Abstract
Emergomycosis is a dimorphic fungal disease that is typically disseminated and fatal among immunocompromised individuals. In the case report, we presented a patient with intermittent fever, night sweats, coughing and phlegm. Chest computed tomography revealed multiple soft-tissue nodules in both lungs. Routine pathological and microbiological tests did not confirm the diagnosis. Therefore, we conducted pathogen detection using metagenomic next-generation sequencing in bronchoalveolar lavage fluid and identified the pulmonary infection caused by Emergomyces orientalis (Es. orientalis). During the antifungal treatment, the patient experienced renal function damage, and we have attempted various antifungal drugs for treatment. Finally, the patient’s condition was brought under control. Therefore, the metagenomic next-generation sequencing pathogen detection was essential.
Plain language summary
We report a case of a rare illness caused by the fungus Emergomyces orientalis (Es. orientalis). The patient had a fever, cough and small lumps were found in his lungs. We diagnosed the illness using a method called metagenomic next-generation sequencing that identified the fungus from the DNA in a patient sample. The drug that was given to the patient worked, but it did cause some issues with his kidneys. This report can help to inform how patients are treated in the future.
Background
Emergomyces orientalis (Es. orientalis) is a rare pathogen.
Case presentation
We report the diagnosis and treatment process of a patient with emergomycosis diagnosed through metagenomic next-generation sequencing detection.
The patient was a 52-year-old male.
Chest enhancement computed tomography (CT) revealed multiple lesions in both lungs. No pathogenic bacteria were found in the sputum cultures.
Metagenomic next-generation sequencing detection in bronchoalveolar lavage fluid indicated the presence of Es. orientalis infection, with 338 detected sequences and a relative abundance of 49.37%.
We administered an injection of amphotericin B cholesterol sulfate complex as an antifungal treatment.
Discussion & conclusion
Emergomycosis often occurs in patients with immune deficiency, but this patient had normal immune function.
At the initial stage of diagnosis, we suspected the patient to be infected with tuberculosis, but the final diagnosis was a rare fungal infection.
Currently, no standard treatments prevail for emergomycosis. Injection with amphotericin B cholesterol sulfate complex was effective. However, the patient had an intermittent renal function injury during the course of treatment. This should be kept in mind for future treatment.
1. Background
Emergomycosis is an emerging dimorphic fungal disease usually observed in immunocompromised individuals [Citation1,Citation2]. It is a biphasic fungus that exists in the environment in the mycelial phase. In mammalian tissues, it forms large, round, thick-walled sterile macrospores or small, round, thin-walled yeast-like cells [Citation3,Citation4]. In 2014, human immunodeficiency virus (HIV) infected people in South Africa were deeply affected by emergomycosis, drawing great attention [Citation5,Citation6]. To date, emergomycosis has been reported in four major areas: Africa [Citation5,Citation6], Asia [Citation7,Citation8], Europe [Citation9] and India [Citation10,Citation11]. However, some researchers believe that the disease could become a global epidemic [Citation12]. According to regional divisions, Emergomyces infection reported in China is caused by Emergomyces orientalis (Es. orientalis) [Citation8].
Studies have shown that three-quarters of the patients with emergomycosis are misdiagnosed with tuberculosis and treated for the latter [Citation13]. Diagnosis can be made using fungal cultures of the affected tissue obtained by histopathological examination and biopsy. Sputum culture and histopathological examination of the patient in question were both negative, and the metagenomic next-generation sequencing (mNGS) pathogen of the bronchoalveolar lavage fluid (BALF) detected Es. orientalis. According to relevant studies, compared with traditional pathogen detection methods, mNGS has a higher pathogen detection rate. It can detect more types and quantities of pathogens, especially those that are rare or difficult to culture [Citation14,Citation15]. Here, we report the diagnosis and treatment process of a patient with emergomycosis diagnosed through mNGS detection.
