with high doses of ~15–20 Gy or greater results in injuries that manifest initially as erythema, followed by blistering, and finally necrosis. Such necrosis occurs 10–30 days after exposure, depending on the radiation dose [Citation60,Citation61]. These radiation-induced skin pathologies are collectively referred to as the cutaneous radiation syndrome. There is continuing interest in developing new types of biotherapies that make use of different types of skin elements (i.e. stromal cells, mesenchymal stem cells, adipose stromal cells, and progenitor cells) either singly or in combination for the treatment of the cutaneous syndrome [Citation62]. The swine model, e.g. the minipig model, has been used extensively to investigate the cutaneous syndrome. By contrast, studies and data from NHPs for cutaneous effects are limited [Citation19].
Several skin-on-chip models have been developed [Citation63,Citation64] and there are platforms which have included vascularization using endothelial cells. However, few have full-thickness skin with both epidermal and dermal-equivalent components [Citation63–65]. The majority of the vascularized skin-on-chip models usually lack dendritic cells and T-cells, and have only a limited number of immunocompetent cells in general. Unfortunately, these deficiencies undermine the utility of ‘skin-on-chip’ models, in terms of mimicking the basic immunologic function(s) of natural skin [Citation66]. These skin-on-chip models need to be applied and thoroughly tested relative to the acute radiation injuries of skin and subsequently, if proven adequate, applied as an investigative, screening tool for cutaneous ARS-specific MCMs.
3.1.4. Liver-on-a-chip
In the human body, the liver is the second largest organ and it regulates energy use, produces bile, hormones, plasma proteins, and metabolizing xenobiotics. Its high degree of vascular perfusion and its active role in metabolizing xenobiotics emphasizes the need for a liver culture system for drug testing purposes [Citation67]. Simply put, vascularization is an important aspect of hepatocyte function and metabolic waste removal. Despite advances in radiation biology, radiation-induced liver disease has not been investigated well. Radiation-induced liver disease is characterized by anicteric ascites, hepatomegaly, decline in liver function, generalized toxicity, and elevation of liver enzymes [Citation68]. Investigations of the Hiroshima and Nagasaki atomic bombings survivors demonstrated disturbing clinical pictures of the delayed effects of acute, radiation exposures, specifically in terms of the increase in incidences of cancer and fatty livers [Citation69]. Such effects of radiation on the liver, however, are also influenced by lifestyle, such as diet, obesity, and alcohol consumption [Citation69]. Liver-on-a-chip platform can be used in the investigation of the drug’s predictive as well as mechanistic aspects.
Investigators have developed the technology to support a perfused liver-on-a-chip culture system that, in part, simulates normal liver physiology [Citation70]. In one such report, a liver-on-a-chip platform has been developed using bioprinted HepG2/C3A spheroids as a 3-D model [Citation71]. Yet another study reported a similar system to generate a liver-on-a-chip using glass covered PDMS micro-wells connected to a micro-perfusion pump [Citation72]. Additional liver-on-a-chip systems have also been reported and with promising results obtained using similar strategies and different types of cells [Citation73,Citation74]. However, primary human hepatocytes are considered the most physiologically relevant cell type for the liver-on-a-chip technology. Studies have suggested that 3-D structures can improve key functions of the hepatic model compared with 2-D cultures [Citation75,Citation76].
Relative to radiobiological studies and the application of liver-on-chip models, there have been few reported studies, and hence, it is difficult to assess and project their long-term utility. Nevertheless, what has been reported is interesting. In one report, a microfluidic chip with liver tissue was used to confirm liver tissue injury from low-dose radiation of 2 Gy and to evaluate the efficacy of a drug. In addition, the well-recognized radioprotective agent, amifostine, was shown to limit the extent of radiation-associated genomic damage, as assessed by the micronuclei assay [Citation77].
3.1.5. Lung-on-a-chip
Understanding how drugs enter the body and reach the bloodstream is an important aspect of drug development. There are many different drug exposure routes, and one important route is pulmonary inhalation. Initially, lung-on-a-chip platforms were fabricated to recreate a specific function of the lung and to mimic blood flow similar to what occurs in vivo [Citation78]. Such lung-on-a-chip did not simulate breathing processes, and as such, represented a major constraint of the model. Breathing movements in the lung are challenging to try to capture with cultured cells. However, the Wyss Institute (Wyss Institute, Harvard University, Cambridge, MA) has had some success in developing lung-on-a-chip technology that mimics the physiology of a breathing lung [Citation79]. This model consists of a microfluidic system with two microchannels separated by a thin, porous, and flexible PDMS membrane lined with fibronectin or collagen, and this emulates the structural, functional, and mechanical properties of the human alveolar–capillary interface. The breathing lung-on-a-chip model has junctional-like complexes (epithelial and endothelial junctional proteins, occludin, and vascular endothelial cadherin) [Citation79]. The strategy for miniaturizing the lung-on-a-chip model involved the construction of a chip comprising three circuits, each having two compartments separated by a thin, porous PDMS membrane [Citation80]. Such miniaturized organ-on-a-chip technology may be adapted to multi-well formats and could be amenable to high-throughput assays. Lung-on-a-chip models might be helpful in the future for studying the late-arising pathological effects of acute radiation exposure, specifically tissue fibrosis in the lung.
Radiation injury is partly mediated through endothelial injury and coagulopathy [Citation81]. Radiation initiates and also accelerates atherosclerosis, leading to vascular injuries and increased levels of proinflammatory cytokines in the blood of long-term survivors of unwanted radiation exposures; all of which suggests that sufficiently intense irradiation can evoke chronic vascular effects. Accordingly, vascularized organ-on-a-chip models might be well-suited to modeling this aspect (vascular mediators) of ARS and related pathologic states (e.g. coagulation events) [Citation82]. It is important to note that similar to lung-on-a-chip, gut-on-a-chip studies reveal a similar key role of endothelium. In brief, this methodology appears to provide a new approach, a new opportunity, to investigate human pathophysiology of pulmonary thrombosis and advance specific MCM development.
Further, a significant effort has been made within the radiobiological research community to identify and develop tissue-specific biomarkers that are not only predictive of ionizing radiation-associated injuries of various tissues and organ systems of the body, but are useful as well for the efficacy testing of newly identified MCMs. In this regard, investigators recently developed human-derived microvascular microfluidic lumens for identifying biomarkers of irradiation. They detected 35 proteins using mass-spectrometry-based proteomics and suggested that these proteins might serve as early biomarkers of ionizing radiation exposure [Citation83]. This suggestion needs to be validated, however.
