1. Introduction
Natural products (NPs) provide an outstanding source of new exciting scaffolds and pharmacophores in drug discovery. Historical evidence demonstrates that life in the ocean began around 3.7 billion years ago which is three times longer than terrestrial life and produces a far greater evolutionary diversity. Notably, out of 34 major animal phyla, 33 are present in the ocean while only 12 are present on land [Citation1]. There are currently 15 marine derived drugs on the EU and/or US pharmaceutical market. Five of these have been approved in the last four years (, ). Ten out of the fifteen are on the market for treatment of cancer, and a small number are approved for the treatment of viral infections, hypertriglyceridemia, and chronic pain. Notably, 38 marine NP inspired pharmaceutical agents are in clinical trials out of around 40,000 isolated marine NPs. Interestingly, this improved success ratio demonstrates its advantage compared to the industry standard where 15,000 molecules are evaluated to get one approved pharmaceutical agent [Citation2–6]. Furthermore, marine biodiversity offers intriguing opportunities for research because around 90% of marine species are undescribed and thus this marine biodiversity can potentially contribute novel solutions to address human life problems [Citation1].
Figure 1. (a) number of marine drugs under clinical trials and already marketed (in use), distributed for therapeutic class; (b) % distribution of all marine drugs (under clinical trials and already marketed) for their use (cancer vs. non cancer). Adapted from [Citation2].
![Figure 1. (a) number of marine drugs under clinical trials and already marketed (in use), distributed for therapeutic class; (b) % distribution of all marine drugs (under clinical trials and already marketed) for their use (cancer vs. non cancer). Adapted from [Citation2].](/cms/asset/c254219c-6c89-4de5-a3c9-73982a79eeba/iedc_a_2285402_f0001_oc.jpg)
Table 1. A perspective of pipeline of marine bioactive molecules. Adapted from [Citation2].
Due to the fascinating diversity of marine natural products, screening thousands of molecules employing traditional experimental and pharmacology models can be expensive and time-consuming. Recent literature articles demonstrate that Artificial Intelligence (AI) plays a crucial role in drug development [Citation7]. AI can be integrated in numerous stages of drug discovery and is expected to potentially reduce drug discovery time and enhance drug development achievement rates. AI has already been integrated in various stages of NP drug discovery and this intervention can assist in marine drug discovery to help elucidating and discovering biologically active molecules. Additionally, AI can help in activity evaluation, target prediction, drug toxicology prediction, drug interaction of marine drugs, and is also crucial to clarify their role in drug repositioning studies [Citation7].
AI-assisted synthesis of marine natural product-based bioactive molecules will help chemists make better decisions and reduce synthesis failures. Ultimately, this will speed up the drug discovery process through pharmacophore-based synthesis of marine natural product derivatives. DSP-1181 is the first example of a drug to enter clinical trials using the AI approach in less than 12 months, compared to 4 years using conventional methods [Citation8]. A researcher team in MIT employed ML algorithm in order to identify a drug called halicin that kills many strains of bacteria [Citation8].
Oceans harbor a more diverse environment compared to terrestrial territory which suggests that oceans generate better and higher biodiversity which further suggests its NPs will lead to a higher occurrence of novel chemistry and remarkable biology [Citation2,Citation4]. Nevertheless, typical bioassay-guided purification techniques are seriously restricting the finding of further novel and bioactive lead NPs from the ocean [Citation9]. Secondly, the difficult ocean environment enhances complications in sampling, which leads to limited material supply and in particular, some rare bioactive NPs which cannot easily be prepared. Thirdly, numerous marine organisms cannot be cultured and some marine species only express their NPs in their natural environment. It has been reported that over 99% of microorganisms cannot be cultivated in nature. In addition, sponges and their microbial fauna have produced numerous NPs with structural and biological diversity which are largely uncultivable [Citation9].
Due to the rapid development of bioinformatics tools, significant differences between the insignificant number of NPs reported and the great potential of biosynthesis, makes one understand that a majority of marine NPs are still largely unexplored. Due to the significant evolution in DNA sequencing technologies along with related bioinformatics tools, a huge amount of genomic sequencing data has been accumulated. These new advances have disclosed further significant approaches for marine NP drug development [Citation9,Citation10], in particular for the establishment of marine microorganisms derived novel and bioactive compounds. Marine metagenomics is a tremendous tool to explore the biosynthetic capability of microorganisms in a few marine environments. For example, sponge metagenomics strategies were employed to identify biosynthetic gene clusters, which manifested in the gene cluster of the anticancer molecule onnamide (1) being isolated from the sponge derived from the uncultivated sponge Theonella swinhoei [Citation9,Citation11].
Another powerful strategy for marine NPs drug development would be genome mining which is becoming a most useful tool to retrieve novel and bioactive marine NPs. Genome mining in the isolation and screening of novel and pharmacological active marine NPs from marine microorganisms can be cultured via suitable strategic protocols [Citation9].