2. Case presentation
The patient was a 52-year-old male. One month before hospitalization, the patient developed a fever reaching a maximum body temperature of 39.1°C, which was more pronounced in the afternoon due to a cold. Additionally, he experienced night sweats accompanied by coughing and black sticky phlegm. After oral administration of ibuprofen, his body temperature decreased to normal. Two weeks prior, he visited our outpatient department for chest enhancement computed tomography (CT), which revealed multiple lesions in both lungs. No pathogenic bacteria were found in the sputum cultures. Levofloxacin was administered orally; however, the symptoms did not improve significantly. He was hospitalized on 23 April 2023. The patient had no history of disease or smoking, but he had been engaged in coal mining work 3 months before the onset of the disease.
Physical examination revealed hypertension. No rashes were found on the whole body, no superficial lymph node enlargement, no positive signs such as hepatosplenomegaly, and no abnormalities were found on other physical examinations.
The following abnormalities in laboratory tests were found: white blood cell count of 7.73 × 109/l and a neutrophil percentage of 72.7%. No abnormalities were detected in squamous cell carcinoma antigen, serum gastrin-releasing peptide precursor, carcinoembryonic antigen, soluble cytokeratin component or neuron-specific enolase. No acid-fast-positive bacteria were detected in the sputum acid-fast staining; gram-negative bacilli and gram-positive streptococci were detected in the sputum smear, and fungal spores and hyphae were not detected. 1,3- β- D-glucan detection, galactomannan detection, cryptococcus capsular polysaccharide antigen detection, and gamma-interferon release test were all negative. No positive results were detected in sputum cultures. An antineutrophil cytoplasmic antibody (ANCA) assay showed that cytoplasmic ANCA, perinuclear ANCA, protease 3 and antiglomerular basement membrane antibodies were all negative, whereas myeloperoxidase was positive. The enhanced chest CT at admission showed multiple soft-tissue nodules in both lungs (CT images are shown in A–C). Superficial lymph node ultrasonography revealed multiple abnormal lymph nodes in the left clavicle. Therefore, lung and supraclavicular lymph node biopsies were performed. The pathological results from the lungs showed a small amount of lung tissue with charcoal deposition, and some alveolar cavities showed tissue cells (A & B). The pathological results of the lymph node needle aspiration smear showed a few visible lymphocytes. To further clarify the diagnosis, we conducted a bronchoscopic examination and found no abnormalities under the microscope. mNGS detection in BALF indicated the presence of Es. orientalis infection, with 338 detected sequences and a relative abundance of 49.37%.
Figure 1. The chest computed tomography images of the patient at the first visit. (A–C) CT images of different transverse sections.
CT: Computed tomography.
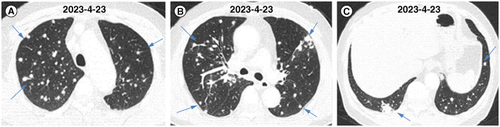
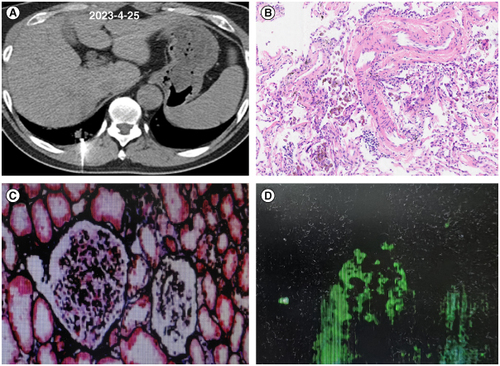
Figure 2. Tissue pathology images of lung biopsy and kidney biopsy. (A) CT images during lung puncture biopsy. (B) Pathological image of hematoxylin-eosin staining in lung tissue. (C) Images of renal tissue stained with periodic acid-silver methe-namine-masson staining. (D) Immunofluorescence detection of IgA in renal tissue.
CT: Computed tomography.
