Abstract
The aim of this study was to examine the effect of pre-exercise low and high glycaemic index (GI) carbohydrate meals on running performance. Eight endurance-trained male runners (mean age 33 years, s
x
=1.7; 63 ml · kg−1 · min−1, s
x
=1.8) completed two trials separated by at least 7 days in a counterbalanced design. Two hours before they were to run and after an overnight fast, each participant consumed an isocaloric meal containing either low (gi=37) or high (gi=77) GI carbohydrate foods (2.4 MJ; 65% carbohydrate; 15% protein; 20% fat) that provided 1.5 g carbohydrate per kilogram of body mass in random order. Each trial consisted of a 21-km performance run on a level treadmill. The participants were required to run at 70%
during the first 5 km of the run. They subsequently completed the remaining 16 km in as short a time as possible. All participants achieved a faster performance time after the consumption of the low GI meal (low vs. high GI: 98.7 min, s
x
=2 vs. 101.5 min, s
x
=2; P<0.01). Blood glucose and serum free fatty acids concentrations were higher throughout the performance run in the low GI trial. Serum insulin concentrations were higher in the high GI trial during the postpandial resting period. However, there were no differences between trials in serum insulin or blood lactate concentrations throughout exercise. Compared with the high GI trial, carbohydrate oxidation was 9.5% lower and fat oxidation was 17.9% higher during exercise in the low GI trial. In conclusion, our results indicate that compared with an isocaloric high GI meal, the consumption of a low GI meal 2 h before a 21-km performance run is a more effective means of improving performance time.
Introduction
The ability to sustain prolonged submaximal exercise is primarily determined by fuel availability. The depletion of carbohydrate reserves is regarded as a major limiting factor in human endurance capacity and performance (Coyle, Citation1995; Williams & Serratosa, Citation2006). Although the consumption of carbohydrate before exercise may result in an improved exercise performance, it has been shown that consuming carbohydrate before exercise causes hyperinsulinaemia, which may lead to hypoglycaemia at the onset of exercise. This insulin surge inhibits lipolysis and increases the reliance on limited muscle glycogen reserve and may cause earlier fatigue during exercise (Sidossis & Wolfe, Citation1996). However, the consumption of a low glycaemic index (GI) meal before exercise may reduce the release of glucose, minimize hypoglycaemia at the onset of exercise, increase the concentration of blood free fatty acids, and attenuate the rate of utilization of muscle glycogen during exercise. It also has the advantages of producing a smaller fluctuation in the blood glucose concentration and less disturbance of the hormonal homeostasis than the consumption of high GI foods (DeMarco, Sucher, Cisar, & Butterfield, Citation1999; Earnest et al., Citation2004; Siu et al., Citation2004; Wee, Williams, Gray, & Horabin, Citation1999).
However, the results obtained relating to the effect of pre-exercise ingestion of low GI foods on exercise performance have been inconsistent. Some studies have shown that endurance performance is improved (DeMarco et al., Citation1999; Earnest et al., Citation2004; Thomas, Brotherhood, & Brand-Miller, Citation1994), whereas others have shown no significant improvement in endurance performance, despite physiological and metabolic changes that occur after the ingestion of such types of food before exercise (Febbraio, Keenan, Angus, Campbell, & Garnham, Citation2000; Sparks, Selig, & Febbraio, Citation1998; Wee et al., Citation1999). The inconsistency in performance improvement might be due to the differences in the amount and time at which the carbohydrate is ingested, the GI values of food, or the type of exercise performance tests used.
In some studies that examined the effect of GI meals (Thomas et al., Citation1994; Wee et al., Citation1999), the variable used to establish endurance performance was the exercise time to exhaustion while maintaining the prescribed exercise intensity (i.e. endurance capacity). This is in contrast to a time-trial protocol, which is a more reliable and practical way of assessing endurance performance (McArdle, Katch, & Katch, Citation2006). In reality, many athletic events involve exercise in which a given distance must be completed in the fastest possible time. Under normal circumstances, athletes seldom exercise to exhaustion but aim to achieve the shortest completion time. Although time to exhaustion is a measure of endurance capacity, it may fail to reflect conventionally the competitive athletic competence (Angus, Hargreaves, Dancey, & Febbraio, Citation2000). Moreover, it has been reported that time-trial protocols produce a smaller coefficient of variation (one-fifth), and therefore provide a better performance evaluation than tests of time to exhaustion (Erlenbusch, Haub, Munoz, MacConnie, & Stillwell, Citation2005; Jeukendrup, Saris, Brouns, & Kester, Citation1996; Palmer, Dennis, Noakes, & Hawley, Citation1996).
