Abstract
It is well known that physiological variables such as maximal oxygen uptake (), exercise economy, the lactate threshold, and critical power are highly correlated with endurance exercise performance. In this review, we explore the basis for these relationships by explaining the influence of these “traditional” variables on the dynamic profiles of the
response to exercise of different intensities, and how these differences in
dynamics are related to exercise tolerance and fatigue. The existence of a “slow component” of
during exercise above the lactate threshold reduces exercise efficiency and mandates a greater consumption of endogenous fuel stores (chiefly muscle glycogen) for muscle respiration. For higher exercise intensities (above critical power), steady states in blood acid–base status and pulmonary gas exchange are not attainable and
will increase with time until
is reached. Here, we show that it is the interaction of the
slow component,
, and the “anaerobic capacity” that determines the exercise tolerance. Essentially, we take the view that an appreciation of the various exercise intensity “domains” and their characteristic effects on
dynamics can be helpful in improving our understanding of the determinants of exercise tolerance and the limitations to endurance sports performance. The reciprocal effects of interventions such as training, prior exercise, and manipulations of muscle oxygen availability on aspects of
kinetics and exercise tolerance are consistent with this view.
Introduction
The physiological determinants of performance in endurance events (athletic events lasting more than approximately 5 min and requiring a substantial and sustained energy transfer from oxidative pathways) have been investigated extensively (for reviews, see Bassett & Howley, Citation2000; Coyle, Citation1995). The most common approach to defining the determinants of performance is to select laboratory-derived parameters of physiological function [such as maximal oxygen uptake (), the lactate threshold, and
] and perform a series of correlations or multiple regression analysis with a measure of performance such as time to complete the event or mean power output (Bassett & Howley, Citation2000; Conley & Krahenbuhl, Citation1980; Coyle, Citation1995; Coyle, Coggan, Hopper, & Walters, 1988; Jones & Doust, Citation1998). A common outcome of these studies is that the power output or oxygen uptake (
) at the lactate threshold (however defined) is very strongly correlated with endurance performance. What such analyses do not provide is a reason why, for example, the power output at the lactate threshold “determines” endurance performance. This has led some authors to question the value of these parameters in determining endurance performance (MacRae, Citation2006; Noakes, Citation1998). It is our contention, however, that none of the aforementioned parameters “determines” endurance performance, or indeed any other type of sports performance. Rather, we suggest that these “traditional” parameters are important because they determine the character of, and place constraints upon, the kinetics of
during exercise. The kinetics, in turn, determines the instantaneous rate of aerobic and anaerobic energy transfer, the mixture and amount of substrate utilized, and the tolerable duration of the exercise. We suggest that only by appreciating how the “traditional” parameters of physiological function interact with the kinetics of
can the physiological determinants of athletic performance be truly understood.
In this review, we outline the “traditional” and contemporary views of the response to exercise, highlighting the key differences in interpretation. In doing so, we aim to provide a gentle introduction to the field of
kinetics, which is sometimes viewed by those not working in it as incomprehensible. We will then explore the relationship between the traditional parameters of physiological function and the
kinetics, highlighting how the lactate threshold and critical power determine the behaviour of the
response, and how
and anaerobic capacity interact to place constraints upon the
response and the time to exhaustion during high-intensity exercise. We provide key examples of how our thesis can be generalized, using the effects of training, warm-up exercise, and manipulation of oxygen delivery on
and performance.
Oxygen uptake response to exercise: Traditional concepts
Since the work of Krogh and Lindhard (Citation1920) and Hill and Lupton (Citation1923), it has been widely appreciated that sustaining exercise beyond a few seconds depends upon the appropriate supply and utilization of oxygen. However, the widespread use of incremental exercise tests on the one hand, and Douglas bag or mixing chamber analyses in which pulmonary gas exchange data are averaged over periods of 30–60 s on the other, has led to the common misconceptions that: (1) following a “lag” in at the onset of constant-load exercise (the “oxygen deficit”), a steady state is always achieved within about 3 min; and (2)
increases linearly as power output increases to
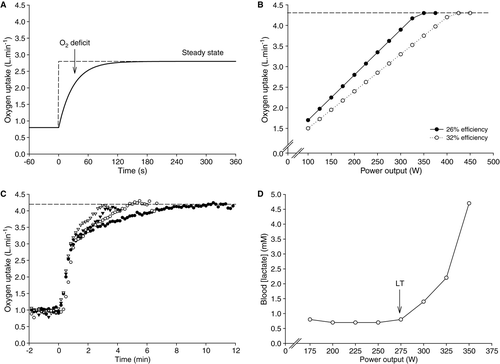
. These ideas are presented in .
Figure 1. Traditional concepts of the oxygen uptake and blood lactate responses to exercise. (A) schematic illustration of the response to cycle exercise performed at 200 W. At the onset of exercise, an oxygen deficit is incurred because the
response lags the energy requirements of the task. A steady-state
of ∼2.80 l · min−1 is achieved within 2–3 min. (B) Schematic illustration of the
response to incremental exercise. Two responses are shown, each yielding the same maximal
(
). However, the mechanical efficiency of the two hypothetical individuals is different, leading to a different relationship between power output and
. The individual with the higher mechanical efficiency achieves
at a higher power output, meaning that they possess a greater scope (in terms of power output) for aerobic work. (C) Real
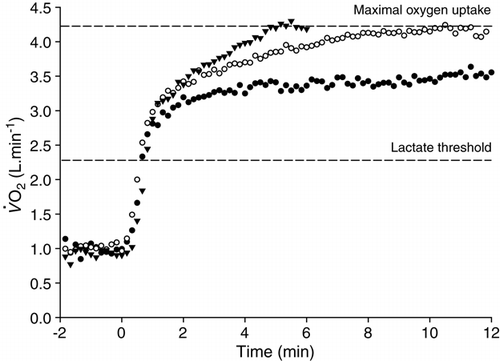
responses to individual bouts of constant power output, high-intensity exercise in a single individual. This panel illustrates that the end-point
is consistent when high-intensity exercise of a short duration (<15 min) is performed. (D) The blood lactate profile during an incremental cycling test. Note that blood lactate concentration remains at baseline concentrations until 275 W (the lactate threshold), but is elevated thereafter.
A shows an idealized response to exercise performed at 200 W. From a baseline value of 0.8 l · min−1 (a typical baseline
if the participant is pedalling against no resistance),
increases in an exponential fashion, reflecting an underlying physiological control process, until a steady state is reached. The
in the steady state in this example is approximately 2.8 l · min−1, meaning that
has increased with a “gain” of ∼10 ml · min−1 · W−1 (i.e. (2800-800)/200). The rapidity with which the steady state is attained depends upon the “speed” or kinetics of the
response to the step increase in external power output. In A, the “time constant” of the
response is 30 s. The time constant is a parameter estimated by fitting an exponential function to the data, and it indicates that after four time constants have elapsed (120 s), the
response has essentially reached its “target”. Therefore, after 2 min the steady state is attained with the difference between the baseline
and the steady state
(the “amplitude” of the response) being 2.0 l · min−1. A smaller value for the time constant (e.g. 20 s) would result in the more rapid attainment of a steady state (in this case, 80 s). This is physiologically useful because a smaller value for the time constant means that the lag in
(i.e. the oxygen deficit) would be smaller, and thus the requirement for “anaerobic” energy provision during the transition from rest to exercise would be reduced. All else being equal, a reduction in the oxygen deficit should be beneficial to exercise performance because it would reduce the depletion of muscle high-energy phosphates (chiefly phosphorylcreatine) and glycogen, and blunt the increase in metabolites that have been associated with the fatigue process and/or which stimulate glycolysis (i.e. adenosine diphosphate, inorganic phosphate, and hydrogen ions). Because the ability to generate energy through substrate-level phosphorylation is considered to be finite (either in capacity or because of the associated accumulation of fatigue-related metabolites), then the exercise tolerance of individuals with very slow Phase II
kinetics, such as those with pulmonary, cardiovascular or metabolic diseases/disorders, might be severely compromised even at very low power outputs (Poole, Kindig, & Behnke, Citation2005). In contrast, elite endurance athletes have remarkably fast Phase II
kinetics that enables them to minimize the magnitude of the oxygen deficit incurred and to limit perturbation to homeostasis in the transition from a lower to a higher metabolic rate (Koppo, Bouckaert, & Jones, Citation2004).