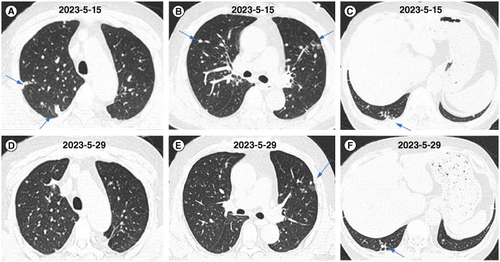
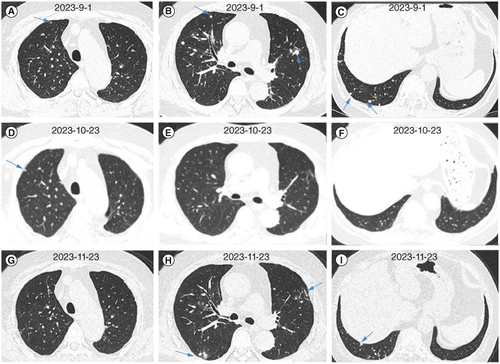
We administered an injection of amphotericin B cholesterol sulfate complex as an antifungal treatment. After 6 days of antifungal treatment, when rechecking the patient’s renal function, the patient’s creatinine was 107.8 mmol/l (normal range: 57–97 mmol/l) and urea nitrogen was 11.1 mmol/l (normal range: 3.1–8 μmol/l). Changes in creatinine and urea levels are shown in ; changes were considered to be caused by the therapeutic drugs. Therefore, we reduced the medication dosage, which was maintained between 100–150 mg per day. After one week of treatment, the patient’s body temperature returned to normal, and cough significantly decreased. After 9 days and 23 days of treatment, chest CT showed a significant reduction in lung lesions in both lungs (A–F). Then, the patient was discharged for treatment. After discharge, due to the inability to administer injectable medication at home, the patient switched to oral itraconazole treatment. After oral administration of itraconazole outside the hospital for 1 month, the patient’s lung lesions increased. Therefore, amphotericin B was administered intravenously outside the hospital for treatment. After the treatment, the patient’s blood creatinine and urea levels increased again.
Figure 3. Trend of blood urea and creatinine detection in the patient undergoing antifungal treatment for 6 months. The X-axis represents time, and the Y-axis represents the numerical value of the detection result. (A) The changing trend of urea. (B) The changing trend of creatinine.
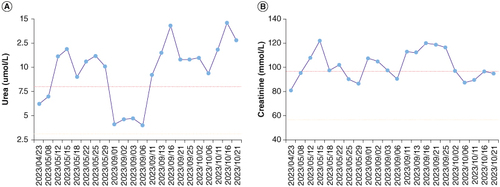
Figure 4. Changes in chest computed tomography images of the patient treated with antifungal therapy within 1 month. (A–C) Chest CT images after 9 days of antifungal treatment. (D–F) Chest CT images after 23 days of antifungal treatment.
CT: Computed tomography.
After nearly 4 months of antifungal treatment, the patient was readmitted to investigate the cause of renal dysfunction. At that time, he underwent chest CT, which still showed nodular lesions in both lungs (A–C). Then, a renal biopsy was performed, and the pathological results were consistent with focal proliferative IgA nephropathy accompanied by acute tubular injury during the recovery period (images shown in C & D). Diagnosis: Nephritis syndrome: Focal proliferative IgA nephropathy (Oxford classification M1S1E0T0C0). Recovery period from acute renal tubular injury. We initially suggested close monitoring of renal function and not administering medication. Based on changes in the patient’s renal function, we continued to administer amphotericin B cholesterol sulfate complex injections at a dose of 100 mg/day. After 1 month of treatment according to the dosage reduction plan, the chest CT scan shown significant absorption of nodules in both lungs (D–F). We then conducted a BALF mNGS test, and the results showed no detection of Es. orientalis. Considering that the nodules in both lungs were not completely absorbed, the patient was given oral voriconazole treatment, administered outside the hospital. No damage to the patient’s renal function was observed during the oral voriconazole treatment. The patient is currently undergoing treatment for the condition (G–I).