Furthermore, most studies that have investigated the effect of pre-exercise GI meals used cycling as the mode of exercise (DeMarco et al., Citation1999; Earnest et al., Citation2004; Febbraio et al., Citation2000; Sparks et al., Citation1998). It should be noted that the study by Wee et al. (Citation1999) is the only published research that used running as the exercise mode to measure the endurance capacity of athletes. Cycling and running have been shown to elicit different physiological responses (Gore, Scroop, Marker, & Catcheside, Citation1992). It has been proposed that carbohydrate ingestion results in more marked elevations in blood glucose and insulin concentrations during running than cycling at the same relative exercise intensity (Tsintzas, Williams, Boobis, & Greenhaff, 1998). However, the influence of the glycaemic index of a pre-exercise carbohydrate meal in running performance has not been investigated. Therefore, the aim of the present study was to examine the effect of the glycaemic index of pre-exercise carbohydrate meals on running performance and metabolic responses. It was hypothesized that consumption of a low GI meal 2 h before exercise could improve subsequent 21-km run performance.
Methods
Participants
Eight endurance-trained male runners were recruited to participate in this study. The mean age, body mass, maximal oxygen uptake (), and maximal heart rate of the eight participants were 33 years (s
x
=1.7), 62 kg (s
x
=1.8), 63 ml · kg−1 · min−1 (s
x
=1.8), and 185 beats · min−1 (s
x
=2) respectively. The present study was approved by the University Clinical Research Ethical Committee. Written informed consent was obtained from each participant. All of the participants were required to complete medical history questionnaires and to provide details of their running ability. None of the participants had a history of diabetes mellitus or other metabolic diseases.
Experimental design
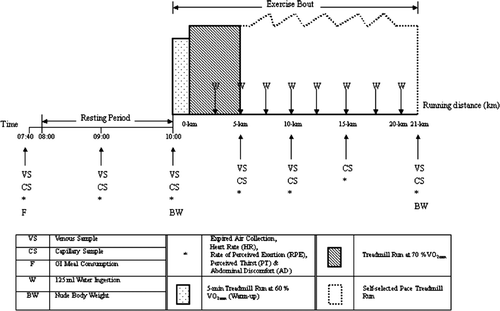
The aim of the protocol used in the main trials of this study was to examine the way in which running performance was affected by pre-exercise low or high GI meals. This protocol consisted of a 2-h rest period followed by one session of exercise. The participants were required to complete a 21-km performance run on a treadmill on two different occasions, separated by at least 7 days. One of the two isocaloric meals providing 1.5 g of carbohydrate per kilogram of body mass, but with a different GI, was consumed 2 h before the run after an overnight fast. The order of these trials was randomized in a counterbalanced crossover design. Neither the running times nor the hypothesis being tested were disclosed to the participants. The time that the participants were required to complete the 21-km run was used as a measure of running performance. To reduce the effects of dehydration, distilled water was made available to the participants before and during exercise. The athletes were asked to follow their normal training schedules during the experimental period and perform the same training schedule 3 days before each main trial. Two days before each main trial, they were required to refrain from strenuous exercise and the consumption of alcoholic beverages (Shirreffs & Maughan, Citation1994) as well as caffeine (Soeren & Graham, Citation1998) to exclude any residual effects before the main trials. Furthermore, the participants were required to complete a consecutive 3-day weighed food diary before each main trial. The dietary record was then analysed using computer software (Food Processor 10.0, ESHA, USA) and they were asked to repeat the same diet before the experimental main trials to minimize the variation in their muscle and liver glycogen concentrations.
Procedures
After the participants were familiarized with treadmill running and the experimental procedures, they took part in a series of preliminary tests to determine: (1) the relationship between oxygen uptake (VO2) and sub-maximal running speed using a 16-min incremental test on a level treadmill; (2) the maximal oxygen uptake () using an uphill incremental treadmill running test to exhaustion; and (3) the relationship between submaximal exercise intensity and blood lactate concentration on a level treadmill. One week before the main trial, the participants completed a 60-min treadmill run to confirm, and if necessary adjust, the running speed for the main trials. Before the two main trials, the post-prandial glycaemic responses of the participants were assessed during a screening session. The purpose of the screening sessions was to ensure that the participants responded normally to the two prescribed GI meals. Data from the screening sessions were assessed by a physician to ensure that no participants were diabetics. Participants reported to the laboratory at 08.00 h after a 10-h fast. The prescribed meal (see detailed description below) was ingested in either a high or low GI trial. Post-prandial blood glucose concentrations were analysed continuously for 2 h after the first consumption. The incremental area under the blood glucose response curve was calculated using the formula of Wolever and colleagues (Citation1991). All of the preliminary tests were conducted in line with the procedures described by Wong and Williams (Citation2000).
On the day of the main trials, the participants reported to the laboratory at about 08.00 h after an overnight fast of 10–12 h. This was to ensure that they had an empty stomach and to minimize the effect of previous meals on the gastric emptying rate of the tests meals. On arrival, the participants rested for 15 min on an examination couch before the insertion of the cannula into the antecubital vein of the forearm. The baseline capillary and venous samples and expired air samples were then collected. Further blood samples and expired air samples were collected at specific times, as shown in .