The response to incremental or ramp exercise can be predicted directly from the above outline. An incremental test is simply a series of steps, each of which follows the above description. A test to determine the lactate threshold directly, for example, typically involves stages of 3–4 min duration with small increments (of 20–30 W) between each stage. A 25-W increment would result in a ∼250 ml · min−1 increase in
between each stage, as shown in B. A ramp test would be similar, except that the increase in
would lag the increase in power output by a constant depending on the time constant of the
response. When performing a ramp test, therefore, adjustment must be made to relate
to power output (Whipp, Citation1987). B shows two other important properties of the
–power output relationship. First, mechanical efficiency will determine the steepness of the relationship between
and power output. If efficiency is low, then the increase in
will be relatively steep (∼11 ml · min−1 · W−1); if the participant's efficiency is high, then the increase in
will be less steep (∼9 ml · min−1 · W−1). In addition to reducing the energy cost of any sub-maximal power output [which clearly benefits exercise tolerance in events where the capacity to oxidize carbohydrate is important to performance outcome (Coyle, Coggan, Hemmert, & Ivy, Citation1986; Karlsson & Saltin, Citation1971)], a high mechanical efficiency also increases the power output at which
is reached (Jones & Carter, Citation2000). The second important property of the
–power output relationship is that there is an upper limit to the increase in
(
). The concept of
was originally established using discontinuous constant-load exercise testing (Åstrand & Saltin, Citation1961a; Hill & Lupton, Citation1923), but it is well known that the peak
observed in the more common ramp test is identical to
measured using constant power output exercise (C; see also Day, Rossiter, Coats, Skasick, & Whipp, Citation2003).
Oxygen uptake response to exercise: Contemporary viewpoint
Before addressing the influence of exercise intensity on the response, which is itself central to the role of
kinetics as a performance determinant, there is one additional feature of the
response that has to be mentioned. That is, the pulmonary
response is dependent on the rate of arrival of blood into the pulmonary capillary network and its degree of deoxygenation. In this regard, pulmonary
cannot directly reflect muscle oxygen uptake, because there is a delay of about 10–20 s between oxygen unloading in the muscle [which can be used to determine muscle oxygen uptake using the Fick principle (Grassi et al., Citation1996)] and the arrival of the same blood in the pulmonary vasculature for gas exchange. At the onset of exercise, therefore, there will be a period in which pulmonary
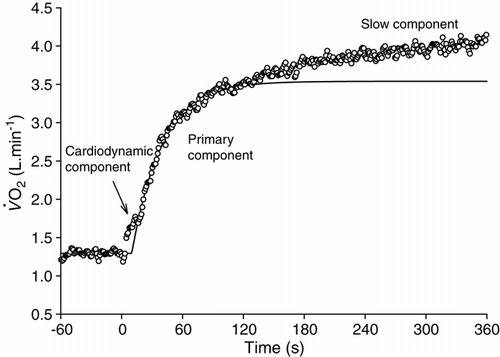
will not reflect muscle oxygen uptake, as was predicted by Barstow and colleagues (Barstow, Lamarra, & Whipp, 1990) and confirmed experimentally by Grassi et al. (Citation1996). As shown in , this period is associated with an abrupt increase in
, chiefly as a result of increased venous return via the muscle pump on the one hand, and increased right ventricular output elevating pulmonary blood flow on the other. As a consequence, this phase of the response (Phase I) has been called the “cardio-dynamic” phase. As the blood returning to the lung in this period has not been subject to increased oxygen extraction by the muscle (having left the muscle microvasculature before capillary PO2 began to fall), venous PO2, PCO2, and the respiratory exchange ratio do not change appreciably from their baseline values. An abrupt change in these variables, in addition to an increase in
that continues in an exponential fashion, signals the onset of the next phase of the
response, which ultimately leads to the attainment of a steady state.
Figure 2. Simultaneous oxygen uptake and phosphorylcreatine (PCr) concentration responses to moderate-intensity knee-extension exercise (from Rossiter et al., Citation1999). (A) The response modelled with no delay (Model 1). (B) The same response modelled with a time delay (Model 2). In (B), the “cardio-dynamic component” is clearly evident. Moreover, the kinetics of the
response using Model 2 (Phase II) is identical to the kinetics of PCr degradation at the onset of exercise (shown in panel C). This figure is reproduced with permission from Rossiter et al. (Citation1999).
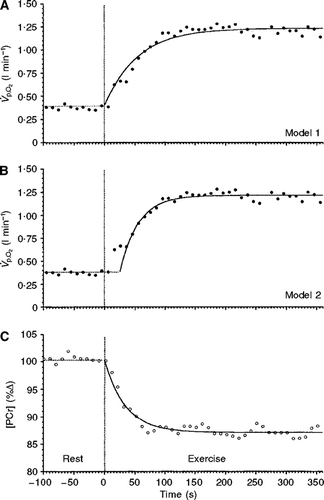
A crucial question, only recently answered, is whether this second period, variously named the fundamental, primary, or fast component (Phase II), reflects the kinetics of muscle oxygen uptake, as was originally proposed by Whipp and colleagues (Whipp, Ward, Lamarra, Davis, & Wasserman, Citation1982) and Barstow et al. (Citation1990)? The answer to this question, provided by comparing direct [i.e. Fick principle (Grassi et al., Citation1996; Koga et al., Citation2005)] or indirect [i.e. 31P-magnetic resonance spectroscopy (Rossiter et al., Citation1999; C] measurements of muscle with simultaneous measurements of pulmonary
, is a qualified “yes” – the qualification being that the time constant of the pulmonary
will reflect the muscle
time constant to within ±10% (Barstow et al., Citation1990). Given the variability in the measurements used (both at the pulmonary and muscle levels), this level of agreement is probably sufficient to use pulmonary
to reflect muscle
(but see Hughson, Tschakovsky, & Houston, Citation2001, for discussion). This relationship is likely to be more robust at higher power outputs, where more of the cardiac output is directed to the exercising muscles, and where the signal-to-noise ratio for the determination of
is higher than at low power outputs. Therefore, the present understanding of the
response to (low- to moderate-intensity) exercise is that it is composed of three distinct phases: Phase I, the cardio-dynamic component; Phase II, the primary or fundamental component; and Phase III, the steady state. Note that this view only differs from that presented in A in that B contains the cardio-dynamic delay, whereas A does not. Much debate surrounds the determinants of the primary
time constant, with discussants falling into one of two camps: those who support the notion that oxygen delivery plays a key role in setting the
time course, and those who suggest that the control of muscle
kinetics has an intracellular origin. We do not address these issues here, as several comprehensive reviews are already available (Poole & Jones, Citation2005; Tschakovsky & Hughson, Citation1999; Whipp & Mahler, Citation1980).
The influence of exercise intensity on the pulmonary oxygen uptake response
It is important to appreciate that the scenario outlined above only holds true for power outputs below the the lactate threshold. Above the lactate threshold, a third component of the response appears that, for reasons explained below, is termed the “slow component”. It remains a source of frustration among those working in the field of
kinetics that this important facet of the
response is, if not unknown to the scientific community, inadvertently or perhaps even deliberately ignored. The blame for its lack of acceptance must lay firmly at the door of textbook writers and teachers of exercise physiology who, with few exceptions, continue to insist that
rises to achieve a steady state at all power outputs up to those requiring
. Our frustration stems from the fact that the existence of the
slow component has been evident for at least 40 years (e.g. Åstrand & Saltin, Citation1961b; Whipp & Wasserman, Citation1972), and its presence during any bout of exercise has major implications for the description and assessment of “exercise intensity” and for computation of the oxygen deficit and rates of substrate utilization (Gaesser & Poole, Citation1996).
The slow component represents a continued increase in
following the primary phase (). This continued rise in
has a considerably slower time course than the Phase II
kinetics, is only evident during exercise above the lactate threshold, and causes
to increase above (as opposed to towards) the anticipated steady-state
value. The slow component appears to be of delayed onset, typically “emerging” 90–180 s after the onset of exercise. Interestingly, although the
slow component has been documented in a variety of activities, its magnitude is less during treadmill running than during some other modes of exercise (Jones & Burnley, Citation2005). It is common to set a power output half-way between the lactate threshold and
to study the
slow component, since the power output at the lactate threshold signifies the highest power output that will not elicit a slow component, and
represents the highest
that can be achieved during exercise. Such a power output would, in
kinetics parlance, be termed “50% delta” – a power output that is calculated as the lactate threshold plus 50% of the difference between the lactate threshold and
. The amplitude of the
slow component during 6–8 min of such exercise typically represents 10–20% of the total
response to exercise (e.g. 200–400 ml · min−1), although the precise amplitude will depend on the experimental conditions, characteristics of the participants, including fitness and muscle fibre type (Barstow, Jones, Nguyen, & Casaburi, Citation1996; Pringle et al., Citation2003), and (most importantly) on the exercise intensity domain in which the exercise takes place; amplitudes in excess of 1 l · min−1 have been observed (Burnley, Doust, & Jones, Citation2005). Although the precise determinants of the
slow component remain obscure, the work of Poole et al. (Citation1991) demonstrated that about 86% of the
slow component could be accounted for by an increase in leg oxygen uptake, suggesting that the exercising muscle is the principal origin of the slow component. In support of this, muscle phosphorylcreatine concentration also demonstrates a slow component-like response during heavy exercise (Haseler, Kindig, Richardson, & Hogan, Citation2004; Rossiter et al., Citation2002). In view of these findings, the intensity- and time-dependent recruitment of muscle fibre types with different metabolic properties has been suggested to one of the most likely mechanisms for the
slow component (for reviews, see Gaesser & Poole, Citation1996; Poole & Jones, Citation2005; Whipp, Ward, & Rossiter, 2005), and this is a working hypothesis of the present review.