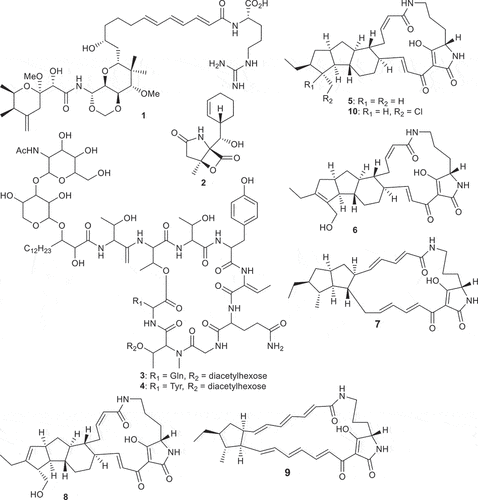
Over the last decade, several genome mining protocols have been established to target particular chemical features or biological effects of bioactive compounds by means of biosynthetic or transporter proteins [Citation12]. These “biosynthetic hooks” allow researchers to search for clusters of biosynthetic genes that are highly likely to encode bioactive molecules that have not yet been discovered. These findings can be used for genome mining efforts to recognize orphan biosynthetic gene clusters (BGCs) that are predicted to furnish secondary metabolites with the chemical moiety associated with the queried bioactivity [Citation12]. Successful examples of marine NPs discovered from the ocean via genome mining include salinosporamide K (2) [Citation13], hassallidins C (3) and D (4) [Citation14], and pactamides A–F (5–10) () [Citation15].
Probably the most astonishing strategies to enhance success in marine NPs drug discovery are the chances offered by synthetic biology (SB). Marine resources have always been tremendous sources of biologically active molecules but such NPs usually exist in low concentration and are usually difficult to prepare in the laboratory due to their structural complexity. Progress in synthetic biology allows for the production of lead molecules in non-natural hosts and this progress has tremendously revolutionized the drug discovery process. Employing computational along with experimental strategies, synthetic biology can assist to design chassis cells as biofactories or as novel drug screening platforms to produce challenging and difficultly obtained bioactive molecules [Citation16].
Another intriguing example is the anticancer diterpene, paclitaxel (taxol) of which the chemical synthesis was both complicated and costly. Interestingly taxol was produced by bacteria (Bacillus and Oceanobacillus species) from non-Taxus host which were isolated from marine macroalgae [Citation17]. Marine carotenoids are used as pharmaceuticals, nutraceuticals, animal feeds and food colors. Synthetic biology, together with metabolic engineering, has established various chimeric pathways for the mass production of natural and modified carotenoids (non-natural carotenoids) [Citation18]. Other successful examples include the production of griseorhodin A and eptidemnamide using synthetic biology protocols.
The pharmaceutical industry produces significant amounts of waste during the preparation and purification of API’s during preclinical and manufacturing operations. Green Chemistry, which minimizes excess solvents and waste through the use of technologies such as bio- or chemo-catalysis, is an important tool for the pharmaceutical industry to achieve its environmental goals while still providing economic benefits. Such approaches, which save time and money and reduce waste, should be used in marine drug discovery.
Ethics and ethical behavior (usually associated with ‘responsible practice’) are the cornerstones of scientific research. Acting ethically and with integrity is important for maintaining academic and research activities. In all walks of life, and particularly in the drug development process, a focus on ethical behavior is essential. The most important reason for the emphasis on ethics in clinical research is integrity. Adherence to ethical standards protects the patients who take part in these trials and ensures that the research results are transparent and reliable. It also assures that patients are not exploited or harmed as a result of their participation they are at a real risk of physiological or psychological harm from unethical clinical research practices. This could also jeopardize the results and lead to mistakes in the development of the drug and could cause irreparable damage when the drug reaches the market. The majority of marine biologists and ecologists conduct experiments without any ethical guidelines, despite the occasional concern expressed by the community about their activities. Marine biologists should take pre-emptive action by encouraging animal ethics committees in their institutes to include environmental ethics.
Studies involving human and animal participants should be conducted in accordance with recognized ethical standards and in compliance with national and international laws and regulations. Scientific results should not be published by publishing organizations without the ethical details of the use of humans and animals being provided. Scientists should seek approval from their affiliated institutions when conducting animal studies on any bioactive molecule. In addition, affiliated institutions should not allow authors to carry out animal experiments or to publish their work without the ethical approval of the institution. Scientists should reconsider the need for ethics or ethical behavior when dealing with marine animals. Since the Nagoya protocol [Citation19], access to the biome, whether marine or otherwise, has been subject to local government permits, a formal negotiated contract, and a local researcher accompanying the extraction.