After the collection of the baseline blood samples, the participants consumed either the low or high isocaloric GI meal. All of the foods had to be consumed within 15–20 min. The participants remained seated in a quiet section of the laboratory and activity levels were minimal (Choi, Cole, Goodpaster, Fink, & Costill, Citation1994), while blood glucose concentrations and the perceived ratings of gut fullness were recorded throughout the 2-h post-prandial period at specific times (i.e. 15, 30, 45, 60, 90, and 120 min after the meal). To ensure that the hydration status of the participants was adequate, each participant ingested 500 ml of distilled water before the exercise session (American College of Sports Medicine, Citation2007). Urine samples obtained during the pre-exercise period were collected in a clean, plastic cylinder for volumetric measurement.
A standardized 5-min warm-up at 60% was performed after the 2-h pre-exercise rest period. The speed of the treadmill was increased to the intensity of 70%
for each participant immediately after the warm-up. For the first 5 km, the participants ran at a fixed speed of 70%
(Williams, Brewer, & Walker, Citation1992). Thereafter, the participants ran at whatever speed they wished for the remaining 16 km of the performance run (Wong, Chan, & Chen, Citation2005). To ensure maximal effort during the performance run, strong verbal encouragement was given to the participants throughout by the main experimenter only, who was unaware of the treatments. Every 2.5 km throughout the run, 125 ml of distilled water at room temperature was given to the participants to minimize the effect of dehydration. During each treadmill run, the participants were cooled by an electric fan and a wet sponge was provided when necessary. Nude body weight was obtained before and after the run following the removal of any sweat from the skin for measuring the body fluid balance. The heart rate of each participant was monitored using short-range radio telemetry (Sport Tester PE 4000, Polar Electro, Finland) throughout the main trials. Expired air samples, capillary blood samples, venous blood samples, ratings of perceived exertion, and ratings of perceived thirst and abdominal discomfort were also obtained at specific times, as shown in . All of the trials were performed under similar conditions of barometric pressure, temperature, and relative humidity (low GI vs. high GI: 760.6 mmHg, s
x
=1.6 vs. 760.2 mmHg, s
x
=1.6; 22.1°C, s
x
=0.4 vs. 22.2°C, s
x
=0.4; 64.8%, s
x
=1.8 vs. 64.4%, s
x
=1.6; P>0.05).
Prescribed glycaemic index meals
Two isocaloric GI meals were used in this study. The glycaemic index of the low GI meal was 37, whereas that of the high GI meal was 77. Each meal was designed and prepared for the participants by a sports dietician. Regarding the macronutrient composition of the meals, carbohydrate contributed 65% of the total caloric intake and protein and fat contributed 15% and 20% respectively. These percentages of macronutrients were recommended by the Chinese Recommended Dietary Allowances, which were passed by the Standing Committee of the Chinese Nutrition Society in Citation1997. The low GI meal was composed of cooked macaroni, apple slices, canned chickpeas, low-fat processed cheese, fruit-flavoured yoghurt, and apple juice. The high GI meal was composed of a baked potato with margarine and tomato sauce, low-fat processed cheese, rice crispies, and a 7-Up soft drink. Both the low and high GI meals provided 1.5 g of carbohydrate per kilogram of body mass for each participant. The energy content and amount of macronutrients that were provided by the low and high GI meals were similar (low vs. high GI: 564.6 kcal, s x =14.2 vs. 562.4 kcal, s x =11.9; 91.6 g carbohydrate, s x =2.4 vs. 91.1 g carbohydrate, s x =2.1; 21.4 g protein, s x =0.5 vs. 21.9 g protein, s x =0.6; 12.4 g fat, s x =0.3 vs. 12.3 g fat, s x =0.2; P>0.05). To avoid the occurrence of bias, the specific purpose of using the GI foods and measuring the weight of the food was not disclosed to the participants.
Analysis
Expired air samples were collected in 150-litre capacity Douglas bags (Harvard Equipment Ltd., USA) for the determination of oxygen uptake and carbon dioxide production. Rates of carbohydrate and fat oxidation were calculated from VO2 and VCO2 values, by applying the equation of McArdle et al. (Citation2006).
Blood lactate and glucose concentrations were determined immediately after the exercise session by the patented immobilized enzyme technology using a lactate analyser (Model 1500, YSI, Ohio, USA) and a glucose analyser (Model 1500 Sidekick, YSI, Ohio, USA). Haematocrit was determined using a haematocrit reader (Clay Adams, Autocrit Ultra 3, New Jersey, USA), and haemoglobin concentration was determined by the cyanmethaemoglobin method (RA-50 Chemistry Analyser, Bayer Diagnostics, Germany). The percentage change in plasma volume from the resting state was estimated from the haemoglobin and haematocrit values (Dill & Costill, Citation1974). The serum samples were analysed for concentrations of insulin (Insulin ELISA, Mercodia, Sweden), free fatty acids (FA 115 kit, Randox Laboratories Ltd., UK), glycerol (GY 105 kit, Randox Laboratories Ltd., UK), and cortisol (Active Cortisol EIA DSL-10-2000, Diagnostic Systems Laboratories, Inc., USA) using commercially available kits.