3.1.6. Brain-on-a-chip
The brain is of special interest because it has a unique structure and function that differs from other organs. It is an understatement that the brain is a complex organ, as highlighted in part by its different regions with special attributes, the variable ratio of the cells in various regions, and a tissue–tissue interface, i.e. the bloodbrain barrier (BBB) [Citation64,Citation65]. An intrinsic property of the blood vessels, especially those CNS-associated vessels, governs the flow of ions and molecules from the blood and into the CNS parenchyma. The brain has several regions with specific anatomy and functions, and each region of the brain demonstrates cellular and proteomic specificities. Once a drug crosses the BBB, it often exerts different effects on different regions of the brain. In sum, all of these characteristics serve to challenge the drug developer in terms of drug-tissue targeting, efficacy, and safety.
The Wyss Institute has created a multi-regional brain-on-a-chip model [Citation84]. The human brain is thought to reach a mature state after the second decade or so of life; prior to this period, brain cells and associated neural network(s) are constantly reorganizing and making new connections required for learning [Citation85]. The latter neural process(es) certainly continue as the individual ages, albeit at reduced paces. Any drug crossing the BBB and affecting brain maturation may impart unwanted responses, thus, posing a risk to normal brain development. Brain-on-a-chip might be used not only to study a host of both normal and abnormal neural conditions (e.g. neuroinflammatory syndromes) but also for investigating the nature and mechanisms of drug-transport across the BBB.
Unlike the rather simple architecture of the liver, the brain has very complex architecture; hence, the complexity of the brain-on-a-chip model and the need for different variations of these brain tissue types depending on the region of the brain to be studied. For example, the capacity of any given drug under test to cross the BBB might be investigated using a specific BBB-on-a-chip, while the impact of the same drug on various regions of the brain might require other types of chips. The selection of cells is important due to the species differences between human and animal neuronal cells, especially astrocytes [Citation86]. An alternative source of human neuronal cells may be iPSCs since they can be induced to form neurons or astrocytes. Research for various disorders and diseases including narcolepsy, Parkinson’s, Alzheimer’s, and Huntington’s diseases may benefit from such approaches [Citation67].
There are reports regarding the possible relationships between radiation exposure and dementia, which underscores the importance of this technology for radiation MCM research [Citation87,Citation88]. At least in theory, it might be more time and cost effective and ultimately easier to conduct mechanistic experiments using the organ-on-a-chip platform rather than using conventional animal models. Although there are several ongoing studies in various laboratories investigating the mechanism of injury and disease induction as well as the development of drugs for various indications including inflammation and neurodegeneration [Citation89–91], currently, there appears to be an absence of reports using this model to evaluate CNS/NV-ARS using supralethal doses of ionizing radiation.
As mentioned above, the BBB models have proven helpful for studying the capacity of drugs to cross the BBB barrier. Early models of BBB comprise endothelial cells on a porous membrane that functioned as a barrier between the apical (the luminal compartment) and basal (the abluminal compartment) side, allowing for the development of apical/basal polarization. Subsequent models involved the co-culture of endothelial cells with astrocytes where astrocytes increased the expression of tight junction and transporter proteins leading to increased cell–cell interactions as well as trans-endothelial resistance [Citation92]. A co-culture of astrocytes and endothelial cells on a porous hollow fiber and exposed to physiological shear stress demonstrates a barrier function with relative permeability comparable to the in vivo situation. Several models of BBB-on-a-chip have been fabricated using similar approaches, and different chips have been used for investigating drugs and improving the knowledge of mechanisms underlying BBB function [Citation93,Citation94]. Improved BBB models may help to better predict the permeability of newly developed drugs and help in advancing BBB-permeant drugs.
3.1.7. Kidney-on-a-chip
The kidney is a complex organ with a dozen or so cell types and millions of nephrons that perform a number of basic functions that include filtration, reabsorption, and secretion in order to regulate levels of compounds, ions, and water in the blood, as well as to produce erythropoietin for red blood cell production, and to help regulate blood pressure by electrolyte and fluid balance. Relative to the subject matter at hand (radiation injury and treatments), the kidney is a radio-responsive organ and is susceptible to late-arising pathologies [Citation95]. Specifically, these radiation-induced kidney injuries involve dynamic and complex interactions between tubular, glomerular, and interstitial cells. The exact pathogenic mechanisms, as well as mediators responsible for such radiation-induced nephropathies are less than clear, but remain under active investigation.
Suitable renal models are vital to better understand the mechanisms of nephrotoxicity and to decrease adverse side effects from certain pharmaceuticals, such as drug-induced nephrotoxicity in the elderly [Citation96]. Each kidney contains millions of basic functional units known as nephrons, and these nephrons are composed of a renal corpuscle for filtration and a tubule for reabsorption. Each segment of the tubule is responsible for a specific function. Initially, in vitro renal cell polarization was recreated using porous membranes as substrates for cells. The attachment of cells to the membrane induced apical/basal polarization and with time, such kidney models have been significantly improved [Citation97]. All current kidney-on-a-chip models employ a porous membrane for support and an apical compartment for medium circulation. An important function of renal cells is their ability to control the reabsorption and secretion of molecules inside the tubules, and recreating this process in vitro is not a simple matter [Citation98]. One major limitation of the kidney-on-a-chip technology is that they only reproduce a specific region of the nephron, either filtration or reabsorption. A nephron-on-a-chip model would be a promising strategy along with 3-D bioprinting to enable spatially controlled distribution of cells [Citation99,Citation100].
As the kidney is an important target organ to relatively intense, ionizing radiation exposure and is susceptible to delayed or late-arising pathologies (e.g. tissue fibrosis that restricts basic organ functions), these kidney-on-a-chip type models need to be evaluated for applicability and utility as an adjunct radiobiological research tool in studying exposure-induced kidney pathologies.
3.1.8. Heart-on-a-chip
Radiation exposure to the heart has been known to lead to significant late-arising radiation-induced myocardial disease, while intense, high doses causing acute pericarditis. Several forms of radiation-induced heart disease manifest a decade or more after exposure and include atherosclerosis, conduction abnormalities, adverse myocardial remodeling, and injury to cardiac valves [Citation101,Citation102]. Atherosclerotic plaques in arteries, fibrotic by nature, are rich in proteoglycans. As a consequence of these cardiovascular syndromes, long-term follow-up is needed for complete assessments of those radiation-associated injuries.
