1. Introduction
Despite billions of dollars invested into cancer drug development each year, studies have shown that oncological clinical trials had a staggeringly low success rate of 3.5% in 2022 [Citation1]. Drug candidates tested in the commonly used murine models often fail to reproduce a comparable efficacy and safety profile in clinical trials. In addition, failure to meet parameters of ADMET (Absorption, Distribution, Metabolism, Excretion, and Toxicity) results in the filtering out of many drug candidates in the preclinical phase. Such sunken costs call for changes in methodology and policy to increase the success rates of cancer drug development. One of the strategies is to implement additional in vivo models to identify suitable hits and filter out inadequate candidates early in the pipeline. From the perspective of clinical application, it is difficult to predict treatment responses due to the genetic predisposition of patients and the heterogenicity of cancer. Patient-derived organoids (PDO) and murine patient-derived xenografts (PDX), the commonly used models to simulate responses, have limitations in practicality and accuracy prediction [Citation2]. This calls for the inclusion of other cancer models to improve the landscape of precision medicine.
The zebrafish was first introduced as a disease model in the 1970s. Owing to its unique strengths, its utilization in cancer research has grown exponentially in recent years. The advantages of zebrafish include 1) high conservation to the human genome and oncologic signaling; 2) high reproduction rates and low cost in animal housing; 3) relative transparency, which enables live-tracking of tumors; and 4) lack of a mature adaptive immune system until four weeks of life, making xenograft studies straightforward. Here, we summarize the current application of zebrafish in the pipeline of cancer drug discovery (). Additionally, we provide examples of how zebrafish helped advance therapeutics developments to various stages of clinical trials ().
Figure 1. Zebrafish’s application in drug discovery and development. The chart outlines the steps of the drug discovery and development pipeline, with the unique strengths of zebrafish described in the shaded boxes.
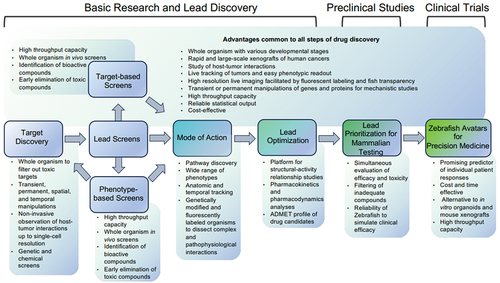
Table 1. Advances in drug discovery, validation, and personalized medicine using zebrafish.
2. Target discovery with reduced toxicities
Although in vitro cell culture systems are the traditional tools used to screen for primary drug targets, zebrafish offer advantages as a whole organism to filter out those that impact development or induce organ toxicity. Zebrafish are especially useful for studying host-tumor interactions in tumor microenvironment and metastasis. Using tools such as mutagenesis and transgenesis, researchers can manipulate molecular targets transiently or permanently, as well as spatially or temporally. In particular, these techniques have been combined with zebrafish’s transparency (e.g. the complete transparent adult fish Casper) to fluorescently label and study tumor cells and tissues of interest [Citation3]. Technological advances in imaging also enable noninvasive observation of host-tumor interaction in live zebrafish up to single-cell resolution [Citation4]. Using zebrafish, researchers can also validate drug targets by modulating the expression, structure, or function of the target protein and determining how the intervention impacts tumor development and oncogenic pathways. For example, Vlecken and Bagowski were able to identify and validate LIMK1 and LIMK2 as potential targets for tumor angiogenesis by using RNAi techniques in a zebrafish xenograft model of human pancreatic cancer [Citation3]. In addition, a genetic screen using transgenic zebrafish identified dihydrolipoamide S-succinyltransferase, a TCA-cycle transferase, as a target for MYC-driven tumors [Citation5]. Devimistat, which inhibits the TCA cycle, has been tested in clinical trials for treating both hematological and solid tumors and recently received a fast-track designation from the FDA for treating acute myeloid leukemia (). Both examples highlight the ability of zebrafish as an in vivo model in identifying drug targets with translational value.