Statistical analysis
All of the data are presented as means and standard errors of the mean (s x ). The dependent variables of the two trials (low vs. high GI), which included performance time, total substrate oxidation, macronutrient intake for 3 days before the main trial, and urinary output, were compared using Student's t-tests for paired data. Changes in blood glucose and blood lactate concentrations, serum insulin, free fatty acids, and glycerol concentrations, plasma volume, respiratory exchange ratio, heart rate, rating of perceived exertion, rating of perceived thirst, rating of abdominal discomfort, and gut fullness scale values were analysed by two-way (treatment×time) repeated-measures analysis of variance (ANOVA). A Tukey post hoc test was used to locate any significant differences. Statistical significance was set at P<0.05. The effect size was also estimated for the strength of relevance for the treatment effect (Cohen, Citation1988).
Results
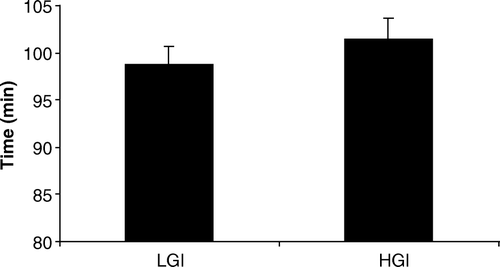
All participants completed the 21-km performance run as directed. The results indicated that the run time was 2.8% faster in the low GI trial than the HGI trial (low GI trial: 98.7 min, s x =2.0, range 94–111; high GI trial: 101.5 min, s x =2.1, range: 96–114; P<0.01, effect size = 0.48). There were no order effects in the run time between trials (Trial 1 vs. Trial 2: 100.2 min, s x =2.4 vs. 100.0 min, s x =1.8; P>0.05) ().
Figure 2. Performance in the 21-km run in the high (HGI) and low (LGI) glycaemic index trials (n=8; mean±s x ). a P<0.01 vs. high GI trial.
Dietary analysis
There were no differences in the daily energy intake or the macronutrient composition of the participants’ diets during the 3 days before each trial (daily energy intake, low GI vs. high GI: 2526 kcal, s x =261.7 vs. 2348 kcal, s x =204.9; carbohydrate, 335 g, s x =28.0 vs. 312 g, s x =24.2; protein, 115 g, s x =16.1 vs. 129 g, s x =23.7; fat, 72 g, s x =9.4 vs. 70 g, s x =7.1; P>0.05).
Physiological changes at rest and during exercise
After consuming the meal, the participants’ blood glucose concentration peaked at 15 min in both the low and high GI trial. In addition, blood glucose concentration was higher in the high than in the low GI trial at 15, 30, and 45 min (P<0.01). The 2-h incremental area under the blood glucose curve after the ingestion of the high GI meal was 66.3% greater than that of the low GI meal (high vs. low GI: 226.3 mmol · min · l−1, s x =13.4 vs. 136.1 mmol · min · l−1, s x =9.7; P<0.01). Blood glucose concentration returned to fasting values before the start of the run on both occasions ().
Figure 3. Blood glucose concentration (mmol · l−1) during the 2-h post prandial period and exercise in the low (LGI) and high (HGI) glycamic index trials (n=8; mean±s x ). a P<0.01 vs. pre-meal; b P<0.01 vs. 120 min; c P<0.05 vs. high GI; d P<0.01 vs. high GI.
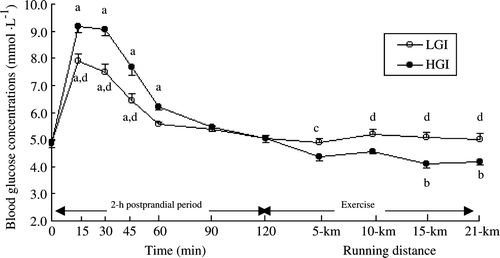
Blood glucose concentration throughout exercise was similar to pre-exercise values in the low GI trial, whereas it decreased gradually in the high GI trial () and was lower than pre-exercise values at 15 km and 21 km of the performance run (P<0.01). The blood glucose concentration of two participants dropped to 3.5 mmol · l−1 at 15 km in the high GI trial. However, no participants reported any of the discomfort associated with hypoglycaemia. There were differences in blood glucose concentration at various stages throughout the 21-km performance run between trials ().
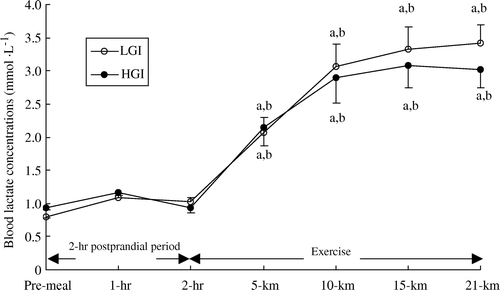
Blood lactate concentration remained unchanged throughout the 2-h post-prandial period and no differences were observed between trials. Blood lactate concentration increased gradually after the onset of exercise and was higher than pre-exercise values in both trials throughout exercise, peaking at the end of the performance run in the low GI trial and at 15 km of the run in the high GI trial (). However, there were no differences in blood lactate concentration between trials.
Figure 4. Blood lactate concentration (mmol · l−1) during the 2-h post-prandial period and exercise in the low (LGI) and high (HGI) glycaemic index trials (n=8; mean±s x ). a P<0.01 vs. pre-meal; b P<0.01 vs. 2-h post-prandial period.