Heart-on-a-chip models are currently used by investigators in the general area of cardiovascular regenerative medicine, as they have been found to be useful in mimicking cardiac muscle structure and function. Heart muscle development is greatly dependent on cellular organization and myotube alignment. Advances in 3D printing have allowed for increased cellular alignment of microcontacts of cardiac muscle cells to be generated in combination with extracellular matrix glycoproteins. This provided the essential framework for muscular contraction and differentiation of myotubes after electrical stimulation [Citation103]. The thin films of cardiac muscle cells have demonstrated potential for muscle contractility [Citation104]. After seeding of muscle cells on a thermally sensitive polymer, ‘muscular’ thin films can be released by the application of heat to the substrate. These thin films have been produced by spin-coating poly(N-isopropylacrylamide) on a glass substrate covered and patterned with a disposable film. The deflections of these films, seeded with cardiomyocytes coming from cell contractions, can be quantified in response to electrical signals. In contrast to other similar in-plane 2D membrane-based approaches for employing stress to adherent cells in other organ-mimicking systems, this muscular thin-film approach measures out-of-plane deflections [Citation105]. Although cardiovascular injuries induced by acute irradiation have been studied experimentally as well as clinically, there appears to be no comparable radiobiological reports in which a heart on-chip model has been employed.
3.2. Multi-organ-on-a-chip
The single-organ-on-a-chip models focus on imitating an individual organ, while multi-organ chips incorporate components of multiple organs in a single chip, thus enabling more comprehensive examination of the interplay of different organs relative to the physiological impact on the whole body. The human body is an elaborate network of complex interacting biological systems comprising individual organs with each having assigned responsibilities for vital and specific functions. Major benefits of the organ-on-chip technology certainly include, but are not limited to the more natural, in vivo-like, three-dimensional architecture of tissues under study, but are also the extension of the ‘functional life’ of those tissues that are beyond what is normally obtainable under conventional culture conditions. However, the downside of their use, especially when a single organ-on-a-chip is used for early-phase efficacy and toxicity testing of given pharmaceuticals, is when the drug under test targets cells/tissues of multiple organs. This may lead to questionable results and, subsequently undesired clinical outcomes when used when the test drug advances into clinical trials in humans. Therefore, the development of the next generation of multi-organs-on-a-chip is of critical importance for the advancement of this technology. The multi-organs-on-a-chip connects two or more tissues with microchannels that allow for the flow of the culture medium in order to accommodate the growth/maintenance requirements of all cultures concurrently. Under such situation, selecting the suitable medium for all cells may be a challenging task. If one organ culture does not get enough nutrients from the selected medium, the entire assay can underperform. Attempts to improve the nutrient systems and, in turn, the function of these multi-organ-on-chip systems are ongoing in various laboratories and include the use of blood or blood products, or its synthetic surrogates that allow for the growth and maintenance all of the compartmentalized organs on the single chip [Citation106].
There are numerous reports describing two organs-on-chip technology; as the liver plays an important role in drug metabolism, the liver serves as the primary organ while being combined with a second organ of interest relative to drug targeting. TissUse reported a chip connecting liver spheroids (HepaRG) and hepatic stellate cells (human skin), and the maintenance of these cultures for four weeks as demonstrated by glucose consumption and lactate release [Citation107]. A similar platform has been used for NTera-2/cl.D1 cell neurospheres and liver spheroids [Citation108]. The connection between the two tissues was shown to enhance the toxicity of 2,5-hexanedione, and this was as a result of necrotic factors secreted by one tissue affecting the other tissue. Another such example is a two-channel PDMS chip to co-culture intestinal CaCo-2 cells and HepG2 cells [Citation109]. In addition to submerged organ cultures, multi-organs-on-chips have also been developed for air–liquid interface (lung) [Citation110]. To facilitate the co-culture of a submerged tissue with another air-exposed tissue, investigators have designed an open multi-organ-on-a chip model composed of several modules; four chambers maintained as lung cultures and one as a liver compartment. The issue of common media was resolved by taking into consideration the special needs of each tissue [Citation111].
There are several approaches ongoing to miniaturize multi-organ-on-a-chip technologies. InSphero has produced a multi-hanging drop plate suspending spheroids of different tissues in a drop of medium and has achieved a degree of success with both liver and cancer spheroids in examining the effects of cyclophosphamide and various metabolites, respectively [Citation112]. In addition to miniaturization, efforts are also being made to increase the number of organs connected; however, this strategy increases system-complexity. TissUse multi-organ-on-a-chip models are available connecting four tissues: liver, skin, intestine, and kidney. The specific genes expressed by each organ and morphology were conserved, suggesting the possibility of connecting multiple organs in a single system [Citation113]. In another report, investigators developed a platform with several compartments to simultaneously culture cardiac, liver, neuronal, and skeletal cells [Citation114]. These cultures remained viable for 2 weeks and appeared to mimic responses of human organs to different drugs including acetaminophen, atorvastatin, and doxorubicin [Citation114].
Besides the limited organ-on-a-chip platforms (either single or multiple organs) discussed above, a sizable number of organ-on-a-chip models have either been developed or are currently being developed for physiological, toxicological, pathological assessment purposes and applied with the intent to support development of pharmaceutical agents. Some of these platforms include lymph node-on-a-chip, spleen-on-a-chip, lymphatic vessel-on-a-chip, nerve-on-a-chip, inflammation-on-a-chip, immunotherapy-on-a-chip, eye-on-a-chip, adipose-on-a-chip, placenta-on-a-chip, vessel-on-a-chip, and cancer-on-a-chip, etc. [Citation2].
4. Commercialization prospects for organ-on chips
Select pharmaceutical companies have a vested interest in developing more efficient and cost-effective MCM screening and testing systems. The organ-on-a-chip technologies can often emulate human physiology and functionality of specific organ systems and are amendable for disease and injury modeling and for MCM development. The in vitro 2D or 3D culture and various animal models are often less than optimal in terms of efficiency and precision testing. In this regard, animal models are the gold standard for the preclinical studies of pharmaceutical agents under development, but reproducibility of results and accuracy of findings are often undermined by response differences between species, i.e. not only in the responses noted between various animal species/models, but also between humans as well. As a result, ~40% of drugs fail clinical trials after prolonged preclinical investigation in various animal models [Citation1]. The organ-on-chip technology can play an important role during different preclinical stages of MCM development, which may lead to a paradigm shift in pharmaceutical development and personalized medicine.
Once the emerging organ-on-a-chip technology enters into the drug discovery area, a significant market for this technology is anticipated. Although the majority of organ-on-a-chip systems share related technology, the need to upgrade these models and differentiation of products for specific use and uniqueness of these platforms will certainly help the start-ups to succeed in capturing market share. Most of the organ-on-a-chip start-up companies prefer to provide standard and limited organ chips based on demand for preclinical investigation. This rather fixed mind set, however, will need to change in order for the full potential of these promising technologies to be realized. Academic researchers, biotech and pharmaceutical companies, and clinical institutions will all have different needs and uses for this technology. With time, a large number of players will be entering into this field. Mimetas, InSphero, TissUse, CN-Bio Innovations, and Emulate are drawing significant attention based on their products. Emulate has also partnered with Takeda, Merck, Johnson & Johnson, and AstraZeneca, and is working with the US FDA for validation of their chips for evaluating safety and efficacy of pharmaceutical agents for regulatory approval and human use.
