1. General observations
Designer peptide drugs have finally come of age. Ten years ago, we surveyed the state-of-the-art of peptide-based drug development and made recommendations to achieve blockbuster peptide therapeutics [Citation1]. By the time of our latest review last year [Citation2], not because of our recommendations, but rather in spite of them, multibillion dollar peptide drugs became a reality. Let’s try again. When peptide drugs were waking up at the beginning of this millenium, everything was on the table driven by peptide chemistry and biology but not patient needs and pharmacology restrictions. Now, as major and minor peptide-based drugs have become a reality, a careful review of the success stories reveals undeniable trends and directions of the landscape. While original early peptide drug research is usually carried out by biotech and later stage by big pharma, the overriding characteristics appear to be the same. Below I support the prevailing concepts, as I see them, by using the examples of ADP355, our adiponectin receptor agonist peptide that has not reached approval, but is in various phases of clinical trials ().
Table 1. Evolving strategies used during the drug development process of the adiponectin receptor agonist peptide ADP355.
The most striking feature of successful and approved peptide-based drugs is that these are predominantly agonists of receptors/targets or, in more general terms, replacement therapies, of naturally missing or low abundance hormones, or other biological regulators (with perhaps exemptions such as Octreotide/Sandostatin, and smaller scale therapeutics including Abarelix/Plenaxis, Enfuvirtide/Fuzeon and Ziconotide/Prialt). Large market replacement therapy drugs include three Glp-1 receptor agonists, (Semaglutide/Ozempic, Dulaglutide/Trulicity and Liraglutide/Victoza), GnRH analogs (Goserelin/Zoladex, Leuprolide/Lupron), GC-C receptor (Linaclotide/Linzess and Plecanatide/Trulance), GRF receptor (Tesamorelin/Egrifta), and MC receptor (Bremanolide/Vyleesi) activators. This fact is the more surprising considering that the basic principles of turning receptor agonists to antagonists have been worked out long a time ago [Citation3]. Our adiponectin receptor agonist ADP355 and our leptin receptor antagonist were discovered at the same time; the peptides are the same size as well as feature similar non-natural amino acid replacements and exhibit very similar efficacy in a number of oncology and anti-inflammatory preclinical models. However, only the adiponectin replacement therapy has thus far made it into human clinical trials. Apparently, antagonist drugs are either smaller than peptides (small molecules) or significantly larger (monoclonal antibodies). The explanation of the limited number of targets above may rest in the druggability of only a set of signaling proteins, a sheer commercial volume of certain receptor agonist therapies (Glp-1), and/or a highly pleiotropic nature of downstream processes allowing multiple unrelated uses (Leuprolide depot is prescribed for endometriosis, uterine fibrosis, advanced prostate cancer, and central precocious puberty), although Glp-1 itself can induce a multitude of downstream effects, including reduction of insulin resistance, decrease in hyperglycemia, loss of body weight, reduction of blood pressure, decrease in reactive oxygen species production, modulation of the inflammatory response, and improvement of reproductive function [Citation4]. Notably, most above-mentioned peptides are GPCR ligands. The success rates of GPCR response modifier drugs, including many peptide-based products, appear to be higher than the average of the new agents submitted for FDA approval [Citation5].
A new way of broadening the pool of disease targets is designing fusion peptides that act as agonists of two receptors, e.g. dual Glp-1 and GIP replacement agents such as Tirzepatide/Mounjaro, or the currently investigated triple Glp-1 – GIP – glucagon receptor agonist Retatrutide. The potential for multiple clinical uses allows label extension after approval or switching the clinical indication if human trials hit a snag. When ADP355 produced unsatisfactory results in phase 3 clinical trials against dry eye disease, thanks to a series of options for adiponectin replacement therapy, the developer Allysta Pharmaceuticals prioritized systemic administration for muscular dystrophy [Citation6] and cardiometabolic diseases.