Figure 5. Changes in chest computed tomography images of the patient treated with antifungal therapy between 4 and 6 months. (A–C) Chest CT images after 4 months of antifungal treatment. (D–F) Chest CT images after 5 months of antifungal treatment. (G–I) Chest CT images after 6 months of antifungal treatment.
CT: Computed tomography.
3. Discussion & conclusion
Literature reports indicate that emergomycosis often occurs in patients with immune deficiency [Citation1,Citation2]. This patient had hypertension before treatment and a history of working in a coal mine. However, it can be seen that patients with normal immune function can also be infected by Emergomyces. In addition, it has been reported in the literature that about 20.8% of emergomycosis patients are uninfected with HIV, and 25% of HIV uninfected patients were immunocompetent [Citation16]. The patient that we are currently reporting on is an immunocompetent patient. At the initial stage of diagnosis, we suspected the patient to be infected with tuberculosis, but the final diagnosis was a rare fungal infection, which was quite unexpected. The patient’s work involved coal mining, and the humid working environment may be the main cause of the disease.
Patients infected with Emergomyces can develop infections in multiple organs, such as the lungs, liver, skin, subcutaneous tissue and bone marrow [Citation5,Citation7,Citation9,Citation10]. Following a lung infection occurs, patients may experience coughing, fever, shortness of breath and other manifestations [Citation7,Citation8]. This patient exhibited these symptoms at an early stage of the disease. The chest CT scan of the patient showed multiple nodular lesions in the two lungs, approximately 1–2 cm in size, which were similar to images of pulmonary sarcoidosis, pulmonary metastases and tuberculosis. Moreover, the chest CT scans of patients diagnosed with Es. orientalis infections mostly showed pulmonary nodule shadows [Citation9]. Therefore, we performed a lung biopsy at the initial stage of treatment; however, the pathological results of the biopsy did not yield a definitive diagnosis. Finally, the infection was diagnosed using mNGS. Subsequently, the patient's two pulmonary nodules significantly reduced after treatment with antifungal drugs, indicating that the treatment was effective. We have summarized the latest case reports on emergomycosis and summarized its epidemiology, clinical manifestations, and treatment in [Citation17–23].
Table 1. Summary of major cases reported in the literatures.
Currently, no standard treatments prevail for emergomycosis. In vitro data suggest that fluconazole resistance is common, but susceptibility to other triazoles and amphotericin B is generally preserved [Citation24,Citation25]. In this case, both pulmonary nodules were reduced after injection of the amphotericin B cholesterol sulfate complex, indicating that the drug was effective. However, the patient had an intermittent renal function injury during the course of treatment. Related clinical study has reported that the main adverse reaction of amphotericin B liposomes is renal dysfunction [Citation26], and the pathological changes in this patient through renal puncture biopsy are also consistent with drug-induced renal injury. Throughout the entire treatment process, we continuously tested creatinine and urea nitrogen to adjust the dosage of medication in a timely manner. The patient was also treated with itraconazole, but the nodules in both lungs were not controlled by this, which further suggests that amphotericin B cholesterol sulfate complex injection, as a broad-spectrum antifungal drug, remains the first choice in antifungal treatment. The efficacy of other antifungal drugs, such as voriconazole and posaconazole, remains to be verified. According to the literature, there is no standard treatment cycle. After 6 months of treatment, chest CT showed that the lesions in both lungs were significantly reduced, and the mNGS pathogen test did not detect Es. orientalis. In the absence of complete absorption of pulmonary lesions, we recommend continued use of antifungal drugs for achieving complete recovery.
In the diagnostic process, we promptly applied mNGS pathogen detection to confirm the disease. After antifungal treatment, the infection was controlled. Therefore, the development of mNGS pathogen detection technology is worth pursuing. Through the treatment of this patient, it can be seen that most of the lung lesions gradually shrink in a short period of time. The reason for the better treatment effect may be related to the patient’s normal immune function. This patient is still under close follow-up, and we also look forward to their early recovery.