Although this technology shows huge potential, there are challenges. One commercialization challenge for the model is limited venture capital investment. Still, several start-ups such as Emulate Inc., InSphero, and Mimetas have received substantial investments [Citation1]. National healthcare system and research organizations can play a vital role in establishing partnerships with academic institutions and companies to support proprietary products and provide funding. The US National Center for Advancing Translational Science has launched a series program for the modeling and nurturing of this technology. Similarly, the National Science Foundation, National Institutes of Health, and the Department of Defense have provided initial seed funding through various mechanisms such as the Small Business Innovation Research (SBIR) and Small Business Technology Transfer (STTR) programs to promote development and commercialization of this technology for use in drug development.
Countries in the Asia-Pacific region (China, Japan, Singapore, and South Korea) appear to be strong emerging markets for organ-on-a-chip technology due to government backing for health care. In 2018, the Chinese Academy of Science initiated a 5-year program called the Organ Reconstruction and Manufacturing program. With steadily growing investment, the technologies required for organ-on-a-chip models are gradually improving and expected to continue to advance, while the cost is expected to shrink. In addition, market demands for organ-on-a-chip products are anticipated to increase significantly and gain recognition by the drug industry.
For developing new ARS MCMs, there has been limited work using these organ-on-a-chip technologies; hence, little has been accomplished. Only two organ models (bone marrow and gut) have been used for studying radiation injuries, and these models are in initial phases of development and validation for ARS MCM-related research and development [Citation3,Citation10,Citation11,Citation27,Citation48]. It will take significant effort and time to bring this technology to the forefront of the ARS MCM area of research. Furthermore, these new, advanced in vitro testing/assessing technologies will need to eventually be folded into the FDA’s regulatory framework governing ARS MCM development following the agency’s Animal Rule [Citation115].
5. Advantages, disadvantages, limitations, challenges, and future prospects
The development during the last few decades of organ-on-a-chip technology has shown its promise as a new technology for drug/MCM discovery and development, and for the better understanding of various disease states/models. At least in theory, the in vitro organ-on-a-chip technologies can better model human systems compared to animal models for determining potential targets of given drugs. Organ chips are specifically valuable for applications that preclude testing in human subjects for initial preclinical safety and efficacy testing of biological MCMs that do not recognize non-human cell targets. The organ-on-a-chip platform is a powerful tool for studying the progression and treatment of cancer, e.g. cell migration and invasion, progression, extracellular signaling, tumor heterogeneity, and biophysical factors in the tumor microenvironment [Citation7,Citation116,Citation117]. Exposure to low doses of radiation and linkages to various types of cancer might be better understood in due course of time using organ-on-chip models [Citation118]. Thus, the organ-on-chip model used to study cancer is relevant from a radiation exposure perspective.
Although the organ-on-a-chip model has much to offer, there are some technical, economical, and social concerns that need to be overcomed before it replaces animal models. Availability of high-quality human cells is usually a challenge, which is getting taken care of by the iPSCs. Additional platforms with advancements in automation, functional ability, integration, manufacturing improvement, and precision medicine have started to emerge and meet the increasing need for improved preclinical MCM development for radiation injury. There are several technical challenges in translating this technology for investigators. Such challenges comprise not only of developing the architectural complexities of both single to multi-organs (also human-on-chip), but also of attaining an appropriate scale of cell number, tissue and organ size, and miniaturization of the system, and developing a common perfusion system for various cells within the same or different organs. The fabrication material for the microfluidic device should not affect cellular response to MCMs under test. There is concern that the PDMS polymer used to fabricate organ chips cannot be used effectively for drug studies owing to drug absorption. PDMS used for the fabrications of a number of organ-on-a-chip models is lipophilic and may bind to molecules in the perfusion medium or MCMs under investigation. Hence, investigators are continuing their efforts to find modifications or alternatives to PDMS that may eliminate or at least reduce such interactions [Citation119]. Over time, this has not turned out to be as major a concern.
While connecting organ-on-chips to microfluidics for perfusion, bubbles may get introduced that disrupt the cell culture. In addition to the challenges linked with the development of organ-on-a-chips, the acceptance of this platform for developing MCMs requires validation before its acceptance by the US FDA or other regulatory agencies [Citation2,Citation120]. The major challenge is a conceptual one: pharmaceutical companies, regulatory agencies and academic investigators have confidence in the way they carry out their work, and they may be hesitant to change their basic assays or work methods. Clear and reproducible results that demonstrate the advantages of organ-on-chips over animal data will be required to convince concerned professionals. Only two organ-on-a-chip models have been utilized to any degree: the bone-marrow-on-a-chip and gut-on-a-chip, which have been used for assessments of acute radiation injury and the evaluation of countermeasures [Citation3,Citation10,Citation11,Citation27,Citation48]. There is a long path forward to get various organ-on-a-chip models properly and fully investigated and validated for the development of MCMs for ARS.
6. Conclusion
The rapid rise in publications and funding from various sources demonstrate great interest in these organ-on-chips technologies across different disciplines of drug development using various, target-specific platforms. Although there are multifaceted challenges for the biochip platforms, the primary one may simply be due to an under appreciation of the strength of these systems in evaluating basic biological problems and processes. The insufficiency of information regarding tissue stability over long periods of time and the results of toxicological evaluation certainly contribute to these blossoming technologies being undervalued [Citation67]. Although an optimal human-on-a-chip (body-on-a-chip) platform with various organs of the human body connected on a device may not be available in the immediate future, multi-organ-on-a-chip technology has already opened a real possibility for such a system to be developed.
Organ-on-a-chip, multi-organs-on-a-chip, and human-on-a-chip technology may become efficient, high-throughput platforms for various steps during pharmaceutical investigations that may be accompanied by decreases in cost due largely to innovation in manufacturing and bulk production. Using organ-level synthetic biology approaches, it may be possible to identify how each cellular, molecular, and physical factor impacts individually and in combination to radiation injury. Such a platform may enable the study of the underlying mechanisms involved in radiation-induced various sub-syndromes and also provide a powerful tool for the discovery of new and novel MCMs for human use in the future.