Finally, from a chemistry perspective, although all these hormone/activator replacement therapies are based on natural products (nota bene none of the peptide drugs above came from high-throughput library screening), all contain non-natural amino acid residues or organic chemistry appendages to extend the circulation time, target given tissues or processes or simply expand the chemical space to improve activity. For example, the long fatty-acid side chain on Semaglutide improves the half-life in the blood by binding to serum albumin [Citation7]. Inclusion of the four non-natural amino acid residues in ADP355 not only improves the pharmacokinetic properties but also improves the in vitro efficacy by 100-fold relative to the native active site sequence [Citation8].
2. Specific considerations
Most currently marketed flavor-of-the-day peptide-based drugs and those under late-stage development are either derivatives of naturally occurring hormones and other signaling bioamides that are not suitable for pharmacological development in their native form (Glp-1, GnRH, GRF), or fragments of non-druggable signaling full-length proteins (adiponectin). The clinical development of these drugs became possible by introducing non-natural amino acid residues that improve stability, circulation characteristics, and/or targeting. Optimizing the amino acid composition is recommended for multiple reasons. In addition to improving drug-like properties and expanding the chemical space, unique sequences enjoy strong intellectual property benefits. While native nucleic acid and protein sequences cannot be patented [Citation9], the general consensus from patent lawyers is that a 30% change in the amino acid composition will make composition-of-matter patenting easier to pursue. Fortunately, a wide range of proteinogenic, amino fatty acid and non-natural residues are commercially available for direct incorporation into synthetic peptides. The nutritional supplement and cosmetics industry helps us in selecting non-natural replacement residues such an ornithine for positively charged [Citation10], norvaline for aliphatic [Citation8], or pyroglutamate for cyclic residues that are metabolites of proteinogenic amino acids. Rather than random combinatorial libraries, lead optimization employs a design-based limited and targeted set of peptide analogs individually synthesized, focusing changes only on those positions that are replaceable without compromising the desired activity level [Citation11]. In our experience, for a 10–12-mer peptide, a pool of 50–60 analogs will provide designer peptides that are superior to native counterparts in pharmacological terms and exhibit a reliable basis for intellectual property filing.
The in vitro and in vivo efficacy screening of peptide drug candidates is not specific for this drug type. What needs attention is the quantification of receptor/target binding. Peptides are small molecules, and any appendage significantly reduces the Kd to the receptor. For all practical purposes, direct radioligand peptide–receptor binding measurements are very infrequently used, probably due to the lack of the availability of radiolabeled amino acid monomers suitable for high-volume peptide synthesis and the reluctance of commercial peptide manufacturers in dealing with radioactive peptide preparation and purification. The two everyday laboratory techniques suitable for direct measuring naked peptide–protein interactions are isothermal calorimetry (ITC) and surface plasmon resonance (SPR). While for 10–12-mers the former frequently fails to produce sufficient heat to quantify, the latter has to employ the naked small peptide in the moving phase resulting in weak signals. Having said that, SPR remains extremely useful for measuring peptide–receptor interaction. Fluorescence polarization using fluorescein-tagged ADP355 (at either termini) identified the Kd to the adiponectin receptor approximately 1 µM [Citation12], hardly at the desired value for pharmaceutical development. By SPR using naked peptide the real figure is a much more appealing 50 nM. The complexity of reliable target binding and PK/PD measurements for peptides renders the implementation of relevant biomarkers even more difficult but extremely important.
Finally, a word about peptide manufacturing. The most frequently used solvents and reagents used for large-scale peptide synthesis are toxic, but apparently the dimethylformamide/piperidine combination is hard to replace to keep production yields high and process time low [Citation13]. Liquid phase synthesis decreases the reagent excess, but the coupling steps take longer, making a negative impact on the labor cost component. Where improvements are made and can be further implemented are the downstream processes such as purification and lyophilization. Automated continuous purification systems now in use shave 40% off time required while increasing the yield by continuously reprocessing the discarded material. Another hot topic is replacement of lyophilization with precipitation and crystallization, especially for orally administered peptides.