Figure 3. The response to heavy-intensity exercise in a healthy individual. The
response is modelled with an exponential function applied from 20 to 120 s to characterize the primary (“fast”)
component. Note that
continues to increase beyond the primary phase, leading to an end-exercise
that is ∼500 ml · min−1 higher than expected.
Exercise intensity domains
Exercise intensity is often defined by using a single parameter of physiological function, such as (i.e.
). This practice, although widespread, is totally inadequate if the goal of exercise testing is to normalize the physiological responses to exercise with respect to the gas exchange and blood acid–base profiles. This is because it is well known that the %
at the lactate threshold varies widely even in a population homogeneous with respect to
, such as trained cyclists (Coyle et al., Citation1988). Thus, studying physiological responses to exercise at 70%
, for example, might well result in some participants exercising below the lactate threshold and others above it. The
slow component would, therefore, be present in some participants but not others, confounding interpretation of the results (Whipp, Citation1994; Whipp et al., Citation2005). As a result, the dynamic behaviour of pulmonary gas exchange (and especially
) and blood acid–base status (which is conveniently tracked using measurements of blood lactate concentration) during constant-load exercise has been used to define three exercise intensity domains, namely moderate, heavy, and severe (). A fourth exercise intensity domain, extreme exercise, has been suggested to account for power outputs at which exhaustion occurs before
is attained (Hill, Poole, & Smith, 2002). The present review will concentrate on the exercise intensity spectrum encompassing moderate, heavy, and severe exercise, since the vast majority of experimental work has been conducted at these intensities.
Table I. Exercise intensity domains
Moderate exercise includes all power outputs below the lactate threshold. In this domain, there is no change or only a transient increase in blood lactate concentration, and attains a steady state following the primary
response (B). Heavy exercise is used to describe power outputs above the lactate threshold, in which blood lactate is elevated but stable over time and a
slow component is evident, leading to an elevated
response. In the heavy domain,
stabilizes at a sub-maximal value after approximately 10–20 min. Heavy exercise is often used as a “catch-all” term for sub-maximal exercise performed above the lactate threshold. In reality, however, there is a third exercise intensity domain, termed “severe”, in which blood lactate concentration is elevated and continues to increase with time, and the
slow component also fails to stabilize. If exercise is continued for long enough,
will reach its maximum value and the individual will fatigue soon thereafter (). The severe-intensity domain is demarcated by the power output associated with the maximal lactate steady state and/or the critical power (the asymptote of the power–duration curve). presents the definitions of these exercise intensity domains, the parameters demarcating their boundaries, and the physiological responses and the likely cause of fatigue in each domain.
Exercise intensity domains and fatigue mechanisms: The power–duration relationship
Hill (Citation1927) and later Monod and Scherrer (Citation1965), Moritani and colleagues (Moritani, Nagata, deVries, & Muro, Citation1981), and Whipp and colleagues (Whipp, Huntsman, Stoner, Lamarra, & Wasserman, Citation1981) described the hyperbolic relationship between force or power output and the tolerable duration of exercise in humans, and it is interesting to note that a similar speed–time relationship is evident across the animal kingdom: horses (Lauderdale & Hinchcliff, Citation1999), mice (Billat, Mouisel, Roblot, & Melki, Citation2005), ghost crabs (Full & Herreid, Citation1983), and lungless salamanders (Full, Citation1986). The pattern shown in therefore appears to be a fundamental property of the physiology of exercise. It is surprising that relatively little direct effort has been expended to understand its physiological basis. The shape of this relationship is thought to be related to the properties of the aerobic and anaerobic energy systems: the aerobic system providing a sustained, but limited, power and having almost unlimited capacity; the anaerobic system providing a power limited only by the force–velocity characteristics of the muscle groups engaged but being limited in capacity. In this scheme, the aerobic system determines the maximum sustainable power output for longer-term exercise, while the anaerobic (non-oxidative) pathways “shape” the curve above the sustained power output (for a recent review, see Morton, Citation2006). It is well known that the power output at the lactate threshold is strongly correlated with performance in events lasting 30–60 min (Coyle, Citation1995). Therefore, an obvious candidate for the maximum sustainable power output would be the lactate threshold. However, athletes are capable of completing a marathon at speeds similar to or in excess of the lactate threshold (Jones, Citation2006). Similarly, 10-km and half-marathon races can be performed considerably above the lactate threshold without significant duress. Theoretically, however, it is the critical power that represents the maximal sustainable power output defined above, and this parameter has therefore been suggested to represent the boundary between the heavy and severe exercise intensity domains (Poole, Ward, Gardner, & Whipp, 1988). The critical power is notionally equivalent to the power output at the so-called “maximal lactate steady state” (for discussion, see Pringle & Jones, Citation2002; Smith & Jones, Citation2001).
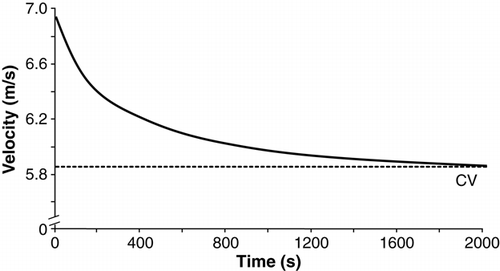
Figure 5. Hyperbolic relationship of running speed to time-to-exhaustion in Paula Radcliffe MBE, the present World record holder for the women's marathon, based upon her personal best times for 800 m, 1500 m, 3000 m, and 5000 m. Using these data, Radcliffe's critical velocity is 5.85 m · s−1 (21.1 km · h−1), which helps to explain some of her exemplary distance running performances.
The above outline poses two important questions: First, why does the lactate threshold correlate with endurance performance, even though athletes consistently exceed the threshold during races? Second, what role does kinetics play in establishing the power–duration relationship? We believe the answer to the second question also provides the answer to the first, and leads to a novel view of the role of
kinetics as a determinant of sports performance. The key to the answer resides in the existence of the
slow component. During heavy-intensity exercise, where exercise can be sustained for a considerable period of time, the
slow component represents an additional oxygen cost that drains the body of its fuel stores more rapidly than if it were not present. Moreover, the slow component will result in a greater rate of heat accumulation during exercise, leading to the earlier attainment of hyperthermia and/or a greater degree of dehydration if the environmental conditions favour uncompensatable heat storage. During severe-intensity exercise, where the
slow component continues to increase until
is attained, the attainment of
signals the imminent termination of exercise. Thus, the
slow component may be at the centre of the fatigue process in both the heavy and severe exercise intensity domains. The importance of the lactate threshold is then obvious: it represents the highest power output at which a
slow component will not develop. Therefore, the longer an athlete wishes their endogenous fuel supplies to last, the closer to the lactate threshold they must exercise. Thus, whether a runner “hits the wall” or not during a marathon could depend, in part, on the presence or absence of the
slow component. A hypothetical example of this is presented in . In this example, it is assumed that all runners are of the same mass, have a similar running economy, and that each attempts to run the marathon at 16 km · h−1. Taking the two most extreme examples, runner 1 runs at the lactate threshold and incurs no slow component. As a result, the runner will expend ∼12 MJ of energy in completing the race. Runner 5, in contrast, must run 2 km · h−1 above their lactate threshold to sustain the same running speed, resulting in a large
slow component that results in an energy requirement of ∼13.3 MJ. Because it is thought that the completion of such an event is dependent, in part, upon the finite muscle glycogen stores (either directly or indirectly) and/or the achievement of a high body temperature, the higher energy requirement of runner 5 is more likely to be associated with premature fatigue and a slowing of the runner's pace late in exercise. In this context, therefore, the lactate threshold determines endurance performance indirectly by determining the highest pace that can be set before the
slow component develops with its negative energetic consequences. It is not surprising, therefore, that marathon runners self-select a running speed that is very close to their lactate threshold during performance (Joyner, Citation1991).