2. Expert opinion
Although the field of marine NP drug discovery is well underway in the 21st century, a number of obstacles remain to the development of NP drugs, especially in the field of marine NP drug discovery. One of the key requirements is the sustainable supply of NPs through the development of efficient processes using fermentation protocols, chemical synthesis or genetic engineering. For example, difficulties in material collection hampered the anti-cancer clinical trial of Cephalostatin 1 [Citation20]. Design and total synthesis of macro- or microbial-derived marine NPs is a common method for producing compounds for clinical trials. However, synthesis of natural products with moderately complex structures remains a major challenge.
AI and machine learning protocols have been heavily applied to Computer-Aided Synthesis Planning (CASP) [Citation21,Citation22], Computer-Aided Drug Design (CADD) [Citation16], and Computer-Assisted Identification of Compounds [Citation23], (identifying known compounds). Recently, computational synthetic routes of NPs including the alkaloid tacamonidine (not yet prepared in the laboratory) have been reported [Citation22]. This has led to the conclusion that computers are now capable of designing syntheses that are crucial to the day-to-day practice of organic synthesis [Citation21,Citation22]. Therefore, computational methods will be important to identify and elucidate the complex structures of marine NPs, to predict the chemical synthesis of marine NPs, to identify and validate the biological targets, and to predict the biological activities of marine NPs [Citation21–23].
Synthetic biology has opened up various possibilities in drug development and currently seven cancer treatments through clinical trials approved by the FDA by July 2022 are being affected [Citation16]. Furthermore, despite these tremendous success stories, synthetic biology inspired cell engineering in drug discovery and medicine still faces numerous challenges including lack of accessible sensors for biomarkers. On the other hand, genome manufacturing and editing is a lengthy and costly process. Therefore, further efforts are necessary in order to integrate chemical, biological and mechanical features for the next generation of cell engineering. Synthetic biology allows drug discovery scientists to manipulate the expression of secondary metabolites to switch on ‘silent’ genes or to make new molecules by shuffling components in biosynthetic pathways.
From 1970 to 2000, relatively large biological sample sizes were used in marine drug discovery. This was required because of the practicalities of elucidating new and often unprecedented types of structures, requiring multi-milligram amounts of the compounds. In the course of time, substantial quantities of isolated natural products are still available which can be evaluated by various biological screening tests. Employing flow-cell NMR spectrometers in combination with increasingly powerful MS methods and algorithms, including de novo sequencing [Citation24], structures of natural products are being elucidated at the nanomolar scale. These technological developments have made it possible to collect ever smaller amounts of biological material which has subsequently allowed the scientific examination of marine species that occur in very low biomass in nature.
Since only microgram quantities of active compounds are isolated and some are subjected to destructive analytical techniques or synthetic modification, there is insufficient left for significant biological screening. As a result, such studies tend to be academic endeavors, except in cases where full chemical synthesis or biosynthesis can furnish the molecule for a crucial biological screening. A long-established problem associated with nanomole scale isolation has been dubbed ’‘crossing the valley of death’’. Although there are various ’‘valleys’‘in the complicated process of drug development, the complication in this context is how to move’‘early stage discoveries’‘into’‘late stage preclinical candidates’’. The requirements for taking a compound to the next stage include SAR determination, mode of action determination, in vivo screening, and determination of pharmacokinetic and pharmaceutical potentials.
The technology for isolation and structure determination has advanced tremendously in the past decade. However, when coupled with a biological assay, this process can sometimes take weeks or months. This is clearly too slow to be competitive with the screening of pure molecules with a known structure. There are two ways to overcome this: a) purification of unique natural products on the basis of criteria of chemical diversity and b) the subsequent screening of these pure products with a defined composition. Alternatively, an even faster structure elucidation technique needs to be developed which should be able to convert screening hits into compound hits in a matter of minutes or hours. To this end, de novo structures based on high-resolution MS data and fragmentation pathways have been developed using a growing number of strategies and algorithms based on MS data, mainly from the metabolomics field. Advances in ion mobility and other related techniques point to the possibility that even stereochemical features of natural products will be able to be determined through mass spectrometry analysis alone [Citation25].