3. Expert opinion
The rise of lifestyle drugs (medications to treat conditions that are not immediately life-threatening and not overwhelmingly disabling including obesity, sexual dysfunction, hair loss, or skin appearance, among others) has completely transformed the prescription and over-the-counter drug market landscape in recent years, and peptide drugs are not exempt to this trend. When patients and consumers want something badly, oral bioavailability is no longer a must, although the less frequently a medication has to be administered, the better (). Now novel Glp-1 agonists are investigated for once-monthly administration timing. When the need for reducing the patient costs of Semaglutide reaches the U.S. Congress, you can bet that fluctuations in personal income levels will not decrease the demand for lifestyle replacement therapy medications. Designer peptide drugs are here to stay. Luckily, receptors for peptide agonist therapy induce pleiotropic signaling events, allowing the optimized peptide ligand to target various conditions and thus helping return of investment projections. The advent of these new drugs has also started a search for effective remedies for associated side effects (muscle loss, sarcopenia, etc), and for developing resistance after long-term use. This could be a new field for peptide-based drug development. While the cost of goods is only a small fraction of total drug development expenses, peptide manufacturing still remains a relatively money-intensive endeavor, uses non-green chemicals, and accordingly limits the expansion of peptide use in consumer products. In spite of the remarkable efficacy of one of the host defense peptides I am familiar with in preclinical models of skin injuries and infections, it could not be added as a component of skin patches due to the unacceptable increase in price of the medicated product in a current competitive over-the-counter market. After this it is surprising that a casein phosphopeptide was made as a component of tooth paste [Citation14] and the widespread marketing of peptide-based beauty products [Citation15], although customers do complain about relatively high retail prices. The author of this Editorial is currently trying both the toothpaste and a multiple palmitoyl oligopeptide-containing skin care product out of curiosity and for the sake of this article. In the highly competitive landscape as alternatives to small molecule therapies, or just compared to other legitimate and copycat peptide drugs, a very strong intellectual property position following a relatively detailed lead optimization is a must. For both large-volume and froufrou small-scale therapies, the end costs have to come down as price pressure works against peptide-based medications [Citation2]. Peptide agonists represent ‘green’ drugs (they are based on natural substances), but peptide manufacturing is far from being that. While it is not mandatory now, for continued public support of peptide drugs, the manufacturing process has to be more environmentally friendly. Nevertheless, the future is rosy for peptide-based therapies as long as they remain patient-focused and not chasing old, unproven theoretical dreams.
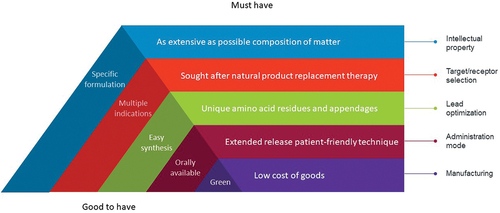
Figure 1. Must have and good to have characteristics to maintain the momentum in peptide-based drug development. A strong intellectual property position is needed to fight off legitimate and copycat competitors; peptide-specific formulation is a plus. The drugs should replace low-abundance natural ligands of receptors or target proteins possibly with potential for multiple clinical applications. The drugs have to be optimized for patentability, efficacy, circulation time, and distribution; easy peptide synthesis is a welcome addition. It is mandatory to administer the peptides as less frequently as possible with contemporary devices and formulations; oral bioavailability is a nice feature, but the lack of it is no longer a game stopper. The manufacturing price has to be reduced, possibly by using green solvents and processes.