Author contributions
P Wang and Y Jiang designed the study, supervised all work, and helped write the manuscript. R Tian, C Zhang, L Zhang, X Cui help to collect patient information. All authors contributed to the article and approved the submitted version.
Financial disclosure
This research received funding from Hebei Provincial Department of Science and Technology of the key research and development program projects (No.22377715D), and Hebei Provincial Health Commission of the Hebei medical application technology tracking project plan (GZ2023038). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Writing disclosure
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
This study was approved by the Ethics Review Committee of the Fourth Hospital of Hebei Medical University, registration number 2023KS206. The participant provided written consent.
Acknowledgments
The authors thank the patient involved in the study for their participation. The authors thank the doctors and nurses from the department of the Fourth Hospital of Hebei Medical University for assistance with follow-up.
Competing interests disclosure
The authors have no competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Additional information
Funding
References
- Schwartz IS, Govender NP, Sigler L, et al. Emergomyces: the global rise of new dimorphic fungal pathogens. PLoS Pathog. 2019;15(9):e1007977. doi:10.1371/journal.ppat.1007977
- Rooms I, Mugisha P, Gambichler T, et al. Disseminated emergomycosis in a person with HIV infection, Uganda. Emerg Infect Dis. 2019;25(9):1750–1751. doi:10.3201/eid2509.181234
- Kenyon C, Bonorchis K, Corcoran C, et al. A dimorphic fungus causing disseminated infection in South Africa. N Engl J Med. 2013;369(15):1416–1424. doi:10.1056/NEJMoa1215460
- Anstead GM, Sutton DA, Graybill JR. Adiaspiromycosis causing respiratory failure and a review of human infections due to Emmonsia and Chrysosporium spp. J Clin Microbiol. 2012;50(4):1346–1354. doi:10.1128/JCM.00226-11
- van Hougenhouck-Tulleken WG, Papavarnavas NS, Nel JS, et al. HIV-associated disseminated emmonsiosis, Johannesburg, South Africa. Emerg Infect Dis. 2014;20(12):2164–2166. doi:10.3201/eid2012.140902
- Heys I, Taljaard J, Orth H. An emmonsia species causing disseminated infection in South Africa. N Engl J Med. 2014;370(3):283–284. doi:10.1056/NEJMc1314277
- Tang XH, Zhou H, Zhang XQ, Han JD, Gao Q. Cutaneous disseminated emmonsiosis due to Emmonsia pasteuriana in apatient with cytomegalovirus enteritis. JAMA Dermatol. 2015;151(11):1263–1264. doi:10.1001/jamadermatol.2015.1792
- Wang P, Kenyon C, de Hoog S, et al. A novel dimorphic pathogen, Emergomyces orientalis (Onygenales), agent of disseminated infection. Mycoses. 2017;60(5):310–319. doi:10.1111/myc.12583
- Gast KB, van der Hoeven A, de Boer MGJ, et al. Two cases of Emergomyces pasteurianus infection in immunocompromised patients in the Netherlands. Med Mycol Case Rep. 2019;24:5–8. doi:10.1016/j.mmcr.2019.01.005
- Malik R, Capoor MR, Vanidassane I, et al. Disseminated Emmonsia pasteuriana infection in India: a case report and areview. Mycoses. 2016;59(2):127–132. doi:10.1111/myc.12437
- Capoor MR, Mishra N, Kolte S, et al. Disseminated Emergomyces pasteurianus infection in India: a case report and a review. Mycopathologia. 2020;185(1):193–200. doi:10.1007/s11046-019-00387-y