Table II. Effects of the  slow component on marathon performance for a group of runners attempting to run at a pace of 16 km · h−1
slow component on marathon performance for a group of runners attempting to run at a pace of 16 km · h−1
The influence of the slow component is even more obvious in the severe-intensity domain, where
rises continuously until
is attained. The rapidity with which the
slow component increases is dependent on the proximity of the power output to the critical power or maximal lactate steady state. The greater the difference between the power output generated during the exercise task and the critical power, the larger the
slow component and the shorter the tolerable duration of the work (). (Note, however, that the amplitude of the
slow component would be truncated at
; in practice, the greatest
slow component amplitudes occur when exercise just above the critical power is continued to fatigue.) The hyperbolic relationship between power output and time to exhaustion is given by the following equation:
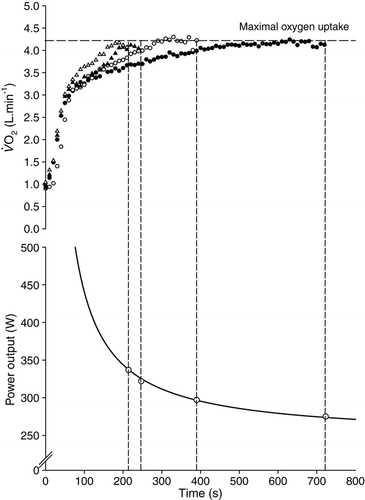
Figure 6. Derivation of the power–duration relationship from bouts of severe-intensity exercise. The upper panel shows the responses to exercise at 275, 297, 322, and 337 W (85–100%
), with the end-point
being equal to
in each case. The power outputs are plotted against time to exhaustion in the lower panel to illustrate the hyperbolic character of the power–duration relationship. These responses resulted in a critical power of 247 W and a W′ (“anaerobic work capacity”) of 19.3 kJ. See text for further details.
where t is time to exhaustion, W′ is a fixed amount of work that can be performed above the critical power (CP), and P is the power output of the task. Hence, if W′ is 16 kJ, CP is 250 W, and P is 300 W, then the tolerable duration of the work will be t=16,000/50 = 320 s, with exhaustion occurring when the participant has accumulated 16 kJ of work above the critical power. During severe exercise, will rise to its maximum value and exhaustion will occur soon thereafter (Hill et al., Citation2002; Poole et al., Citation1988). Therefore, the tolerable duration of exercise in the severe domain depends upon the interaction of three parameters: the anaerobic capacity [thought to be one, if not the, major determinant of W′ (Miura, Kino, Kajitani, Sato, & Fukuba, 1999; Miura, Sato, Sato, Fukuba, & Whipp, 2000)],
, and the
slow component. Increasing
and keeping the other parameters constant would have the effect of increasing the scope for the
slow component to develop, which should extend the time to exhaustion. Similarly, increasing the anaerobic capacity would increase time to exhaustion by increasing the amount of non-oxidative energy available throughout exercise and certainly once
has been attained (this would be equivalent to an increased W′). Furthermore, decreasing the rate at which the
slow component develops would extend the time required before
is reached. Once again, therefore, the
slow component is central to exercise tolerance, this time not because of its influence on the total energy expenditure, but because its behaviour in relation to other physiological parameters determines the tolerable duration of exercise. Given the above, it is not surprising that the critical power and/or maximal lactate steady state (which demarcate the severe domain from the heavy domain) have been reported to be highly correlated with endurance exercise performance, albeit in a surprisingly small number of studies (Jones & Doust, Citation1998; Kolbe, Dennis, Selley, Noakes, & Lambert, Citation1995). Similarly, the
(the highest rate at which adenosine triphosphate can be re-synthesized through oxidative processes) will be highly correlated with endurance performance, as attested to by a wealth of studies (e.g. Bassett & Howley, Citation2000; Karlsson & Saltin, Citation1971), at least in part because it will dictate the power output and/or time at which a continuation of exercise depends entirely on the (limited) anaerobic capacity.
To summarize the above as succinctly as possible, we are suggesting that exercise tolerance is directly or indirectly determined by the kinetics of across a wide variety of exercise durations (i.e. all exercise tasks performed at power outputs above the lactate threshold). In this context, the proximity of the power output to the lactate threshold determines whether or not a
slow component occurs. If the power output is above the lactate threshold, then the proximity of the power output to the critical power determines the behaviour of the
slow component (i.e. whether or not it will stabilize). If the power output is above the critical power, then
interacts with the trajectory of the
slow component and the magnitude of the anaerobic capacity to determine the tolerable duration of the task (and by extension the W′). The long-established correlations between each of the lactate threshold, critical power/maximal lactate steady state, and
with endurance performance might therefore be viewed as a direct consequence of these parameters influencing the behaviour of the
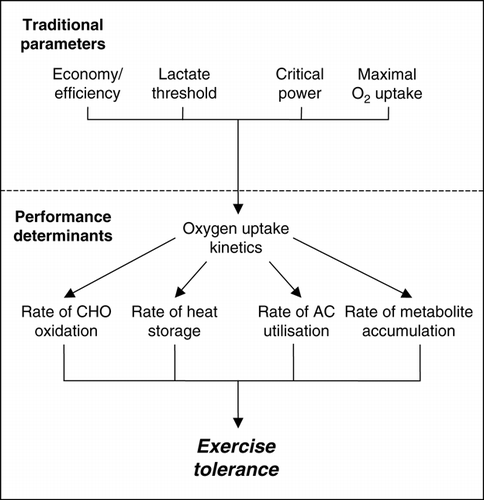
response and its corollaries. This model is presented in . Note that in this model the
kinetics serves as the “conduit” linking the traditional parameters of aerobic fitness to exercise tolerance, by determining the rate of carbohydrate oxidation (in heavy exercise, above the lactate threshold) or the rate of anaerobic capacity utilization/metabolite accumulation (in severe exercise, above the critical power). It must be stressed that the model presented is intended to broadly illustrate the above concepts, rather than to be statistically or mathematically defensible in its current form. However, because
kinetics is a field of study whose basis is in control systems theory, it should be possible to formulate a mathematical model with the appropriate structure in terms of variables, constants, and parameters to achieve the latter aim, though to attempt to do so is beyond the scope of the present review. Below we address some important experimental findings that provide the essential evidence to support our conceptual model.
Figure 7. The role of kinetics in heavy- and severe-intensity exercise tolerance. In this model, the “traditional parameters” of physiological function (exercise economy/efficiency, lactate threshold, critical power, and
) combine to determine the character of the
response to exercise. That is, exercise performed above the lactate threshold results in the
slow component emerging, the proximity of the imposed power output to critical power determines if the
slow component will stabilize, and
provides both the maximum “amplitude” of the primary
response, and determines the extent to which the
slow component can develop. The oxygen uptake kinetics determines exercise tolerance by determining the rate of carbohydrate (CHO) oxidation and/or the rate of heat storage during heavy-intensity exercise. Manipulating fuel stores (glycogen loading/depletion) or the capacity for heat storage (pre-heating/cooling, hypohydration) will influence exercise tolerance by determining when the rate of carbohydrate oxidation or heat storage can no longer be maintained. During severe-intensity exercise, the kinetics determines the rate of anaerobic capacity (AC) utilization, and the related rate of metabolite accumulation. Altering the oxygen uptake kinetics can be achieved directly (using, for example, prior exercise or a pacing strategy) or indirectly (by manipulating the “traditional parameters” through training or altered oxygen transport), resulting in predictable effects on exercise tolerance. Lastly, exercise tolerance could also be influenced by manipulating the anaerobic capacity (creatine monohydrate supplementation, severe prior exercise) or rate of metabolite accumulation (sodium bicarbonate ingestion).
Responses to training
The physiological responses to endurance training have been well studied (for a review, see Jones & Carter, Citation2000) and it is known that an appropriate combination of training session duration, intensity, and frequency, when carried out for a sufficient length of time, leads to an enhancement of the parameters of aerobic function including , exercise economy or efficiency, lactate threshold, critical power, and
kinetics. As will be considered below, each of these improvements can be considered to enhance exercise tolerance through the model outlined in .
A characteristic feature of the physiological adaptation to endurance training is a rightward shift in the blood lactate concentration– relationship with a commensurate increase in the
(and power output) at the lactate threshold (Davis, Frank, Whipp, & Wasserman, Citation1979; Denis, Fouquet, Poty, Geyssant, & Lacour, Citation1982; Jones, Citation2006; Jones & Carter, Citation2000). The mechanistic basis for this effect need not detain us. However, the functional consequence of the enhanced lactate threshold, in relation to
kinetics, is that it extends the range of power outputs that can be described as “moderate” – that is, post training, higher power outputs can be sustained without the occurrence of a
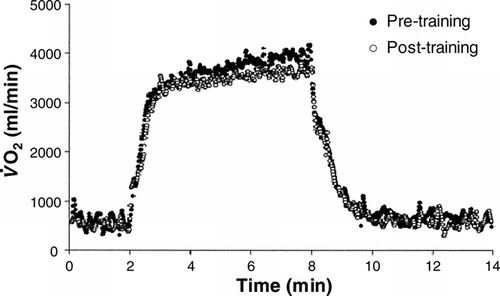
slow component. For reasons outlined earlier, this should be associated with enhanced exercise tolerance. Endurance training also increases the critical power (Jenkins & Quigley, Citation1992; Poole, Ward, & Whipp, 1990) and an important effect of this is to increase the range of power outputs that might be termed “heavy” rather than “severe”. In other words, an increased critical power will allow higher power outputs to be sustained that enable the (eventual) attainment of steady states in pulmonary gas exchange and blood acid–base status. Because exercise at power outputs above the critical power leads to predictable but relatively limited (i.e.<30–45 min) times to exhaustion, an increased critical power with endurance training can have a profound impact on exercise tolerance and hence sports performance. This is clearly seen when the amplitude of the
slow component is compared at the same power output or running speed before and after a period of endurance training (). Several studies have shown that the
slow component is significantly attenuated after only a few weeks of training (Berger, Tolfrey, Williams, & Jones, Citation2006c; Carter et al., Citation2000; Casaburi, Storer, Ben-Dov, & Wasserman, Citation1987; Womack et al., Citation1995). An increased lactate threshold and critical power with training might mean that a power output that could be classified as severe before training commenced might only be characterized as heavy or even moderate after training (with commensurate effects on the
and acid–base response profiles). Even if the power output remains in the severe domain after training, a delayed or more slowly developing
slow component should be associated with enhanced exercise tolerance because it would take longer for
to be attained. Sodium bicarbonate ingestion appears to have similar qualitative, but smaller absolute, effects on the development of the
slow component (Berger, MacNaughton, Keatley, Wilkerson, & Jones, Citation2006b; Kolkhorst, Rezende, Levy, & Buono, Citation2004). Presumably, greater extrusion of H+ ions from the muscle with an enhanced blood buffering capacity reduces muscle fatigue and delays or reduces the requirement for higher-order, less-efficient muscle fibres to be recruited with time as exercise proceeds. A delayed
slow component response and a greater anaerobic capacity (related to enhanced buffering capacity) with sodium bicarbonate ingestion would be expected to extend time to exhaustion during severe-intensity exercise, although this hypothesis remains to be tested directly. Consistent with this notion, however, is evidence that an increased W′ brought about by a period of high-intensity interval training enables a prolongation of the time to fatigue at a fixed supra-critical power output (Jenkins & Quigley, Citation1993).