3. In vivo lead screens
Zebrafish-based screens often utilize transgenic or xenograft models of cancer. The output of administered drugs can be measured in zebrafish models for morphologic, therapeutic, pathway, or behavioral changes [Citation6]. Due to the high fecundity and small size of zebrafish, these drug screens feature high throughput capabilities and reliable statistical outputs. Researchers have also ventured into optimizing the screens by automating steps of the process such as microinjections, embryo sorting, phenotype identification, and image acquisition.
3.1. Target-based screens
Zebrafish are suitable for screens to uncover the leads that inhibit well-studied targets from libraries composed of thousands of biochemical compounds. For example, a transgenic zebrafish screen of pharmacologically active compounds identified two antidepressants that target the β-catenin pathway in hepatocellular carcinoma [Citation6]. A zebrafish blastomere screen of 3,840 bioactive small molecules identified retinoid acid agonists with potent anti-cancer properties, which reduce the aberrant MYB expression in adenoid cystic carcinoma and leukemia [Citation7]. Retinoid acid is tested in clinical trials for treating multiple types of cancers including advanced adenoid cystic carcinoma, neuroblastoma, and brain cancers (NCT03999684, NCT00135135, and NCT00528437; ). The third example is the discovery of 16,16-dimethyl-PGE2 (dmPGE2; ProHema) through a screen for compounds targeting the prostanoid E receptors [Citation8]. DmPGE2 was found to regulate vertebrate hematopoietic stem cells by increasing their numbers in the aorta-gonad-mesonephros region of zebrafish. Subsequently, dmPGE2 has progressed to a phase II clinical trial for treating hematologic malignancies (NCT00890500; ) [Citation8].
3.2. Phenotype-based screens
Zebrafish are also ideal for screening leads based on phenotypic changes (e.g. cancer-suppressing) when the molecular target is unknown. This phenotype-based screen has several strengths over target-based drug discovery. It can identify entirely new classes of therapeutics and reveal previously unsuspected ‘druggable’ pathways or molecular targets [Citation9]. Moreover, candidate drugs identified through this method must fulfill parameters of both anticancer efficacy and minimal in vivo toxicity. ‘Traditional’ target-based drug discovery often fails due to in vivo toxicities that are typically tested later in the process. Zebrafish phenotypic screens bypass this issue with toxicity assessment as the first filtering criterion, saving both cost and time.
Drugs discovered by this approach include Lenaldekar that was identified from a library of 26,400 molecules [Citation10]. Lenaldekar exhibits selective killing of multiple types of leukemia with potential for clinical utility. Another example is the discovery of perphenazine for killing T-cell leukemia cells through a similar phenotype-based screen of 4,880 FDA-approved drugs or drug-like molecules [Citation11]. Notably, NSC210627 was discovered by screening a 2,000-chemical library based on melanoma-inhibiting phenotypes [Citation12]. Chemoinformatic structural analysis revealed that NSC210627 resembles dihydroorotate dehydrogenase (DHODH) inhibitors and exerts its anti-melanoma effects through DHODH suppression. DHODH was then validated to be a new druggable target for melanoma. Leflunomide, an FDA-approved DHODH inhibitor for treating rheumatoid arthritis, was evaluated in preclinical studies and a phase I trial for melanoma treatment (NCT01611675) [Citation9]. Leflunomide is now tested in clinical trials for treating multiple myeloma and metastatic triple-negative breast cancer (), demonstrating the ability of zebrafish phenotypic screens to simultaneously identify new targets and therapeutics.
Both target and phenotype-based screens can result in drug repurposing, which is time and cost efficient as the drug is already well characterized. Using zebrafish gastrulation as a readout in a screen, Nakayama et al. identified 20 FDA-approved drugs that can inhibit tumor cell invasion [Citation13]. Their follow-up studies using zebrafish and mouse xenografts led to the identification of pizotifen, an antagonist of serotonin receptor 2C, as a metastasis-suppressing drug. Wang et al. demonstrated that Rosuvastatin, an FDA-approved drug for treating hypercholesterolemia and cardiovascular diseases, impacts endothelial cell function and suppresses prostate tumor growth [Citation14]. Rosuvastatin is now being tested in clinical trials for treating multiple cancers, including metastatic breast cancer, rectal cancer, squamous cell carcinoma, and prostate cancer ().