- Samaddar A, Sharma A. Emergomycosis, an emerging systemic mycosis in immunocompromised patients: current trends and future prospects. Front Med (Lausanne). 2021;8:670731. doi:10.3389/fmed.2021.670731
- Schwartz IS, Govender NP, Corcoran C, et al. Clinical characteristics, diagnosis, management and outcomes of disseminated emmonsiosis: a retrospective case series. Clin Infect Dis. 2015;61(6):1004–1012. doi:10.1093/cid/civ439
- Jiang J, Yang W, Wu Y, et al. Metagenomic next-generation sequencing for identifying pathogens in patients with rheumatic diseases and diffuse pulmonary lesions: a retrospective diagnostic study. Front Cell Infect Microbiol. 2022;12:963611. doi:10.3389/fcimb.2022.963611
- Yan H, Li Z, Xia H, Li Q, Bai H. A case report on mixed pulmonary infection of nocardia nova, mycobacterium tuberculosis, and aspergillus fumigatus based on metagenomic next-generation sequencing. Front Public Health. 2022;10:927338. doi:10.3389/fpubh.2022.927338
- Vinayagamoorthy K, Gangavaram DR, Skiada A, Prakash H. Emergomycosis, an emerging thermally dimorphic fungal infection: a systematic review. J Fungi (Basel). 2023;9(10):1039. doi:10.3390/jof9101039
- Schwartz IS, Sanche S, Wiederhold NP, Patterson TF, Sigler L. Emergomyces canadensis, a dimorphic fungus causing fatal systemic human disease in North America. Emerg Infect Dis. 2018;24(4):758–761. doi:10.3201/eid2404.171765
- Feng P, Yin S, Zhu G, et al. Disseminated infection caused by Emmonsia pasteuriana in a renal transplant recipient. J Dermatol. 2015;42(12):1179–1182. doi:10.1111/1346-8138.12975
- Crombie K, Spengane Z, Locketz M, et al. Paradoxical worsening of Emergomyces africanus infection in an HIV-infected male on itraconazole and antiretroviral therapy. PLoS Negl Trop Dis. 2018;12(3):e0006173. doi:10.1371/journal.pntd.0006173
- Capoor MR, Mishra N, Kolte SG, et al. Disseminated Emergomyces pasteurianus Infection in India: a case report and a review. Mycopathologia. 2020;185(1):193–200. doi:10.1007/s11046-019-00387-y
- Chik KK, To WK. Autochthonous Emergomyces pasteurianus pneumonia in an immunocompromised patient in Hong Kong: a case report. Hong Kong Med J. 2020;26(5):446–448. doi:10.12809/hkmj198280
- He D, Quan M, Zhong H, et al. Emergomyces orientalis emergomycosis diagnosed by metagenomic next-generation sequencing. Emerg Infect Dis. 2021;27(10):2740–2742. doi:10.3201/eid2710.210769
- Mah J, Bakker A, Tseng C, et al. Isolated pulmonary emergomycosis in an immunocompetent patient in Alberta, Canada. Open Forum Infect Dis. 2022;9(3):ofac021. doi:10.1093/ofid/ofac021
- Dukik K, Al-Hatmi AMS, Curfs-Breuker I, Faro D, de Hoog S, Meis JF. Antifungal susceptibility of emerging dimorphic pathogens in the family Ajellomycetaceae. Antimicrob. Agents Chemother. 2017;62(1):e01886–17. doi:10.1128/AAC.01886-17
- Maphanga TG, Britz E, Zulu TG, Mpembe RS, Naicker SD, Schwartz IS. In vitro antifungal susceptibility of yeast and mold phases of isolates of dimorphic fungal pathogen Emergomyces africanus (formerly Emmonsia sp.) from HIV-infected South African patients. J Clin Microbiol. 2017;55(6):1812–1820. doi:10.1128/JCM.02524-16
- Youngs J, Low JM, Whitney L, et al. Safety and efficacy of intermittent high-dose liposomal amphotericin B antifungal prophylaxis in haemato-oncology: an eight-year single-centre experience and review of the literature. J Fungi (Basel). 2020;6(4):385. doi:10.3390/jof6040385