Figure 8. Influence of 6 weeks of endurance running training on kinetics during heavy-intensity treadmill running in a representative individual. Note the similar primary component response but the marked attenuation of the
slow component. This effect could bey related to the effects of endurance training on the lactate threshold and critical power and would be expected to result in an enhanced exercise tolerance. See text for further information. Reproduced with permission from Carter et al. (Citation2000).
An increased with endurance training would increase the highest power output that could be sustained within the severe exercise intensity domain. Furthermore, an increased
, assuming no change in critical power, would increase the time to exhaustion during exercise in the severe domain because there would be “more room” for a
slow component to develop before
was attained – that is, the point at which the continuation of exercise becomes wholly dependent on the finite anaerobic energy reserves would be delayed. This latter scenario is perhaps more likely to occur in the early stages of a training programme in previously sedentary individuals; with long-term training, the
appears to be more resistant to change and improved exercise tolerance occurs through continued effects on sub-maximal factors such as the lactate threshold and critical power (Jones, Citation2006; Jones & Carter, Citation2000). Another effect of longer-term endurance training is to enhance exercise economy or efficiency – that is, reduce the
requirement for exercising at a particular running speed or power output (Coyle, Citation2005; Jones, Citation2006). It should be noted here that, owing to the
slow component, economy and efficiency measurements can only be considered valid for exercise performed below the lactate threshold. An improvement in economy or efficiency will not, in itself, alter the metabolic rate at which the lactate threshold or critical power occur, but will enable higher running speeds or power outputs to be sustained at those metabolic rates. Again, this will extend the range of power outputs in the “moderate” and “heavy” exercise intensity domains with predictable, and positive, consequences for exercise tolerance.
The changes in the lactate threshold, critical power, , and exercise economy with endurance training alluded to above collectively increase the highest power outputs that can be attained within the moderate, heavy, and severe exercise intensity domains with important effects on
slow component behaviour and thus exercise tolerance. However, endurance training also has important effects on the fundamental component of the
kinetics. A reduction in the amplitude of this response would signify an improved exercise economy/efficiency, but effects on the time constant of the response are even more profound. Studies have shown that the Phase II
time constant can be reduced by up to 50% even after only 4–6 weeks of endurance training (Berger et al., Citation2006c; Fukuoka et al., Citation2002; Phillips, Green, MacDonald, & Hughson, Citation1995). A 50% speeding of the Phase II
kinetics means that, for the same increase in metabolic rate above baseline, the magnitude of the oxygen deficit would be halved. This effect, in itself, might be expected to enhance exercise tolerance at any power output because a reduced oxygen deficit would be associated with a reduction both in substrate-level phosphorylation and in the accumulation of metabolites that have been linked to the fatigue process (e.g. inorganic phosphate and H+). This effect might be particularly important during exercise above the critical power, since a sparing of the finite W′ would be predicted to extend the time to exhaustion. From the above discussion, therefore, it appears that the enhanced exercise tolerance that attends endurance training is fundamentally linked to changes in both the “fast” and “slow” components of the
kinetics.
“Warm-up” exercise and pacing strategies: Influence on oxygen uptake kinetics and performance
The performance of prior “warm-up” exercise is common in sport, and prior exercise has been used extensively as a means of altering the physiological responses to subsequent exercise. Recently, it has become clear that prior exercise can profoundly alter the kinetic response to subsequent exercise. Moreover, careful design of the exercise protocol, in terms of prior exercise intensity, recovery duration, and the intensity of the subsequent or “criterion” bout, can result in predictable changes in exercise tolerance. In short, prior exercise represents an intervention in which the
kinetics or the anaerobic capacity, or both, can be altered – with predictable consequences for performance.
The early work of Pendergast and colleagues (Pendergast, Leibowitz, Wilson, & Cerretelli, Citation1983) demonstrated that the response to exercise could be altered by prior exercise. Gerbino and colleagues (Gerbino, Ward, & Whipp, Citation1996) presented similar findings using a protocol that has been adopted and adapted in most subsequent studies into the effect of prior exercise on
kinetics. It involved 6 min of prior exercise (of either moderate or heavy intensity) followed by 6 min of recovery and a further 6-min bout of exercise (again, of either moderate or heavy intensity). These authors demonstrated that prior heavy exercise “speeded” the
kinetics during the subsequent heavy exercise bout, but that prior moderate and prior heavy exercise had no effect on the
kinetics during subsequent moderate exercise (for a review, see Jones, Koppo, & Burnley, Citation2003a). The overall “speeding” of the response has been demonstrated to be caused by an elevation of the primary component amplitude and a reduction of the
slow component amplitude (). In other words, the
response is significantly increased early in the second bout of heavy exercise, and the amplitude (or trajectory) of the
slow component significantly reduced, a response suggested to be related to altered motor unit recruitment patterns (Burnley, Doust, Ball, & Jones, Citation2002). It is important to stress, however, that the precise physiological mechanism for the effects of prior exercise on the
kinetics is unclear. Nevertheless, reasonable predictions about the effects of prior exercise on exercise tolerance can be made given the known physiology.
Figure 9. Oxygen uptake responses to two identical 6-min bouts of severe-intensity exercise separated by 20 min of passive recovery. Note that as a consequence of the performance of the first bout (solid circles) the response to the second (open circles) is increased early in exercise (primary phase) and the trajectory (and amplitude) of the slow component is reduced. The response profile in bout 2 would be expected to increase the tolerable duration of exercise providing that the prior exercise does not reduce the anaerobic capacity.
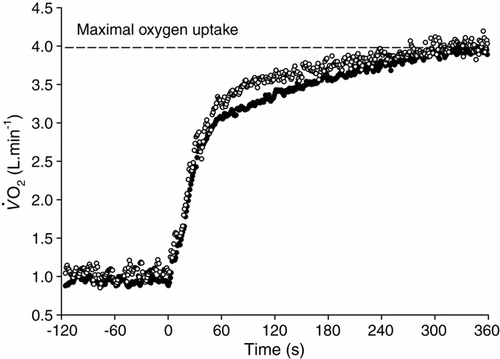
Using the model presented in , a response profile that was closer to mono-exponential with a blunted
slow component would be considered to be beneficial providing that there was no change in
or the anaerobic capacity, because it should increase the time taken to attain
. Alternatively, for exercise performed above
, the increased primary
response would increase the oxidative metabolic contribution early in exercise. Both of these response profiles would be expected to spare the finite anaerobic capacity and hence extend the time to exhaustion. The work of Jones and colleagues (Jones, Wilkerson, Burnley, & Koppo, Citation2003b) seems to be consistent with this scenario: time to exhaustion during exercise performed at 100–120%
was significantly increased when preceded by a bout of heavy exercise. The enhancement of
early in exercise is also evident in the study of Burnley et al. (Citation2005), who showed that prior moderate and prior heavy exercise increased subsequent performance (measured as mean power output during a 7-min “performance trial”) by 2–3%.
Although the ergogenic effects of prior exercise are now well documented, it is important to note that both Jones et al. (Citation2003b) and Burnley et al. (Citation2005) used the same priming exercise (6 min at 50% delta) and the same recovery duration between the priming bout and the criterion exercise (10 min). Other studies that have used prior severe exercise (Carter et al., Citation2005; Koppo & Bouckaert, Citation2002) or prior sprint exercise (Wilkerson, Koppo, Barstow, & Jones, Citation2004) have shown no change, or a decrement, in time to exhaustion, respectively. Burnley et al. (Citation2005) showed that prior sprint exercise led to a non-significant 1.8% reduction in performance using the same performance trial described above. Interestingly, all studies (positive, negative, and neutral with respect to performance effects) showed that the kinetics was altered in a similar way. This is good evidence that if altered
kinetics can indeed influence performance, then it must do so by interacting with other physiological parameters. Prior exercise may result in a small increase in
(Jones and Carter, Citation2004), or increase the peak
achieved at exhaustion without altering
(Jones et al., Citation2003b), suggesting that changes in this parameter are unlikely to account for the disparate results (only a reduction in
could explain poorer exercise tolerance). More likely, severe or sprint exercise probably reduces the anaerobic capacity so that even if
kinetics is altered in a way that would predispose to an extended time to exhaustion, the capacity for anaerobic exercise is expended too soon for the benefit to be realized. In contrast, prior exercise performed below the critical power (i.e. moderate and heavy exercise) should not significantly alter the anaerobic capacity, and so changes in
kinetics (if observed) would be likely to enhance time to exhaustion or performance. Whether priming exercise improves performance, therefore, could depend upon striking a balance between the effect of priming on the
kinetics on the one hand, and the extent to which the anaerobic capacity has been depleted on the other.