4. Mechanistic elucidation of the drug candidates
Zebrafish are optimal for studying complex molecular mechanisms that impact organ and system development. Zhu et al. investigated the spatial and temporal role of an activated anaplastic lymphoma kinase (ALK) mutation to neuroblastoma pathogenesis [Citation15]. Their results demonstrated the prosurvival effects of the ALK F1174L mutation that collaborates with MYCN in tumor development, providing implications for targeted therapy. Additionally, compared to in-vitro systems, zebrafish offer a wider range of phenotypes that aid mechanistic studies of drug candidates. For example, fumagillin, an anti-angiogenic natural product that inhibits methionine aminopeptidase type 2 enzyme, induced a gastrulation phenotype in zebrafish embryos like those caused by gene mutations in the noncanonical Wnt5 pathway [Citation16]. This led to further elucidation of the mechanism of action of fumagillin and the discovery of the targetability of the noncanonical Wnt signaling pathway in cancer angiogenesis [Citation16].
5. Lead optimization
Zebrafish are suitable for pharmacokinetics and pharmacodynamics studies using mass spectroscopy and liquid/gas chromatography [Citation6,Citation9]. Therefore, an ADMET profile of selected drugs can be generated using zebrafish. This shows the strong potential of using zebrafish as a platform for structural-activity relationship studies and drug optimization, another vital step in drug discovery [Citation9]. Hence, zebrafish can facilitate the development of the drug prototype into those possessing optimal potency, bioavailability, and minimal toxicity.
6. Lead prioritization for mammalian testing
Mammalian models, particularly mice, are the current gold standard of preclinical testing. However, murine models of cancer are time-consuming to study and are often kept in an artificial sterile environment. Due to cost and regulatory constraints, murine experiments, including toxicity studies, are mostly short-term and predict clinical outcomes inconsistently. On the other hand, the predictability of zebrafish for various toxicity parameters has been validated, with sufficient to good accuracy of 60–100% [Citation17]. Examples of these include cardiotoxicity, developmental toxicity, and seizure liability. The zebrafish is thus suitable for in vivo toxicity screens, which should complement and precede mammalian studies to eliminate toxic compounds early on and save costs.
Efficacy is another major reason for drug candidate attrition in the drug discovery process. Indeed, an estimated 40–50% of drug failure is due to a lack of clinical efficacy [Citation18]. As none of the animal models can perfectly simulate human diseases, employing multiple in vivo systems in preclinical studies can help increase the confidence of a drug candidate. The zebrafish is suitable for this purpose as its physiology and pathophysiology are highly conserved. It can sometimes simulate drug effects in humans better than mice do, as shown in the study of thalidomide’s ability to cause morphological limb defects in zebrafish while lacking any teratogenic effects in mice [Citation9]. Combined with the ease to assess the ADMET profiles of drugs, zebrafish can ascertain and prioritize drug candidates for further testing in mammalian models.
7. Zebrafish avatars to advance precision medicine
Over the past 10 years, precision medicine has emerged to personalize and improve treatment for cancer patients. Cancer avatars, which historically rely on in vitro organoids and mice xenografts, serve as a key tool to predict responses and select drugs for individual patients. However, these avatars have substantial limitations including biological fidelity and logistical constraints in cost and time [Citation2,Citation19]. Zebrafish avatars can overcome the limitation of in vitro organoid models by simulating complex physiological environments. Additionally, due to their high fecundity and small size, zebrafish cancer avatars can complement murine models with increased statistical outputs, reduced patient tissue usage, and rapid readouts [Citation19]. Currently, zebrafish avatars are often established through transplanting patient-derived tumor samples into zebrafish embryos. High throughput screening with a variety of therapeutic agents is then conducted and readouts are often available within just 4–7 days, enabling the selection of high-efficacy treatments to guide clinical decisions.