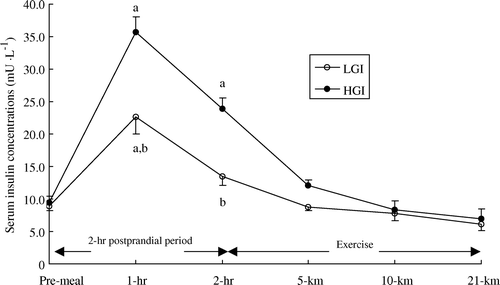
After consumption of the GI meals, serum insulin concentration was significantly higher than fasting values after 1 h of the post-prandial period on both occasions (). Serum insulin concentration was higher in the high GI trial than in the low GI trial after 1 h and 2 h of the post-prandial period (P<0.01). At the onset of exercise, serum insulin concentration was similar to fasting values in the low GI trial (13.5 mU · l−1, s x =1.5 vs. 8.9 mU · l−1, s x =0.7; P>0.05), whereas it remained 150% higher in the high GI trial (23.8 mU · l−1, s x =1.7 vs. 9.5 mU · l−1, s x =1.0; P < 0.01) (). Serum insulin concentration decreased gradually after the start of exercise in both trials. Concentrations returned to fasting values at the end of the 21-km performance run and no differences were found between the two experimental conditions.
Figure 5. Serum insulin concentration (mU · l−1) during the 2-h post prandial period and exercise in the low (LGI) and high (HGI) glycaemic index trials (n=8; mean±s x ). a P<0.01 vs. pre-meal; b P<0.01 vs. high GI.
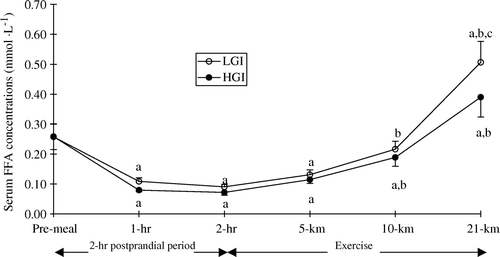
Serum free fatty acid concentration decreased and was lower than fasting values throughout the 2-h post-prandial period (P<0.01). However, no differences were observed between trials (). After the onset of exercise, serum free fatty acid concentration gradually increased and peaked at the end of the performance run in both trials. At the end of exercise, serum free fatty acid concentration was higher in the low GI than in the high GI trial (P<0.01).
Figure 6. Serum free fatty acids (FFA) concentration (mmol · l−1) during the 2-h post-prandial period and exercise in the low (LGI) and high (HGI) glycaemic index trials (n=8; mean±s x ). a P<0.01 vs. pre-meal; b P<0.01 vs. 2-h post-prandial period; c P<0.01 vs. high GI.
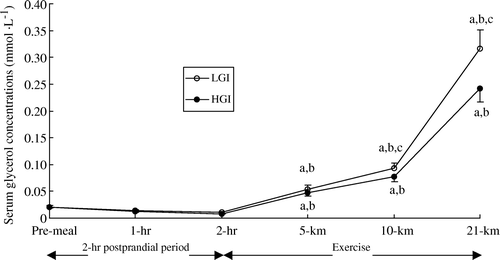
During the 2-h post-prandial period, serum glycerol concentration was similar to fasting values and no differences were observed between the two experimental conditions (). Serum glycerol concentration rose gradually after the onset of exercise and differences were observed at 10 km and at the end of the performance run between trials (P < 0.01).
Figure 7. Serum glycerol concentration (mmol · l−1) during the 2-h post-prandial period and exercise in the low (LGI) and high (HGI) glycaemic index trials (n=8; mean±s x ). a P<0.01 vs. pre-meal; b P<0.01 vs. 2-h post-prandial period; c P < 0.01 vs. high GI.
There was no difference in urinary output throughout the 2-h post-prandial period in the two experimental conditions (low vs. high GI: 508 ml, s x =53 vs. 492 ml, s x =48; P>0.05). In addition, the changes in plasma volume (low vs. high GI: −3.91%, s x =0.40 vs. −3.88%, s x =0.37; P>0.05) and sweat loss (low vs. high GI: 1.5 kg, s x =0.1 vs. 1.5 kg, s x =0.1; P>0.05) during exercise were similar in both trials.
shows the changes in respiratory exchange ratio, carbohydrate, and the fat oxidation rate. After the consumption of meals, the respiratory exchange ratio at 1 h and 2 h of the post-prandial period was higher than fasting values in both trials (P<0.01) but there were no differences between the two experimental conditions. The respiratory exchange ratios at 5 km and 10 km of the performance run were lower in the low GI than high GI trial (P<0.05). Carbohydrate oxidation rates at 1 h and 2 h of the post-prandial period were also higher than fasting values in both trials (P<0.01). The rate of carbohydrate oxidation at 1 h of the post-prandial was lower in the low than in the high GI trial (P<0.05). During the performance run, carbohydrate oxidation rates at 5 km and 10 km were lower in the low than high GI trial (P<0.05). Overall, the total amount of carbohydrate utilized during exercise in the low GI trial was 9.5% lower than that in the high GI trial (low GI vs. high GI: 230.37 g, s x =8.9 vs. 254.56 g, s x =15.9; P<0.05, effect size = 0.54).