Declaration of interest
The author has no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Sigwart JD, Blasiak R, Jaspars M, et al. Unlocking the potential of marine biodiscovery. Nat Prod Rep. 2021;38(7):1235–1242. doi: 10.1039/D0NP00067A
- Cappello E, Nieri P. From life in the sea to the clinic: the marine drugs approved and under clinical trial. Life. 2021;11(12):1390. doi: 10.3390/life11121390
- The global marine pharmaceuticals pipeline. USA: Midwestern University. Available from: https://www.marinepharmacology.org/
- Almaliti J, Gerwick WH. Methods in marine natural product drug discovery: what’s new? Expert Opin Drug Discov. 2023;18(7):687–691. doi: 10.1080/17460441.2023.2214360
- Mayer AMS, Pierce M, Reji M, et al. J Pharmacol Exp Ther 2023;385(S3):299. doi:10.1124/jpet.122.527010
- Guo FW, Zhang Q, Gu YC, et al. Sulfur-containing marine natural products as leads for drug discovery and development. Curr Opin Chem Biol. 2023;75:102330. doi: 10.1016/j.cbpa.2023.102330
- Gupta R, Srivastava D, Sahu M, et al. Artificial intelligence to deep learning: machine intelligence approach for drug discovery. Mol Divers. 2021;25(3):1315–1360. doi: 10.1007/s11030-021-10217-3
- Farghali H, Canová NK, Arora M. The potential applications of artificial intelligence in drug discovery and development. Physiol Res. 2021;70:S715–S722. doi: 10.33549/physiolres.934765
- Chu L, Huang J, Muhammad M, et al. Genome mining as a biotechnological tool for the discovery of novel marine natural products. Crit Rev Biotechnol. 2020;40(5):571–589. doi: 10.1080/07388551.2020.1751056
- Gomez-Escribano J, Alt S, Bibb M. Next generation sequencing of actinobacteria for the discovery of novel natural products. Mar Drugs. 2016;14(4):78. doi: 10.3390/md14040078
- Joern P, Dequan H, Gaiping W. Antitumor polyketide biosynthesis by an uncultivated bacterial symbiont of the marine sponge theonella swinhoei. Proc Natl Acad Sci USA. 2004;101(46):16222–16227. doi: 10.1073/pnas.0405976101
- Bauman KD, Butler KS, Moore BS, et al. Genome mining methods to discover bioactive natural products. Nat Prod Rep. 2021;38(11):2100–2129. doi: 10.1039/d1np00032b
- Eustaquio AS, Nam SJ, Penn K, et al. The discovery of salinosporamide K from the marine bacterium “Salinispora pacifica” by genome mining gives insight into pathway evolution. Chembiochem. 2011;12(1):61–64. doi: 10.1002/cbic.201000564
- Vestola J, Shishido TK, Jokela J, et al. Hassallidins, antifungal glycolipopeptides, are widespread among cyanobacteria and are the end-product of a nonribosomal pathway. Proc Natl Acad Sci USA. 2014;111(18):E1909–E1917. doi: 10.1073/pnas.1320913111
- Saha S, Zhang W, Zhang G, et al. Activation and characterization of a cryptic gene cluster reveals a cyclization cascade for polycyclic tetramate macrolactams. Chem Sci. 2017;8(2):1607–1612. doi: 10.1039/c6sc03875a
- Zhao N, Song Y, Xie X, et al. Synthetic biology-inspired cell engineering in diagnosis, treatment, and drug development. Signal Transduct Target Ther. 2023;8(1):112. doi: 10.1038/s41392-023-01375-x
- Subramanian M, Marudhamuthu M. Hitherto unknown terpene synthase organization in taxol‑producing endophytic bacteria isolated from marine macroalgae. Curr Microbiol. 2020;77(6):918–923. doi: 10.1007/s00284-020-01878-8
- Wang C, Kim JH, Kim SW. Synthetic biology and metabolic engineering for marine carotenoids: nNew opportunities and future prospects. Mar Drugs. 2014;12(9):4810–4832. doi: 10.3390/md12094810
- Sherman B, Henry RJ. The Nagoya Protocol and historical collections of plants. Nat Plants. 2020;6(5):430–432. doi: 10.1038/s41477-020-0657-8
- Dey P, Kundu A, Chakraborty HJ, et al. Therapeutic value of steroidal alkaloids in cancer: current trends and future perspectives. Int J Cancer. 2019;145(7):1731–1744. doi: 10.1002/ijc.31965
- Coley CW, Green WH, Jensen KF. Machine learning in computer-aided synthesis planning. Acc Chem Res. 2018;51(5):1281–1289. doi: 10.1021/acs.accounts.8b00087
- Szymkuc S, Gajewska EP, Klucznik T, et al. Computer-assisted synthetic planning: the end of the beginning. Angew Chem Int Ed. 2016;55(20):5904–5937. doi: 10.1002/anie.201506101
- Pereira F, Aires-de-Sousa J. Computational methodologies in the exploration of marine natural product leads. Mar Drugs. 2018;16(7):236. doi: 10.3390/md16070236
- Ng J, Bandeira N, Liu WT, et al. Dereplication and de novo sequencing of nonribosomal peptides. Nat Methods. 2009;6(8):596–599. doi: 10.1038/nmeth.1350
- Bai L, Romanova EV, Sweedler JV. Distinguishing endogenous D-amino acid-containing neuropeptides in individual neurons using tandem mass spectrometry. Anal Chem. 2011;83(7):2794–2800. doi: 10.1021/ac200142m