The possible influence on exercise tolerance of differences in the manner in which power output is imposed and an individual's energetic resources are utilized (i.e. the pacing strategy) has received limited research attention. However, some research appears to suggest that “fast-start” or “all-out” strategies have the potential to enhance exercise performance (Bishop et al., 2002). A fast-start pacing strategy, relative to the mean power output that can be sustained for a given distance or duration, would increase the “error signal” between the instantaneously “required” and the actual
, and might be predicted to result in a more rapid absolute increase in
following the onset of exercise (Whipp & Mahler, Citation1980). As outlined earlier, faster
kinetics could predispose to enhance exercise tolerance by reducing the magnitude of the oxygen deficit incurred across the transient, assuming that the anaerobic capacity is not prematurely expended. In a recent study, Jones et al. (Citationin press) examined this possibility by having participants perform exercise bouts to fatigue at a mean power output that was predicted, from the pre-determined power–time relationship, to result in exhaustion in 120 s (∼145% critical power). On one occasion, this power output was set constant from the onset of exercise (even-pace); on one occasion, the power output was increased from 10% below to 10% above the mean power output over 120 s (slow-start); and on the one other occasion, the power output was reduced from 10% above to 10% below the mean power output over 120 s (fast-start). As would be predicted from the critical power concept, the participants’ time to exhaustion was not significantly different from 120 s for either the even-pace or slow-start strategies. However, time to exhaustion with the fast-start strategy was significantly increased (to 174 s, on average). Importantly, the work rate at the termination of exercise in the fast-start condition remained substantially above the critical power, and so the results cannot be explained simply by the continually falling work rate beyond 120 s. The fast-start strategy was also associated with a reduced mean response time for
(i.e.
was reached more rapidly) but
and blood lactate concentration measured at the end of exercise were not different between the three conditions. Interestingly, the energy equivalent of the additional oxygen consumption that occurred in the first 120 s of exercise with the fast-start strategy was not significantly different from the additional work done above the critical power in this condition compared to the other conditions. We interpret these data to indicate that a fast-start pacing strategy might facilitate the oxidative contribution to energy metabolism early in the transition to high-intensity exercise, thus sparing the finite anaerobic capacity and enabling exercise to be sustained for longer. This example serves to illustrate further how understanding the interaction between
kinetics,
, and the anaerobic capacity can provide insight into the limitations to exercise performance. However, we stress that additional research into the influence of pacing strategy on
kinetics and exercise performance is required before firm conclusions can be drawn.
Effect of manipulating muscle oxygen delivery on oxygen uptake and performance
In recent studies, we have used several interventions in an attempt to either enhance or impair the potential for oxygen delivery to muscle (Berger, Campbell, Wilkerson, & Jones, Citation2006a; Burnley, Roberts, Thatcher, Doust, & Jones, Citation2006; Wilkerson, Berger, & Jones, Citation2006; Wilkerson, Rittweger, Berger, Naish, & Jones, Citation2005). The results of these studies support our proposed model relating kinetics to exercise tolerance. In the study of Burnley et al. (Citation2006), the withdrawal of 450 ml of whole blood reduced both haemoglobin concentration and
by ∼5%. During a step test to a severe-intensity power output, blood withdrawal did not alter either the amplitude or the time constant of the fundamental
response. However, the amplitude of the
component was significantly reduced and the magnitude of the reduction (∼0.14 l · min−1) was proportional to the reduction in
caused by the intervention. Time to exhaustion was reduced by 14%. It would appear that the reduction in
resulting from the blood withdrawal limited the extent to which
could rise in the slow component phase of the response; and because
increased at approximately the same rate during the slow component region of the response both before and after the intervention,
was reached more rapidly following blood withdrawal and the time to exhaustion was reduced. On the other hand, interventions that increase
, such as the administration of recombinant human erythropoietin (Wilkerson et al., Citation2005), inspiration of hyperoxic gas (Wilkerson et al., Citation2006), or acute expansion of the plasma volume (Berger et al., Citation2006a), enable an extended time to exhaustion during high-intensity exercise, for the reasons outlined earlier. For example, Berger et al. (Citation2006a) reported that acute expansion of the plasma volume did not significantly affect
kinetics but caused a 6% increase in
and a 16% increase in time to exhaustion during severe exercise (). Strikingly, these results are almost a “mirror image” of those of Burnley et al. (Citation2006) for blood donation.
Figure 10. Representative oxygen uptake responses to severe-intensity exercise in the studies of Burnley et al. (Citation2006) (panels A and B) and Berger et al. (Citation2006a) (panels C and D). In (A), a participant performed a bout of cycle exercise at 80% delta to exhaustion, and in (B) completed the same task 24 h after the removal of 1 unit (450 ml) of whole blood. The kinetics in the primary adaptive phase was unchanged, but peak
was reduced. With “less room” for the
slow component to develop, the time to exhaustion was reduced by ∼24%. In (C), a participant performed a bout of cycle exercise to exhaustion at 70% delta, and in (D) performed the same task 10 min after acute plasma volume expansion. The
kinetics in the primary phase was also unaffected in this condition, but the
peak was increased. This provided the
slow component with a greater scope to increase, and time to exhaustion was increased by ∼50%. In other words, blood donation and plasma volume expansion had directly opposite effects on
peak and time to exhaustion. See text for details.
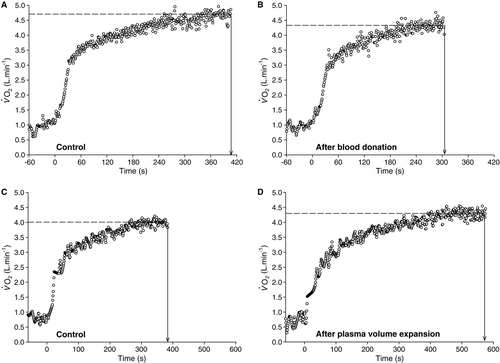
Practical implications and conclusions
A key implication of the above model for the interpretation of the relationship between power output and the tolerable duration of exercise is that the curvature constant parameter W′, so often referred to as the “anaerobic work capacity”, may be nothing of the sort. Although it is true that interventions such as creatine loading, glycogen depletion, and endurance or high-intensity interval training have predictable effects on the parameters of the power–duration relationship (Jenkins & Quigley, Citation1992, Citation1993; Miura et al., Citation1999, Citation2000; Poole et al., Citation1990), it is much harder to explain the influence of prior exercise and pacing on W′ if one accepts the assumption that W′ represents the anaerobic work capacity. We would argue that, while contributing by far the most significant component of W′, the anaerobic work capacity is not its only determinant. Rather, our admittedly limited evidence suggests that W′ is a parameter that depends upon the anaerobic capacity, the conditions at the onset of exercise (whether the oxidative machinery is primed or not primed), the , and the pattern of power output imposition (i.e. pacing). We suggest that changing any one of these will result in predictable changes in W′ provided, of course, that the critical power is not changed also. The most basic definition of W′ (the amount of work that can be done above the critical power) is clearly mathematically consistent and, given the above, conceptually accurate. We therefore suggest that labelling this parameter the “anaerobic work capacity” should be abandoned.
In summary, it is our contention that the “traditional” parameters of aerobic function (, exercise economy, lactate threshold, critical power) are strongly related to endurance exercise performance, in part because they collectively delineate the power outputs surrounding the various exercise intensity domains and hence determine the behaviour of
. The determinants of exercise tolerance and the limitations to sports performance can therefore be better understood through an appreciation of the physiological significance of the fast and slow components of the dynamic
response to exercise.
References
- Åstrand , P.-O. and Saltin , B. 1961a . Maximal oxygen uptake and heart rate in various types of muscular activity . Journal of Applied Physiology , 16 : 977 – 981 .
- Åstrand , P.-O. and Saltin , B. 1961b . Oxygen uptake during the first minutes of heavy muscular exercise . Journal of Applied Physiology , 16 : 971 – 976 .
- Barstow , T. J. , Jones , A. M. , Nguyen , P. and Casaburi , R. 1996 . Influence of muscle fiber type and pedal frequency on oxygen uptake kinetics of heavy exercise . Journal of Applied Physiology , 81 : 1642 – 1650 .
- Barstow , T. J. , Lamarra , N. and Whipp , B. J. 1990 . Modulation of muscle and pulmonary O2 uptakes by circulatory dynamics during exercise . Journal of Applied Physiology , 68 : 979 – 989 .