Table I. Respiratory exchange ratios (RER), carbohydrate oxidation (CHO), and fat oxidation rates during the 2-h post-prandial period and exercise in the low and high GI trials (n=8; mean±s x )
Fat oxidation rate 1 h after the high GI meal was lower than fasting values (P<0.01). Fat oxidation rates at 5 km, 10 km, and 15 km were higher in the low than high GI trial (P<0.05). Overall, the total amount of fat utilized during exercise in the low GI trial was 17.9% higher than in the high GI trial (low GI vs. high GI: 54.25 g, s x =5.2 vs. 46.03 g, s x =4.8; P<0.05, effect size = 0.61). No differences were observed in total carbohydrate oxidation or fat oxidation during the 2-h post prandial period between the two trials (low GI vs. high GI: carbohydrate, 22.61 g, s x =2.3 vs. 24.45 g, s x =2.6; P>0.05; fat, 11.52 g, s x =1.2 vs. 10.44 g, s x =0.8; P>0.05).
Changes in perceptual variables and heart rates at rest and during exercise
Compared with fasting values, the gut fullness scale peaked 15 min post-prandial in both trials (P<0.01). The gut fullness scales remained higher than fasting values throughout the 2-h post-prandial period in both trials (P<0.01). However, no differences were observed between the two experimental conditions ().
Table II. Gut fullness scales during the 2-h post-prandial period in the low and high GI trials (n=8; mean±s x )
summarizes heart rates, ratings of perceived exertion, ratings of perceived thirst, and ratings of abdominal discomfort throughout the 21-km performance run. Heart rates were higher in the low GI trial than high GI trial at 15 km and at the end of the performance run (P<0.05). Ratings of perceived exertion were higher than pre-exercise values throughout exercise in both trials (P<0.01), but there were no differences between trials. No differences were observed in abdominal discomfort or perceived thirst between the two experimental conditions.
Table III. Heart rate, ratings of perceived exertion, perceived thirst, and abdominal discomfort during the 21-km performance run in the low and high GI trials (mean±s x )
Discussion
The most significant finding of this study is that the consumption of a low glycaemic index meal that provides 1.5 g of carbohydrate per kilogram of body mass 2 h before exercise can improve the subsequent performance of a 21-km run by 2.8% compared with consumption of an isocaloric high glycaemic index meal. The improvement in running performance in the low GI trial was accompanied by a 9.5% lower carbohydrate oxidation and 17.9% higher fat oxidation during exercise compared with the high GI meal.
The effect of the glycaemic index of a pre-exercise carbohydrate meal on endurance performance has attracted considerable interest in recent years in the area of sports nutrition. The results of the present study demonstrate further that low GI foods offer modest post-prandial insulinaemic and glycaemic responses over high GI foods when consumed before prolonged submaximal exercise. Results of the present study are consistent with previous findings that a pre-exercise low GI carbohydrate meal results in a shift of substrate utilization from carbohydrate to fat during exercise (DeMarco et al., Citation1999; Thomas et al., Citation1994). In addition, the concentrations of free fatty acids and glucose are higher during the latter stages of exercise after consumption of a low GI meal than a high GI meal (DeMarco et al., Citation1999; Earnest et al., Citation2004; Febbraio et al., Citation2000; Thomas et al., Citation1994; Wee et al., Citation1999). It is also intriguing to note that such metabolic responses were accompanied by improvements in endurance performance. This was not observed in most previous studies, apart from those of Thomas and colleagues (Thomas, Brotherhood, & Brand, Citation1991), DeMarco et al. (Citation1999), and Wu and Williams (Citation2006); in contrast to the present study, however, time-to-exhaustion protocols were adopted by these authors. It has been argued that the time-to-exhaustion measure of exercise performance is not sufficiently sensitive to detect differences between treatments, because endurance competitions often require athletes to complete a set amount of work in the fastest possible time (McClellan, Cheung, & Jacobs, Citation1995). Consequently, the design of the present study was one that required the participants to complete a fixed distance run in the shortest possible time. This enabled the investigators to conclude that the pre-exercise ingestion of low GI meals is beneficial to endurance performance compared with the ingestion of high GI meals.
It is noteworthy that the two test meals were both isocaloric and provided an average 91 g of carbohydrate. The 66.3% greater incremental area under the post-prandial blood glucose curve after the high GI meal was therefore not the result of differences in the energy or fat and protein contents between the two trials. During the post-prandial period, 23 g and 25 g of carbohydrate were oxidized in the low and high GI trials, respectively. A portion of the remaining ∼68 g of unoxidized carbohydrate from the meals in the two trials might have been stored as muscle and liver glycogen before the start of exercise (Coyle, Coggan, Hemmert, Lowe, & Walters, Citation1985), whereas some undigested carbohydreate might still have been present in the gastrointestinal tract (Thorne, Thompson, & Jenkins, 1983) during the early part of exercise. Serum insulin concentrations 2 h after the ingestion of the meal were still elevated above pre-meal values, which supports the suggestion that the digestion and absorption processes were still in progress (Wee et al., Citation1999).