7. Expert opinion
Due to heightened animal welfare issues and the growing concern over using experimental animals in preclinical research, the organ-on-a-chip platform may be a helpful option to reduce both the need and number of animals utilized, and in turn, serve to minimize ethical issues arising from the use of large numbers of animals in studies conducted to better understand the etiology and pathogenesis of various diseases and associated injuries, as well as for those needed for drug discovery, screening, and development essential for regulatory approval. This is particularly important for MCMs being developed for approval for human use following the US FDA Animal Rule, specifically for CBRN (chemical, biological, radiological, and nuclear) agents, where clinical studies for drug efficacy under phase II and III cannot be conducted with patients in clinical studies due to ethical reasons [Citation115].
One of the major drawbacks of the organ-on-a-chip technology, as it is generally and currently applied, is that it models the physiological and tissue architecture of only single organs and not multiple organs. With the advent of multi-organ-on-a-chip models, a new paradigm in drug development has presented itself; complex interactions of several organs and the capacity can be observed holistically and monitored, specifically in reference to drug uptake, metabolism, excretion, pharmacokinetic/pharmacodynamic parameters, and given biological effects [Citation67]. With computational modeling to convert in vitro data into in vivo predictions, multi-organs-on-a-chip may be helpful in reducing the rate of clinical failure of drugs. Currently, the main reason for the high failure rate of new pharmaceuticals in clinical study is our inadequate knowledge of human pathophysiology and the underlying mechanisms. The development of a more improved in vitro system, known as body-on-a-chip or human-on-a-chip, is moving forward to imitate the entire body physiology in a single platform for disease modeling and drug development [Citation1].
The primary goal of all organ-on-a-chip models is to recreate physiological processes observed in vivo. In vitro grown primary cells and tissues systems continue to be improved and substantially so, relative to the mimicking life cycles, morphologies, and metabolic and related physiologic patterns of naturally occurring cells in situ. Nevertheless, the use of the organ-on-a-chip model for drug discovery remains underutilized and is largely employed to confirm prior results. Though this field is somewhat still in infancy, a number of investigators have begun to explore this technology’s next step, namely multi-organ-on-a-chip systems.
In due course of time, sustained integration of innovative concepts and technologies into the promising platform of organs-on-a-chip may connect technical and biological gaps between preclinical, translational, and clinical studies for drug development and approvals by regulatory bodies such as the US FDA, European Medical Agency (EMA), and Japanese Pharmaceuticals and Medical Devices Agency (PMDA) [Citation120–122].
Article highlights
The attrition rate in drug development continues to be high and the success rate can be increased by using disease-relevant models.
Organ-on-a-chip is a promising and interdisciplinary technology for drug screening, disease modeling, and precision medicine.
This platform with microfluidics and controllable culture within an organotypic architectural environment offer physiologically important options to interrogate human biology.
The development of a personalized organ-on-a-chip platform will significantly promote its biomedical use and commercialization.
Organ-on-a-chip platforms may be a helpful alternative and serve to avoid ethical issues for use of animals in drug development and regulatory approvals.
The organ-on-a-chip technology has immense potential to advance radiation medical countermeasure discovery and development.
This box summarizes key points contained in the article.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose
Authors’ contributions
VKS and TMS did literature search, drafted the manuscript, revised, and finalized for publication.
Acknowledgments
The opinions or assertions contained herein are the private views of the authors and are not necessarily those of the Uniformed Services University of the Health Sciences, or the Department of Defense. The mention of specific therapeutic agents does not constitute endorsement by the U.S. Department of Defense, and trade names are used only for the purpose of clarification. We apologize to those having contributed substantially to the topics discussed herein that we were unable to cite because of space constraints. We are thankful to Ms. Alana Carpenter for editing the manuscript.
Additional information
Funding
References
- Ma C, Peng Y, Li H, et al. Organ-on-a-chip: a new paradigm for drug development. Trends Pharmacol Sci. 2021;42:119–133.
- Shanti A, Teo J, Stefanini C. In vitro immune organs-on-chip for drug development: a review. Pharmaceutics. 2018;10:278.
- Torisawa YS, Tung YC. Editorial for the special issue on organs-on-chips. Micromachines (Basel). 2020;11:369.
- Bissell MJ, Ewald A. Goodbye flat biology - time for the 3rd and the 4th dimensions. J Cell Sci. 2017;130:3–5.
- Khademhosseini A, Langer R. A decade of progress in tissue engineering. Nat Protoc. 2016;11:1775–1781.
- Chen CS. 3D biomimetic cultures: the next platform for cell biology. Trends Cell Biol. 2016;26:798–800.
- Ma C, Witkowski MT, Harris J, et al. Leukemia-on-a-chip: dissecting the chemoresistance mechanisms in B cell acute lymphoblastic leukemia bone marrow niche. Sci Adv. 2020;6. DOI:10.1126/sciadv.aba5536.
- Prantil-Baun R, Novak R, Das D, et al. Physiologically based pharmacokinetic and pharmacodynamic analysis enabled by microfluidically linked organs-on-chips. Annu Rev Pharmacol Toxicol. 2018;58:37–64.
- Quan Y, Sun M, Tan Z, et al. Organ-on-a-chip: the next generation platform for risk assessment of radiobiology. RSC Adv. 2020;10:39521–39530.
- Torisawa YS, Spina CS, Mammoto T, et al. Bone marrow-on-a-chip replicates hematopoietic niche physiology in vitro. Nat Methods. 2014;11:663–669.
- Torisawa YS, Mammoto T, Jiang E, et al. Modeling hematopoiesis and responses to radiation countermeasures in a bone marrow-on-a-chip. Tissue Eng Part C Methods. 2016;22:509–515.
- Singh VK, Newman VL, Berg AN, et al. Animal models for acute radiation syndrome drug discovery. Expert Opin Drug Discov. 2015;10:497–517.
- Singh VK, Olabisi AO. Nonhuman primates as models for the discovery and development of radiation countermeasures. Expert Opin Drug Discov. 2017;12:695–709.
- Williams JP, Jackson IL, Shah JR, et al. Animal models and medical countermeasures development for radiation-induced lung damage: report from an NIAID workshop. Radiat Res. 2012;177:e0025–39.
- Williams JP, Brown SL, Georges GE, et al. Animal models for medical countermeasures to radiation exposure. Radiat Res. 2010;173:557–578.
- Hasegawa A, Tanigawa K, Ohtsuru A, et al. Health effects of radiation and other health problems in the aftermath of nuclear accidents, with an emphasis on Fukushima. Lancet. 2015;386:479–488.