- Bassett , D. R. and Howley , E. T. 2000 . Limiting factors for maximum oxygen uptake and determinants of endurance performance . Medicine and Science in Sports and Exercise , 32 : 70 – 84 .
- Berger , N. J. , Campbell , I. T. , Wilkerson , D. P. and Jones , A. M. 2006a . Influence of acute plasma volume expansion on kinetics, peak, and performance during high-intensity cycle exercise . Journal of Applied Physiology , 101 : 707 – 714 .
- Berger , N. J. , MacNaughton , L. R. , Keatley , S. , Wilkerson , D. P. and Jones , A. M. 2006b . Sodium bicarbonate ingestion alters the slow but not the fast phase of kinetics . Medicine and Science in Sports and Exercise , 38 : 1909 – 1917 .
- Berger , N. J. , Tolfrey , K. , Williams , A. G. and Jones , A. M. 2006c . Influence of continuous and interval training on oxygen uptake on-kinetics . Medicine and Science in Sports and Exercise , 38 : 504 – 512 .
- Billat , V. L. , Mouisel , E. , Roblot , N. and Melki , J. 2005 . Inter- and intrastrain variation in mouse critical running speed . Journal of Applied Physiology , 98 : 1258 – 1263 .
- Burnley , M. , Doust , J. H. , Ball , D. and Jones , A. M. 2002 . Effects of prior heavy exercise on kinetics during heavy exercise are related to changes in muscle activity . Journal of Applied Physiology , 93 : 167 – 174 .
- Burnley , M. , Doust , J. H. and Jones , A. M. 2005 . Effects of prior warm-up regime on severe-intensity cycling performance . Medicine and Science in Sports and Exercise , 37 : 838 – 845 .
- Burnley , M. , Roberts , C. L. , Thatcher , R. , Doust , J.H. and Jones , A. M. 2006 . Influence of blood donation on O2 uptake on-kinetics, peak O2 uptake and time to exhaustion during severe-intensity cycle exercise in humans . Experimental Physiology , 91 : 499 – 509 .
- Carter , H. , Grice , Y. , Dekerle , J. , Brickley , G. , Hammond , A. J. and Pringle , J. S. 2005 . Effect of prior exercise above and below critical power on exercise to exhaustion . Medicine and Science in Sports and Exercise , 37 : 775 – 781 .
- Carter , H. , Jones , A. M. , Barstow , T. J. , Burnley , M. , Williams , C. and Doust , J. H. 2000 . Effect of endurance training on oxygen uptake kinetics during treadmill running . Journal of Applied Physiology , 89 : 1744 – 1752 .
- Casaburi , R. , Storer , T. W. , Ben-Dov , I. and Wasserman , K. 1987 . Effect of endurance training on possible determinants of during heavy exercise . Journal of Applied Physiology , 62 : 199 – 207 .
- Conley , D. L. and Krahenbuhl , G. S. 1980 . Running economy and distance running performance of highly trained athletes . Medicine and Science in Sports and Exercise , 12 : 357 – 360 .
- Coyle , E. F. 1995 . Integration of the physiological factors determining endurance performance ability . Exercise and Sport Sciences Reviews , 23 : 25 – 63 .
- Coyle , E. F. 2005 . Improved muscular efficiency displayed as Tour de France champion matures . Journal of Applied Physiology , 98 : 2191 – 2196 .
- Coyle , E. F. , Coggan , A. R. , Hemmert , M. K. and Ivy , J. L. 1986 . Muscle glycogen utilization during prolonged strenuous exercise when fed carbohydrate . Journal of Applied Physiology , 61 : 165 – 172 .
- Coyle , E. F. , Coggan , A. R. , Hopper , M. K. and Walters , T. J. 1988 . Determinants of endurance in well-trained cyclists . Journal of Applied Physiology , 64 : 2622 – 2630 .
- Davis , J. A. , Frank , M. H. , Whipp , B. J. and Wasserman , K. 1979 . Anaerobic threshold alterations caused by endurance training in middle-aged men . Journal of Applied Physiology , 46 : 1039 – 1046 .
- Day , J. R. , Rossiter , H. B. , Coats , E. M. , Skasick , A. and Whipp , B. J. 2003 . The maximally attainable during exercise in humans: The peak vs. maximum issue . Journal of Applied Physiology , 95 : 1901 – 1907 .
- Denis , C. , Fouquet , R. , Poty , P. , Geyssant , A. and Lacour , J. R. 1982 . Effect of 40 weeks of endurance training on the anaerobic threshold . International Journal of Sports Medicine , 3 : 208 – 214 .
- Fukuoka , Y. , Grassi , B. , Conti , M. , Guiducci , D. , Sutti , M. Marconi , C. 2002 . Early effects of exercise training on on- and off-kinetics in 50-year-old subjects . Pflügers Archive , 443 : 690 – 697 .
- Full , R. J. 1986 . Locomotion without lungs: Energetics and performance of a lungless salamander . American Journal of Physiology , 251 : R775 – R780 .
- Full , R. J. and Herreid , C. F. 1983 . Aerobic response to exercise of the fastest land crab . American Journal of Physiology , 244 : R530 – R536 .
- Gaesser , G. A. and Poole , D. C. 1996 . The slow component of oxygen uptake kinetics in humans . Exercise and Sport Sciences Reviews , 24 : 35 – 71 .
- Gerbino , A. , Ward , S. A. and Whipp , B. J. 1996 . Effects of prior exercise on pulmonary gas-exchange kinetics during high-intensity exercise in humans . Journal of Applied Physiology , 80 : 99 – 107 .
- Grassi , B. , Poole , D. C. , Richardson , R. S. , Knight , D. R. , Erickson , B. K. and Wagner , P. D. 1996 . Muscle O2 uptake kinetics in humans: Implications for metabolic control . Journal of Applied Physiology , 80 : 988 – 998 .
- Haseler , L. J. , Kindig , C. A. , Richardson , R. S. and Hogan , M. C. 2004 . The role of oxygen in determining phosphocreatine onset kinetics in exercising humans . Journal of Physiology , 558 : 985 – 992 .
- Hill , A. V. 1927 . Muscular movement in man , New York : McGraw-Hill .
- Hill , A. V. and Lupton , H. 1923 . Muscular exercise, lactic acid, and the supply and utilisation of oxygen . Quarterly Journal of Medicine , 16 : 135 – 171 .
- Hill , D. W. , Poole , D. C. and Smith , J. C. 2002 . The relationship between power and the time to achieve . Medicine and Science in Sports and Exercise , 34 : 709 – 714 .
- Hughson , R. L. , Tschakovsky , M. E. and Houston , M. E. 2001 . Regulation of oxygen consumption at the onset of exercise . Exercise and Sport Sciences Reviews , 29 : 129 – 133 .
- Jenkins , D. G. and Quigley , B. M. 1992 . Endurance training enhances critical power . Medicine and Science in Sports and Exercise , 24 : 1283 – 1289 .
- Jenkins , D. G. and Quigley , B. M. 1993 . The influence of high-intensity exercise training on the Wlim–Tlim relationship . Medicine and Science in Sports and Exercise , 25 : 275 – 282 .
- Jones , A. M. 2006 . The physiology of the world record holder for the women's marathon . International Journal of Sports Science and Coaching , 1 : 101 – 116 .
- Jones , A. M. and Burnley , M. 2005 . “ Effect of exercise modality on kinetics ” . In Oxygen uptake kinetics in sport, exercise and medicine , Edited by: Jones , A. M. and Poole , D. C. 95 – 114 . London : Routledge .
- Jones , A. M. and Carter , H. 2000 . The effect of endurance training on parameters of aerobic fitness . Sports Medicine , 29 : 373 – 386 .
- Jones , A. M. and Carter , H. 2004 . Oxygen uptake–work rate relationship during two consecutive ramp exercise tests . International Journal of Sports Medicine , 25 : 415 – 420 .
- Jones , A. M. and Doust , J. H. 1998 . The validity of the lactate minimum test for determination of the maximal lactate steady state . Medicine and Science in Sports and Exercise , 30 : 1304 – 1313 .
- Jones , A. M. , Koppo , K. and Burnley , M. 2003a . Effects of prior exercise on metabolic and gas exchange responses to exercise . Sports Medicine , 33 : 949 – 971 .
- Jones , A. M. , Wilkerson , D. P. , Burnley , M. and Koppo , K. 2003b . Prior heavy exercise enhances performance during subsequent perimaximal exercise . Medicine and Science in Sports and Exercise , 30 : 2085 – 2092 .
- Jones , A. M. , Wilkerson , D. P. , Vanhatalo , A. , & Burnley , M. (in press) . Influence of pacing strategy on O2 uptake and tolerance to high-intensity exercise . Scandinavian Journal of Medicine and Science in Sports .
- Joyner , M. J. 1991 . Modeling: Optimal marathon performance on the basis of physiological factors . Journal of Applied Physiology , 70 : 683 – 687 .
- Karlsson , J. and Saltin , B. 1971 . Diet, muscle glycogen, and endurance performance . Journal of Applied Physiology , 31 : 203 – 206 .