Despite being relatively new, the predictability of zebrafish avatars has been validated in several types of cancers, such as lung cancer, breast cancer, colorectal cancer, and leukemia [Citation20]. Recent work demonstrated that zebrafish avatars can faithfully recapitulate radiosensitivity of colorectal cancer and chemosensitivity of both breast cancer and colorectal cancer in patients [Citation20]. Yan et al. discovered the combination therapy of Olaparib and Temozolomide against human rhabdomyosarcoma while validating the biological fidelity of immunodeficient adult zebrafish xenografts [Citation21]. This work subsequently led to the initiation of a clinical trial (NCT01858168). In addition, a trial enrolling 120 cancer patients is currently ongoing to evaluate the ability of zebrafish PDXs to predict individual responses (NCT03668418). This trial is expected to provide valuable insights into the overall utility of zebrafish cancer avatars, highlighting their potential in precision medicine [Citation19].
8. Expert opinion
The zebrafish has demonstrated its unique strengths in cancer research and its suitability to improve the process of drug discovery and development. For instance, it can speed up the discovery of drug targets and the identification of therapeutic leads through phenotypic screens. With its high reproducibility, mechanistic studies in this versatile organism can dissect complex oncogenic pathways and facilitate the selection of combination treatments in a cost-effective manner. The rapid embryonic development of zebrafish can be leveraged to filter out toxic drug candidates and increase success rates of preclinical and clinical testing. Unlike mice, zebrafish are raised in a non-sterile environment. They also manifest telomere shortening, a feature central to human cancer biology yet lacking in mice. Therefore, zebrafish aptly complement the murine models for preclinical testing to decrease the failure rates of drug candidates in clinical trials. Recent evidence emerging from zebrafish avatars also supports their utility in precision medicine to help stratify patient populations for personalized treatment.
The application of zebrafish in drug discovery and development is still in its early stages, having only been introduced into cancer research in the past two decades. As zebrafish are vertebrates, but not mammals, hesitation in their inclusion in preclinical testing and doubts about their relevance to patients still require additional evidence to be overcome. In addition, even though zebrafish can be used to study ADMET of drugs, their pharmacokinetics may be different from those in humans and should be compared carefully. Despite concerns about potential unphysiological responses in zebrafish avatars, studies show that fish tolerate 32–34°C, a temperature conducive to human tumor cell growth.
Future technological advances, such as the generation of fish suited to live at 37°C, will optimize the utility of zebrafish avatars. Pairing zebrafish with other model systems should hasten the process of drug discovery while increasing its success rates. Enhanced communications among the zebrafish research community, the industry, funding agencies, and policymakers will help steer and prioritize translational research using this model system. We hope that the accumulated scientific evidence collected through collaboration between academia and industry will soon convince policymakers to favor zebrafish as an essential tool for drug discovery and development.
Declaration of interest
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
The authors would like to thank D Kashy in the Feng laboratory for his helpful discussions.
Additional information
Funding
References
- Aitken M, Kleinrock M, Connely N, et al. Global Oncology Trends-2023. Parsippany, NJ: IQVIA Institute; 2023.