- Marzaleh MA, Rezaee R, Rezaianzadeh A, et al. Design and validation of a hospital emergency department preparedness questionnaire for radiation accidents, nuclear accidents, and nuclear terrorism in Iran. Am J Disaster Med. 2020;15:283–292.
- MacVittie TJ, Farese AM, Jackson WE 3rd. A systematic review of the Hematopoietic Acute Radiation Syndrome (H-ARS) in canines and non-human primates: acute mixed neutron/gamma vs. reference quality radiations. Health Phys. 2020;119:527–558.
- Singh VK, Seed TM. A review of radiation countermeasures focusing on injury-specific medicinals and regulatory approval status: part I. Radiation sub-syndromes, animal models and FDA-approved countermeasures. Int J Radiat Biol. 2017;93:851–869.
- Girgis M, Li Y, Jayatilake M, et al. Short-term metabolic disruptions in urine of mouse models following exposure to low doses of oxygen ion radiation. J Environ Sci Health C Toxicol Carcinog. 2021; 39: 234–249.
- Li Y, Girgis M, Wise SY, et al. Analysis of the metabolomic profile in serum of irradiated nonhuman primates treated with Ex-Rad, a radiation countermeasure. Sci Rep. 2021;11:11449.
- Dissmore T, DeMarco AG, Jayatilake M, et al. Longitudinal metabolic alterations in plasma of rats exposed to low doses of high linear energy transfer radiation. J Environ Sci Health C Toxicol Carcinog. 2021; 39: 219–233.
- Girgis M, Li Y, Ma J, et al. Comparative proteomic analysis of serum from nonhuman primates administered BIO 300: a promising radiation countermeasure. Sci Rep. 2020;10:19343.
- Hall EJ, Giaccia AJ. Radiobiology for the radiobiologist. 7th ed. Philadelphia: Lippincott Williams and Wilkins; 2012.
- Stewart FA, Akleyev AV, Hauer-Jensen M, et al. ICRP publication 118: ICRP statement on tissue reactions and early and late effects of radiation in normal tissues and organs–threshold doses for tissue reactions in a radiation protection context. Ann ICRP. 2012;41:1–322.
- Seed TM. Acute effects. The Health Risks of Extraterrestrial Environments; 2011 [cited 2021 Jul 20]. Available from: https://three.jsc.nasa.gov/articles/SeedAcuteEffects.pdf
- Jalili-Firoozinezhad S, Prantil-Baun R, Jiang A, et al. Modeling radiation injury-induced cell death and countermeasure drug responses in a human gut-on-a-chip. Cell Death Dis. 2018;9:223.
- Frohlich E. Issues with cancer spheroid models in therapeutic drug screening. Curr Pharm Des. 2020;26:2137–2148.
- Tung YC, Hsiao AY, Allen SG, et al. High-throughput 3D spheroid culture and drug testing using a 384 hanging drop array. Analyst. 2011;136:473–478.
- Timm DM, Chen J, Sing D, et al. A high-throughput three-dimensional cell migration assay for toxicity screening with mobile device-based macroscopic image analysis. Sci Rep. 2013;3:3000.
- Devarasetty M, Mazzocchi AR, Skardal A. Applications of bioengineered 3D tissue and tumor organoids in drug development and precision medicine: current and future. Biodrugs. 2018;32:53–68.
- In JG, Foulke-Abel J, Estes MK, et al. Human mini-guts: new insights into intestinal physiology and host-pathogen interactions. Nat Rev Gastroenterol Hepatol. 2016;13:633–642.
- Fujii M, Matano M, Toshimitsu K, et al. Human intestinal organoids maintain self-renewal capacity and cellular diversity in niche-inspired culture condition. Cell Stem Cell. 2018;23:787–93 e6.
- Kim J, Koo BK, Knoblich JA. Human organoids: model systems for human biology and medicine. Nat Rev Mol Cell Biol. 2020;21:571–584.
- Rowe RG, Daley GQ. Induced pluripotent stem cells in disease modelling and drug discovery. Nat Rev Genet. 2019;20:377–388.
- Whitesides GM. The origins and the future of microfluidics. Nature. 2006;442:368–373.
- Haeberle S, Zengerle R. Microfluidic platforms for lab-on-a-chip applications. Lab Chip. 2007;7:1094–1110.
- World Econmic Forum. Top Ten Emerging Technologies. 2016 [cited 2022 Jan 22]. Available from: https://www.weforum.org/agenda/2016/06/top-10-emerging-technologies-2016
- Sano E, Mori C, Matsuoka N, et al. Tetrafluoroethylene-propylene elastomer for fabrication of microfluidic organs-on-chips resistant to drug absorption. Micromachines (Basel). 2019;10. DOI:10.3390/mi10110793.
- Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature. 2014;505:327–334.
- Sugiyama T, Nagasawa T. Bone marrow niches for hematopoietic stem cells and immune cells. Inflamm Allergy Drug Targets. 2012;11:201–206.
- Yu VW, Scadden DT. Hematopoietic stem cell and its bone marrow niche. Curr Top Dev Biol. 2016;118:21–44.
- Dexter TM, Allen TD, Lajtha LG. Conditions controlling the proliferation of haemopoietic stem cells in vitro. J Cell Physiol. 1977;91:335–344.
- Dexter TM, Coutinho LH, Spooncer E, et al. Stromal cells in haemopoiesis. Ciba Found Symp. 1990;148:76–86. discussion 86-95.
- Laver J, Ebell W, Castro-Malaspina H. Radiobiological properties of the human hematopoietic microenvironment: contrasting sensitivities of proliferative capacity and hematopoietic function to in vitro irradiation. Blood. 1986;67:1090–1097.
- Sieber S, Wirth L, Cavak N, et al. Bone marrow-on-a-chip: long-term culture of human haematopoietic stem cells in a three-dimensional microfluidic environment. J Tissue Eng Regen Med. 2018;12:479–489.
- Bruce A, Evans R, Mezan R, et al. Three-dimensional microfluidic tri-culture model of the bone marrow microenvironment for study of acute lymphoblastic leukemia. PLoS One. 2015;10:e0140506.
- Chou DB, Frismantas V, Milton Y, et al. On-chip recapitulation of clinical bone marrow toxicities and patient-specific pathophysiology. Nat Biomed Eng. 2020;4:394–406.
- McDermott AJ, Huffnagle GB. The microbiome and regulation of mucosal immunity. Immunology. 2014;142:24–31.
- Bein A, Shin W, Jalili-Firoozinezhad S, et al. Microfluidic organ-on-a-chip models of human intestine. Cell Mol Gastroenterol Hepatol. 2018;5:659–668.
- Xiang Y, Wen H, Yu Y, et al. Gut-on-chip: recreating human intestine in vitro. J Tissue Eng. 2020;11:2041731420965318.