Blood glucose concentration remained higher throughout the exercise in the low GI trial. However, it dropped below pre-exercise values towards the end of the run in the high GI trial. The latter response was thought to be mediated by a higher pre-exercise serum insulin concentration, which led to a reduction in hepatic glucose production and increased muscle glucose uptake (Marmy-Conus, Fabris, Proietto, & Hargreaves, Citation1996). Although serum insulin concentration was similar in the two trials throughout the performance run, several studies have demonstrated that the effects of insulin on peripheral tissues appear to be long lasting, even though insulin concentration returns to basline values (Coyle, Citation1991; Montain, Hopper, Coggan, & Coyle, Citation1991). This persistent hyper-insulinaemic effect probably increased muscle glucose uptake during the high GI trial to a greater extent than in the low GI trial (Wee et al., Citation1999). Providing that the liver glycogen store is sufficient, the exercise-induced rise in hepatic glucose output can match the increased glucose uptake by the muscles during exercise, which could have been the case in the high GI trial. As the exercise continued, the liver glycogen store might not have been sufficient to cover the increased glucose uptake, which resulted in the sharp drop in blood glucose concentration towards the end of exercise. This may explain why blood glucose concentration was maintained in the low GI trial, but dropped below baseline values towards the end of exercise in the high GI trial. Although direct evidence (e.g. liver glycogen data) is lacking in this study, previous studies have shown that a greater portion of unoxidized carbohydrate remained in the gastrointestinal space at the onset of exercise for later release into the blood circulation when low GI foods had been previously consumed (Burke, Collier, & Hargreaves, Citation1998; Thorne et al., Citation1983). Similar effects on blood glucose concentration were observed in the low GI trial when compared with the high GI trial in the present study.
The reduction in carbohydrate reserves is one of the major causes of the deterioration of performance and increased fatigue during prolonged, submaximal running (Tsintzas et al., Citation1996; Williams & Serratosa, Citation2006; Wong & Williams, Citation2000). The total amount of carbohydrate that was oxidized during the performance run in the low GI trial was ∼10% lower than that in the high GI trial. The same amount of carbohydrate was consumed by the participants before exercise following an overnight fast on both occasions and the total pre-exercise carbohydrate oxidation was similar in the two trials. Consequently, it was anticipated that similar carbohydrate reserves existed before exercise under the two experimental conditions. Therefore, it is reasonable to suggest that glycogen sparing might have occurred during exercise in the low GI trial. Some support for these proposals is provided by the results from a recent study by Wee and colleagues (Wee, Williams, Tsintzas, & Boobis, Citation2005), in whch muscle biopsy samples were obtained from a group of runners before and 3 h after they consumed high and low GI breakfasts, and again after 30 min of treadmill running at 70% . There was an increase in muscle glycogen concentration (15%) in the vastus lateralis muscle after the high GI meal but not after the low GI meal. Furthermore, there was a greater rate of degradation of muscle glycogen during the 30-min treadmill run after the high GI pre-exercise meal than after the low GI pre-exercise meal. Therefore, we can speculate that carbohydrate sparing might be one of the factors that enhanced the running performance following a low GI meal in the present study.
Our results are similar to those of previous studies. For example, a relative shift in substrate utilization from carbohydrate to fat was observed in the low GI trial compared with the high GI trial (Febbraio et al., Citation2000; Wee et al., Citation1999). This observation was supported by the lower respiratory exchange ratios during the first 10 km of the low GI trial. Conversely, the metabolic responses in the high GI trial seemed to be mediated by the persistent effect of hyperinsulinaemia, which resulted in suppressed lipolysis, increased carbohydrate oxidation, and increased muscle glycogen utilization (Kuipers, Fransen, & Keizer, Citation1999). Moreover, lower serum free fatty acids and glycerol concentrations were observed in the high GI trial during exercise. Evidence showed that insulin stimulates the reduction of the lipolytic rate of the adipose cells, and thus limits the availability of free fatty acids and glycerol in the blood circulation (Wolfe, Nadel, Shaw, Stephenson, & Wolfe, Citation1986). However, serum free fatty acids and glycerol concentrations gradually increased towards the end of the run. As the duration of exercise is extended, blood epinephrine rises progressively. Epinephrine stimulates the process of lipolysis, and this may explain the gradual increase in serum free fatty acids and glycerol concentrations (Horowitz & Klein, Citation2000).
Sweat loss and the change in plasma volume during exercise were similar between trials. This suggests that the glycaemic index of pre-exercise carbohydrate meals has no effect on hydration during exercise. Therefore, the hydration status of the participants during exercise did not account for the difference in endurance performance between trials. A limitation of this study is that a control trial (i.e. a fasted condition) was not included. However, the benefits of pre-exercise carbohydrate supplementation on endurance performance compared with a fasting condition have been well established previously (El-Sayed, Balmer, & Rattu, Citation1997; Schabort, Bosch, Weltan, & Noakes, Citation1999; Sherman et al., Citation1989). These studies clearly showed that an increased carbohydrate supply resulted in higher respiratory exchange ratios during exercise, compared with fasting condition.