- Koga , S. , Poole , D. C. , Shiojiri , T. , Kondo , N. , Fukuba , Y. Miura , A. 2005 . Comparison of oxygen uptake kinetics during knee extension and cycle exercise . American Journal of Physiology: Regulatory, Integrative and Comparative Physiology , 288 : R212 – R220 .
- Kolbe , T. , Dennis , S. C. , Selley , E. , Noakes , T. D. and Lambert , M. I. 1995 . The relationship between critical power and running performance . Journal of Sports Sciences , 13 : 265 – 269 .
- Kolkhorst , F. W. , Rezende , R. S. , Levy , S. S. and Buono , M. J. 2004 . Effects of sodium bicarbonate on kinetics during heavy exercise . Medicine and Science in Sports and Exercise , 36 : 1895 – 1899 .
- Koppo , K. and Bouckaert , J. 2002 . The decrease in slow component induced by prior exercise does not affect the time to exhaustion . International Journal of Sports Medicine , 23 : 262 – 267 .
- Koppo , K. , Bouckaert , J. and Jones , A. M. 2004 . Effects of training status and exercise intensity on phase II kinetics . Medicine and Science in Sports and Exercise , 36 : 225 – 232 .
- Krogh , A. and Lindhard , J. 1920 . The changes in respiration at the transition from work to rest . Journal of Physiology , 53 : 431 – 439 .
- Lauderdale , M. A. and Hinchcliff , K. W. 1999 . Hyperbolic relationship between time-to-fatigue and workload . Equine Veterinary Journal Supplement , 30 : 586 – 590 .
- MacRae , H. S.-H. 2006 . Does laboratory testing have predictive and practical value for cycling performance? . International Journal of Sports Science and Coaching , 1 : 389 – 397 .
- Miura , A. , Kino , F. , Kajitani , S. , Sato , H. and Fukuba , Y. 1999 . The effect of oral creatine supplementation on the curvature constant parameter of the power–duration curve for cycle ergometry in humans . Japanese Journal of Physiology , 49 : 169 – 174 .
- Miura , A. , Sato , H. , Sato , H. , Fukuba , Y. and Whipp , B. J. 2000 . The effect of glycogen depletion on the curvature constant parameter of the power–duration curve for cycle ergometry . Ergonomics , 43 : 133 – 141 .
- Monod , H. and Scherrer , J. 1965 . The work capacity of a synergic muscle group . Ergonomics , 8 : 329 – 338 .
- Moritani , T. , Nagata , A. , deVries , H. A. and Muro , M. 1981 . Critical power as a measure of physical work capacity and anaerobic threshold . Ergonomics , 24 : 339 – 350 .
- Morton , R. H. 2006 . The critical power and related whole-body bioenergetic models . European Journal of Applied Physiology , 96 : 339 – 354 .
- Noakes , T. D. 1998 . Maximal oxygen uptake: “Classical” versus “contemporary” viewpoints. A rebuttal . Medicine and Science in Sports and Exercise , 30 : 1381 – 1398 .
- Pendergast , D. , Leibowitz , R. , Wilson , D. and Cerretelli , P. 1983 . The effect of preceding anaerobic exercise on aerobic and anaerobic work . European Journal of Applied Physiology and Occupational Physiology , 52 : 29 – 35 .
- Phillips , S. M. , Green , H. J. , MacDonald , M. J. and Hughson , R. L. 1995 . Progressive effect of endurance training on kinetics at the onset of submaximal exercise . Journal of Applied Physiology , 79 : 1914 – 1920 .
- Poole , D. C. and Jones , A. M. 2005 . “ Towards an understanding of the mechanistic bases of kinetics ” . In Oxygen uptake kinetics in sport, exercise and medicine , Edited by: Jones , A. M. and Poole , D. C. 294 – 328 . London : Routledge .
- Poole , D. C. , Kindig , C. A. and Behnke , B. J. 2005 . “ kinetics in different disease states ” . In Oxygen uptake kinetics in sport, exercise and medicine , Edited by: Jones , A. M. and Poole , D. C. 353 – 372 . London : Routledge .
- Poole , D. C. , Schaffartzik , W. , Knight , D. R. , Derion , T. , Kennedy , B. Guy , H. J. 1991 . Contribution of exercising legs to the slow component of oxygen uptake kinetics in humans . Journal of Applied Physiology , 71 : 1245 – 1260 .
- Poole , D. C. , Ward , S. A. , Gardner , G. W. and Whipp , B. J. 1988 . Metabolic and respiratory profile of the upper limit for prolonged exercise in man . Ergonomics , 31 : 1265 – 1279 .
- Poole , D. C. , Ward , S. A. and Whipp , B. J. 1990 . The effects of training on the metabolic and respiratory profile of high-intensity cycle ergometer exercise . European Journal of Applied Physiology and Occupational Physiology , 59 : 421 – 429 .
- Pringle , J. S. and Jones , A. M. 2002 . Maximal lactate steady state, critical power and EMG during cycling . European Journal of Applied Physiology , 88 : 214 – 226 .
- Pringle , J. S. , Doust , J. H. , Carter , H. , Tolfrey , K. , Campbell , I. T. Sakkas , G. K. 2003 . Oxygen uptake kinetics during moderate, heavy and severe intensity “submaximal” exercise in humans: The influence of muscle fibre type and capillarisation . European Journal of Applied Physiology , 89 : 289 – 300 .
- Rossiter , H. B. , Ward , S. A. , Doyle , V. L. , Howe , F. A. , Griffiths , J. R. and Whipp , B. J. 1999 . Inferences from pulmonary O2 uptake with respect to intramuscular [phosphocreatine] kinetics during moderate exercise in humans . Journal of Physiology , 518 : 921 – 932 .
- Rossiter , H. B. , Ward , S. A. , Howe , F. A. , Kowalchuk , J. M. , Griffiths , J. R. and Whipp , B. J. 2002 . Dynamics of intramuscular 31P-MRS P(i) peak splitting and the slow components of PCr and O2 uptake during exercise . Journal of Applied Physiology , 93 : 2059 – 2069 .
- Smith , C. G. and Jones , A. M. 2001 . The relationship between critical velocity, maximal lactate steady-state velocity and lactate turnpoint velocity in runners . European Journal of Applied Physiology , 85 : 19 – 26 .
- Tschakovsky , M. E. and Hughson , R. L. 1999 . Interaction of factors determining oxygen uptake at the onset of exercise . Journal of Applied Physiology , 86 : 1101 – 1113 .
- Whipp , B. J. 1987 . Dynamics of pulmonary gas exchange . Circulation , 76 : VI18 – VI28 .
- Whipp , B. J. 1994 . The slow component of O2 uptake kinetics during heavy exercise . Medicine and Science in Sports and Exercise , 26 : 1319 – 1326 .
- Whipp , B. J. , Huntsman , D. J. , Stoner , N. , Lamarra , N. and Wasserman , K. 1981 . A constant which determines the duration of tolerance to high-intensity work . Federation Proceedings , 41 : 1591
- Whipp , B. J. and Mahler , M. 1980 . “ Dynamics of gas exchange during exercise ” . In Pulmonary gas exchange , Edited by: West , J. B. Vol. II , 33 – 96 . Mew York : Academic Press .
- Whipp , B. J. , Ward , S. A. , Lamarra , N. , Davis , J. A. and Wasserman , K. 1982 . Parameters of ventilatory and gas exchange dynamics during exercise . Journal of Applied Physiology , 52 : 1506 – 1513 .
- Whipp , B. J. , Ward , S. A. and Rossiter , H. B. 2005 . Pulmonary O2 uptake during exercise: Conflating muscular and cardiovascular responses . Medicine and Science in Sports and Exercise , 37 : 1574 – 1585 .
- Whipp , B. J. and Wasserman , K. 1972 . Oxygen uptake kinetics for various intensities of constant-load work . Journal of Applied Physiology , 33 : 351 – 356 .
- Wilkerson , D. P. , Berger , N. J. and Jones , A. M. 2006 . Influence of hyperoxia on pulmonary O2 uptake kinetics following the onset of exercise in humans . Respiration Physiology and Neurobiology , 153 : 92 – 106 .
- Wilkerson , D. P. , Koppo , K. , Barstow , T. J. and Jones , A. M. 2004 . Effect of prior multiple-sprint exercise on pulmonary O2 uptake kinetics following the onset of perimaximal exercise . Journal of Applied Physiology , 97 : 1227 – 1236 .
- Wilkerson , D. P. , Rittweger , J. , Berger , N. J. , Naish , P. F. and Jones , A. M. 2005 . Influence of recombinant human erythropoietin treatment on pulmonary O2 uptake kinetics during exercise in humans . Journal of Physiology , 568 : 639 – 652 .
- Womack , C. J. , Davis , S. E. , Blumer , J. L. , Barrett , E. , Weltman , A. L. and Gaesser , G. A. 1995 . Slow component of O2 uptake during heavy exercise: Adaptation to endurance training . Journal of Applied Physiology , 79 : 838 – 845 .