- Shah P, Fritz JV, Glaab E, et al. A microfluidics-based in vitro model of the gastrointestinal human-microbe interface. Nat Commun. 2016;7:11535.
- Shim KY, Lee D, Han J, et al. Microfluidic gut-on-a-chip with three-dimensional villi structure. Biomed Microdevices. 2017;19:37.
- Kim HJ, Li H, Collins JJ, et al. Contributions of microbiome and mechanical deformation to intestinal bacterial overgrowth and inflammation in a human gut-on-a-chip. Proc Natl Acad Sci U S A. 2016;113:E7–15.
- Hollingsworth BA, Cassatt DR, DiCarlo AL, et al. Acute radiation syndrome and the microbiome: impact and review. Front Pharmacol. 2021;12:643283.
- Jalili-Firoozinezhad S, Gazzaniga FS, Calamari EL, et al. A complex human gut microbiome cultured in an anaerobic intestine-on-a-chip. Nat Biomed Eng. 2019;3:520–531.
- Tovaglieri A, Sontheimer-Phelps A, Geirnaert A, et al. Species-specific enhancement of enterohemorrhagic E. coli pathogenesis mediated by microbiome metabolites. Microbiome. 2019;7:43.
- Cheema AK, Li Y, and Singh J, et al. Microbiome study in irradiated mice treated with BIO 300, a promising radiation countermeasure. Anim Microbiome. 2021;3:71.
- Ingber DE. Human organs-on-chips for disease modelling, drug development and personalized medicine. Nat Rev Genet. 2022. DOI:10.1038/s41576-022-00466-9
- Peter RU, Gottlober P, Nadeshina N, et al. Radiation lentigo. A distinct cutaneous lesion after accidental radiation exposure. Arch Dermatol. 1997;133:209–211.
- Peter RU, Gottlober P. Management of cutaneous radiation injuries: diagnostic and therapeutic principles of the cutaneous radiation syndrome. Mil Med. 2002;167:110–112.
- Lataillade JJ, Doucet C, Bey E, et al. New approach to radiation burn treatment by dosimetry-guided surgery combined with autologous mesenchymal stem cell therapy. Regen Med. 2007;2:785–794.
- O’Neill AT, Monteiro-Riviere NA, Walker GM. Characterization of microfluidic human epidermal keratinocyte culture. Cytotechnology. 2008;56:197–207.
- Abaci HE, Gledhill K, Guo Z, et al. Pumpless microfluidic platform for drug testing on human skin equivalents. Lab Chip. 2015;15:882–888.
- Mori N, Morimoto Y, Takeuchi S. Skin integrated with perfusable vascular channels on a chip. Biomaterials. 2017;116:48–56.
- Ramadan Q, Ting FC. In vitro micro-physiological immune-competent model of the human skin. Lab Chip. 2016;16:1899–1908.
- Bovard D, Iskandar A, Luettich K, et al. Organs-on-a-chip: a new paradigm for toxicological assessment and preclinical drug development. Toxicol Res Appl. 2017;1:1–16.
- Munoz-Schuffenegger P, Ng S, Dawson LA. Radiation-induced liver toxicity. Semin Radiat Oncol. 2017;27(4):350–357.
- Nakajima T, Ninomiya Y, Nenoi M. Radiation-induced reactions in the liver - Modulation of radiation effects by lifestyle-related factors. Int J Mol Sci. 2018;19:3855.
- Ware BR, Khetani SR. Engineered liver platforms for different phases of drug development. Trends Biotechnol. 2017;35:172–183.
- Bhise NS, Manoharan V, Massa S, et al. A liver-on-a-chip platform with bioprinted hepatic spheroids. Biofabrication. 2016;8:014101.
- Bavli D, Prill S, Ezra E, et al. Real-time monitoring of metabolic function in liver-on-chip microdevices tracks the dynamics of mitochondrial dysfunction. Proc Natl Acad Sci USA. 2016;113:E2231–40.
- Ong LJY, Chong LH, Jin L, et al. A pump-free microfluidic 3D perfusion platform for the efficient differentiation of human hepatocyte-like cells. Biotechnol Bioeng. 2017;114:2360–2370.
- Ju SM, Jang HJ, Kim KB, et al. High-throughput cytotoxicity testing system of Acetaminophen using a microfluidic device (MFD) in HepG2 cells. J Toxicol Environ Health. 2015;78:1063–1072.
- Ramaiahgari SC, den Braver MW, Herpers B, et al. A 3D in vitro model of differentiated HepG2 cell spheroids with improved liver-like properties for repeated dose high-throughput toxicity studies. Arch Toxicol. 2014;88:1083–1095.
- Takahashi Y, Hori Y, Yamamoto T, et al. 3D spheroid cultures improve the metabolic gene expression profiles of HepaRG cells. Biosci Rep. 2015;35:e00208.
- Snyder JE, Hamid Q, Wang C, et al. Bioprinting cell-laden matrigel for radioprotection study of liver by pro-drug conversion in a dual-tissue microfluidic chip. Biofabrication. 2011;3:034112.
- Kniazeva T, Hsiao JC, Charest JL, et al. A microfluidic respiratory assist device with high gas permeance for artificial lung applications. Biomed Microdevices. 2011;13:315–323.
- Huh D, Matthews BD, Mammoto A, et al. Reconstituting organ-level lung functions on a chip. Science. 2010;328:1662–1668.
- Stucki AO, Stucki JD, Hall SR, et al. A lung-on-a-chip array with an integrated bio-inspired respiration mechanism. Lab Chip. 2015;15:1302–1310.
- Venkatesulu BP, Mahadevan LS, Aliru ML, et al. Radiation-induced endothelial vascular injury: a review of possible mechanisms. JACC Basic Transl Sci. 2018;3:563–572.
- Jain A, Barrile R, van der Meer AD, et al. Primary human lung alveolus-on-a-chip model of intravascular thrombosis for assessment of therapeutics. Clin Pharmacol Ther. 2018;103:332–340.
- Millet LJ, Giannone RJ, Greenwood MS, et al. Identifying candidate biomarkers of ionizing radiation in human pulmonary microvascular lumens using microfluidics-a pilot study. Micromachines (Basel). 2021;12. DOI:10.3390/mi12080904.
- Dauth S, Maoz BM, Sheehy SP, et al. Neurons derived from different brain regions are inherently different in vitro: a novel multiregional brain-on-a-chip. J Neurophysiol. 2017;117:1320–1341.
- Stiles J, Jernigan TL. The basics of brain development. Neuropsychol Rev. 2010;20:327–348.