- Ciucci A, Buttarelli M, Fagotti A, et al. Preclinical models of epithelial ovarian cancer: practical considerations and challenges for a meaningful application. Cell Mol Life Sci. 2022 Jun 16;79(7):364. doi: 10.1007/s00018-022-04395-y
- Astell KR, Sieger D. Zebrafish in vivo models of cancer and metastasis. Cold Spring Harb Perspect Med. 2020 Aug 3;10(8):a037077. doi: 10.1101/cshperspect.a037077
- Loveless R, Shay C, Teng Y. Unveiling tumor microenvironment interactions using zebrafish models. Front Mol Biosci. 2020;7:611847. doi: 10.3389/fmolb.2020.611847
- Anderson NM, Li D, Peng HL, et al. The TCA cycle transferase DLST is important for MYC-mediated leukemogenesis. Leukemia. 2016 Jun;30(6):1365–1374. doi: 10.1038/leu.2016.26
- Letrado P, de Miguel I, Lamberto I, et al. Zebrafish: speeding up the cancer drug discovery process. Cancer Res. 2018 Nov 1;78(21):6048–6058. doi: 10.1158/0008-5472.CAN-18-1029
- Mandelbaum J, Shestopalov IA, Henderson RE, et al. Zebrafish blastomere screen identifies retinoic acid suppression of MYB in adenoid cystic carcinoma. J Exp Med. 2018 Oct 1;215(10):2673–2685. doi: 10.1084/jem.20180939
- Lee HC, Lin CY, Tsai HJ. Zebrafish, an in vivo platform to screen drugs and proteins for biomedical use. Pharmaceuticals (Basel). 2021 May 24;14(6):500. doi: 10.3390/ph14060500
- Patton EE, Zon LI, Langenau DM. Zebrafish disease models in drug discovery: from preclinical modelling to clinical trials. Nat Rev Drug Discov. 2021 Aug;20(8):611–628. doi: 10.1038/s41573-021-00210-8
- Ridges S, Heaton WL, Joshi D, et al. Zebrafish screen identifies novel compound with selective toxicity against leukemia. Blood. 2012 Jun 14;119(24):5621–5631. doi: 10.1182/blood-2011-12-398818
- Gutierrez A, Pan L, Groen RW, et al. Phenothiazines induce PP2A-mediated apoptosis in T cell acute lymphoblastic leukemia. J Clin Invest. 2014 Feb;124(2):644–655.
- White RM, Cech J, Ratanasirintrawoot S, et al. DHODH modulates transcriptional elongation in the neural crest and melanoma. Nature. 2011 Mar 24;471(7339):518–522. doi: 10.1038/nature09882
- Nakayama J, Tan L, Li Y, et al. A zebrafish embryo screen utilizing gastrulation identifies the HTR2C inhibitor pizotifen as a suppressor of EMT-mediated metastasis. Elife. 2021 Dec 17;10. doi: 10.7554/eLife.70151
- Wang C, Tao W, Wang Y, et al. Rosuvastatin, identified from a zebrafish chemical genetic screen for antiangiogenic compounds, suppresses the growth of prostate cancer. Eur Urol. 2010 Sep;58(3):418–426. doi: 10.1016/j.eururo.2010.05.024
- Zhu S, Lee JS, Guo F, et al. Activated ALK collaborates with MYCN in neuroblastoma pathogenesis. Cancer Cell. 2012 Mar 20;21(3):362–373. doi: 10.1016/j.ccr.2012.02.010
- Zhang Y, Yeh JR, Mara A, et al. A chemical and genetic approach to the mode of action of fumagillin. Chem Biol. 2006 Sep;13(9):1001–1009.
- Bauer B, Mally A, Liedtke D. Zebrafish embryos and larvae as alternative animal models for toxicity testing. Int J Mol Sci. 2021 Dec 14;22(24):13417. doi: 10.3390/ijms222413417
- Sun D, Gao W, Hu H, et al. Why 90% of clinical drug development fails and how to improve it? Acta Pharm Sin B. 2022 Jul;12(7):3049–3062.
- Fazio M, Ablain J, Chuan Y, et al. Zebrafish patient avatars in cancer biology and precision cancer therapy. Nat Rev Cancer. 2020 May;20(5):263–273. doi: 10.1038/s41568-020-0252-3
- Al-Hamaly MA, Turner LT, Rivera-Martinez A, et al. Zebrafish cancer avatars: a translational platform for analyzing tumor heterogeneity and predicting patient outcomes. Int J Mol Sci. 2023 Jan 24;24(3):2288. doi: 10.3390/ijms24032288
- Yan C, Brunson DC, Tang Q, et al. Visualizing engrafted human cancer and therapy responses in immunodeficient zebrafish. Cell. 2019 Jun 13;177(7):1903–1914 e14. doi: 10.1016/j.cell.2019.04.004