Abbreviations
FDA, U.S. Food and Drug Administration; GC-C, guanylate cyclase-C; Glp-1, glucagon-like peptide 1; GIP, gastric inhibitory polypeptide; GnRH, gonadotropin hormone-releasing hormone; GPCR, G-protein-coupled receptor, GRF, growth regulating factor; IP, intellectual property; ITC, isothermal calorimetry; Kd, dissociation constant; PD, pharmacodynamics; PK, pharmacokinetics; SPR, surface plasmon resonance
Declaration of interest
L Otvos Jr. is a paid consultant and scientific advisor to Allysta Pharmaceuticals focusing on developing adiponectin receptor agonists for various clinical applications as well as a employee/consultant with OLPE Pharmaceutical Consultants. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Otvos L Jr, Wade JD. Current challenges in peptide-based drug discovery. Front Chem. 2014;2:62. doi: 10.3389/fchem.2014.00062
- Otvos L Jr, Wade JD. Big peptide drugs in a small molecule world. Front Chem. 2023;11:1302169. doi: 10.3389/fchem.2023.1302169
- Hruby V. Designing peptide receptor agonists and antagonists. Nat Rev Drug Discov. 2002;1(11):847–858. doi: 10.1038/nrd939
- Pandey S, Mangmool S, Parichatikanond W. Multifaceted roles of GLP-1 and its analogs: a review on molecular mechanisms with a cardiotherapeutic perspective. Pharmaceuticals. 2023;16(6):836. doi: 10.3390/ph16060836
- Hauser AS, Attwood MM, Rask-Andersen M, et al. Trends in GPCR drug discovery: new agents, targets and indications. Nat Rev Drug Discov. 2017;16(12):829–842. doi: 10.1038/nrd.2017.178
- Bellissimo CA, Gandhi S, Castellani LN, et al. The slow-release adiponectin analog ALY688-SR modifies early-stage disease development in the D2.Mdx mouse model of Duchenne muscular dystrophy. Am J Physiol Cell Physiol. 2024;326:C1011–C1026. doi: 10.1152/ajpcell.00638.2023
- Lau J, Bloch P, Schäffer L, et al. Discovery of the once-weekly glucagon-like peptide-1 (GLP-1) analogue Semaglutide. J Med Chem. 2015;58(18):7370–7380. doi: 10.1021/acs.jmedchem.5b00726
- Otvos LJ, Haspinger E, La RF, et al. Design and development of a peptide-based adiponectin receptor agonist for cancer treatment. BMC Biotechnol. 2011;11:90. doi: 10.1186/1472-6750-11-90
- Klusty T, Weinmeyer R. Supreme court to myriad genetics: Synthetic DNA is patentable but isolated genes are not. AMA J Ethics. 2015;17:849–853. doi: 10.1001/journalofethics.2015.17.9.hlaw1-1509
- Bluhm ME, Schneider VA, Schäfer I, et al. N-Terminal Ile-Orn- and Trp-Orn-motif repeats enhance membrane interaction and increase the antimicrobial activity of Apidaecins against Pseudomonas aeruginosa. Front Cell Dev Biol. 2016;4:39. doi: 10.3389/fcell.2016.00039
- Hansen S, Nile AH, Mehta SC, et al. Lead optimization yields high affinity frizzled 7-targeting peptides that modulate clostridium difficile Toxin B pathogenicity in epithelial cells. J Med Chem. 2019;62:7739–7750. doi: 10.1021/acs.jmedchem.9b00500
- Sun Y, Zang Z, Zhong L, et al. Identification of adiponectin receptor agonist utilizing a fluorescence polarization based high throughput assay. PLOS ONE. 2013;8(5):e63354. doi: 10.1371/journal.pone.0063354
- Ferrazzano F, Catani M, Cavazzini A, et al. Sustainability in peptide chemistry: current synthesis and purification technologies and future challenges. Green Chem. 2022;24(3):975–1020. doi: 10.1039/D1GC04387K
- Reise M, Kranz S, Heyder M, et al. Effectiveness of casein phosphopeptide-amorphous calcium phosphate (CPP-ACP) compared to fluoride products in an in-vitro demineralization model. Materials. 2021;14:5974. doi: 10.3390/ma14205974
- Ngoc LTN, Moon J-Y, Lee Y-C. Insights into bioactive peptides in cosmetics. Cosmetics. 2023;10(4):111. doi: 10.3390/cosmetics10040111