- Oberheim NA, Takano T, Han X, et al. Uniquely hominid features of adult human astrocytes. J Neurosci. 2009;29:3276–3287.
- Yamada M, Mimori Y, Kasagi F, et al. Incidence of dementia, Alzheimer disease, and vascular dementia in a Japanese population: radiation effects research foundation adult health study. Neuroepidemiology. 2008;30:152–160.
- Riudavets MA, Mena H, Bouffard JP, et al. Relationship between radiation injury and Alzheimer-related neurodegenerative changes. Clin Neuropathol. 2005;24:236–238.
- Gijzen L, Marescotti D, Raineri E, et al. An intestine-on-a-chip model of plug-and-play modularity to study inflammatory processes. SLAS Technol. 2020;25:585–597.
- Esch EW, Bahinski A, Huh D. Organs-on-chips at the frontiers of drug discovery. Nat Rev Drug Discov. 2015;14:248–260.
- Peters MF, Choy AL, Pin C, et al. Developing in vitro assays to transform gastrointestinal safety assessment: potential for microphysiological systems. Lab Chip. 2020;20:1177–1190.
- Abbott NJ. Astrocyte-endothelial interactions and blood-brain barrier permeability. J Anat. 2002;200:629–638.
- Cho H, Seo JH, Wong KH, et al. Three-dimensional blood-brain barrier model for in vitro studies of neurovascular pathology. Sci Rep. 2015;5:15222.
- Xu H, Li Z, Yu Y, et al. A dynamic in vivo-like organotypic blood-brain barrier model to probe metastatic brain tumors. Sci Rep. 2016;6:36670.
- Baradaran-Ghahfarokhi M. Radiation-induced kidney injury. J Renal Injury Prev. 2012;1:49–50.
- Naughton CA. Drug-induced nephrotoxicity. Am Fam Physician. 2008;78:743–750.
- Justice BA, Badr NA, Felder RA. 3D cell culture opens new dimensions in cell-based assays. Drug Discov Today. 2009;14:102–107.
- Hoenig MP, Zeidel ML. Homeostasis, the milieu interieur, and the wisdom of the nephron. Clin J Am Soc Nephrol. 2014;9:1272–1281.
- Homan KA, Kolesky DB, Skylar-Scott MA, et al. Bioprinting of 3D convoluted renal proximal tubules on perfusable chips. Sci Rep. 2016;6:34845.
- Sochol RD, Gupta NR, Bonventre JV. A role for 3D printing in kidney-on-a-chip platforms. Curr Transplant Rep. 2016;3:82–92.
- Taunk NK, Haffty BG, Kostis JB, et al. Radiation-induced heart disease: pathologic abnormalities and putative mechanisms. Front Oncol. 2015;5:39.
- Darby SC, Cutter DJ, Boerma M, et al. Radiation-related heart disease: current knowledge and future prospects. Int J Radiat Oncol Biol Phys. 2010;76:656–665.
- Agarwal A, Goss JA, Cho A, et al. Microfluidic heart on a chip for higher throughput pharmacological studies. Lab Chip. 2013;13:3599–3608.
- Balachandran K, Alford PW, Wylie-Sears J, et al. Cyclic strain induces dual-mode endothelial-mesenchymal transformation of the cardiac valve. Proc Natl Acad Sci USA. 2011;108:19943–19948.
- Park J, Wetzel I, and Dreau D, et al. 3D miniaturization of human organs for drug discovery. Adv Healthc Mater. 2018;7:1700551.
- Vernetti LA, Vogt A, Gough A, et al. Evolution of experimental models of the liver to predict human drug hepatotoxicity and efficacy. Clin Liver Dis. 2017;21:197–214.
- Wagner I, Materne EM, Brincker S, et al. A dynamic multi-organ-chip for long-term cultivation and substance testing proven by 3D human liver and skin tissue co-culture. Lab Chip. 2013;13:3538–3547.
- Materne EM, Ramme AP, Terrasso AP, et al. A multi-organ chip co-culture of neurospheres and liver equivalents for long-term substance testing. J Biotechnol. 2015;205:36–46.
- Choe A, Ha SK, Choi I, et al. Microfluidic Gut-liver chip for reproducing the first pass metabolism. Biomed Microdevices. 2017;19:4.
- Castell JV, Donato MT, Gomez-Lechon MJ. Metabolism and bioactivation of toxicants in the lung. The in vitro cellular approach. Exp Toxicol Pathol. 2005;57(Suppl 1):189–204.
- Coppeta JR, Mescher MJ, Isenberg BC, et al. A portable and reconfigurable multi-organ platform for drug development with onboard microfluidic flow control. Lab Chip. 2016;17:134–144.
- Frey O, Misun PM, Fluri DA, et al. Reconfigurable microfluidic hanging drop network for multi-tissue interaction and analysis. Nat Commun. 2014;5:4250.
- Maschmeyer I, Lorenz AK, Schimek K, et al. A four-organ-chip for interconnected long-term co-culture of human intestine, liver, skin and kidney equivalents. Lab Chip. 2015;15:2688–2699.
- Oleaga C, Bernabini C, Smith AS, et al. Multi-Organ toxicity demonstration in a functional human in vitro system composed of four organs. Sci Rep. 2016;6:20030.
- U.S. Food and Drug Administration. Guidance document: product development under the animal rule; 2015 [cited 2020 Oct 20]. Available from: http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm399217.pdf.
- Witkowski MT, Dolgalev I, Evensen NA, et al. Extensive remodeling of the immune microenvironment in B cell acute lymphoblastic leukemia. Cancer Cell. 2020;37:867–82 e12.
- Marturano-Kruik A, Nava MM, Yeager K, et al. Human bone perivascular niche-on-a-chip for studying metastatic colonization. Proc Natl Acad Sci U S A. 2018;115:1256–1261.
- Beir V. Mechanisms of radiation-induced cancer. Health risks from exposure to low levels of ionizing radiation. Washington: The National Academies Press; 1980.
- Low LA, and Tagle DA. Organs-on-chips: progress, challenges, and future directions. Experimental biology and medicine. Exp Biol Med. 2017;242:1573–1578.
- Oye KA, Eichler HG, Hoos A, et al. Pharmaceuticals licensing and reimbursement in the European Union, United States, and Japan. Clin Pharmacol Ther. 2016;100:626–632.
- Langhans SA. Using 3D in vitro cell culture models in anti-cancer drug discovery. Expert Opin Drug Discov. 2021;16:841–850.
- Moysidou CM, Owens RM. Advances in modelling the human microbiome-gut-brain axis in vitro. Biochem Soc Trans. 2021;49:187–201.