In conclusion, the consumption of a low GI meal 2 h before a 21-km performance run is more effective in improving run time than an isocaloric high GI meal. Compared with the high GI meal, the ingestion of low GI foods resulted in a shift in substrate utilization from carbohydrate to fat and a higher blood glucose concentration was maintained in the low GI trial throughout exercise.
References
- American College of Sports Medicine (2007) . Position stand on exercise and fluid replacement . Medicine and Science in Sports and Exercise , 39 , 377 – 390 .
- Angus , D. J. , Hargreaves , M. , Dancey , J. and Febbraio , M. A. 2000 . Effect of carbohydrate or carbohydrate plus medium-chain triglyceride ingestion on cycling time trial performance . Journal of Applied Physiology , 88 : 113 – 119 .
- Burke , L. M. , Collier , G. R. and Hargreaves , M. 1998 . Glycemic index – a new tool in sport nutrition? . International Journal of Sport Nutrition , 8 : 401 – 415 .
- Chinese Nutrition Society . 1997 . “ Chinese Recommended Dietary Allowances (passed by the standing committee in October 1988) ” . In The composition of Chinese foods , Edited by: Wang G , P. B. and Wen , Z . 314 – 321 . Washington, DC : International Life Sciences Institute Press .
- Choi , D. , Cole , K. J. , Goodpaster , B. H. , Fink , W. J. and Costill , D. L. 1994 . Effect of passive and active recovery on the resynthesis of muscle glycogen . Medicine and Science in Sports and Exercise , 26 : 992 – 996 .
- Cohen , J. 1988 . Statistical power analysis for the behavioral sciences , 2nd edn. , Hillsdale, NJ : Erlbaum .
- Coyle , E. F. 1991 . Timing and method of increased carbohydrate intake to cope with heavy training, competition and recovery . Journal of Sports Sciences , 9 : 29S – 52S .
- Coyle , E. F. 1995 . Integration of the physiological factors determining endurance performance ability . Exercise and Sport Sciences Reviews , 23 : 25 – 63 .
- Coyle , E. F. , Coggan , A. R. , Hemmert , M. K. , Lowe , R. C. and Walters , T. J. 1985 . Substrate usage during prolonged exercise following a pre-exercise meal . Journal of Applied Physiology , 59 : 429 – 433 .
- DeMarco , H. M. , Sucher , K. P. , Cisar , C. J. and Butterfield , G. E. 1999 . Pre-exercise carbohydrate meals: Application of glycemic index . Medicine and Science in Sports and Exercise , 31 : 164 – 170 .
- Dill , D. B. and Costill , D. L. 1974 . Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration . Journal of Applied Physiology , 37 : 247 – 248 .
- Earnest , C. P. , Lancaster , S. L. , Rasmussen , C. J. , Kerksick , C. M. , Lucia , A. Greenwood , M. C. 2004 . Low vs. high glycemic index carbohydrate gel ingestion during simulated 64-km cycling time trial performance . Journal of Strength and Conditioning Research , 18 : 466 – 472 .
- El-Sayed , M. S. , Balmer , J. and Rattu , A. J. 1997 . Carbohydrate ingestion improves endurance performance during a 1 hour simulated cycling time trial . Journal of Sports Sciences , 15 : 223 – 230 .
- Erlenbusch , M. , Haub , M. , Munoz , K. , MacConnie , S. and Stillwell , B. 2005 . Effect of high-fat or high-carbohydrate diets on endurance exercise: A meta-analysis . International Journal of Sport Nutrition and Exercise Metabolism , 15 : 1 – 14 .
- Febbraio , M. A. , Keenan , J. , Angus , D. J. , Campbell , S. E. and Garnham , A. P. 2000 . Preexercise carbohydrate ingestion, glucose kinetics, and muscle glycogen use: Effect of the glycemic index . Journal of Applied Physiology , 89 : 1845 – 1851 .
- Gore , C. , Scroop , G. C. , Marker , J. D. and Catcheside , P. 1992 . Plasma volume, osmolarity, total protein and electrolytes during treadmill running and cycle ergometer exercise . European Journal of Applied Physiology , 65 : 302 – 310 .
- Horowitz , J. and Klein , S. 2000 . Lipid metabolism during endurance exercise . American Journal of Clinical Nutrition , 72 : 558S – 563S .
- Jeukendrup , A. E. , Saris , W. H. M. , Brouns , F. and Kester , A. D. M. 1996 . A new validated endurance performance test . Medicine and Science in Sports and Exercise , 28 : 266 – 270 .
- Kuipers , H. , Fransen , E. J. and Keizer , H. A. 1999 . Pre-exercise ingestion of carbohydrate and transient hypoglycemia during exercise . International Journal of Sports Medicine , 20 : 227 – 231 .
- Marmy-Conus , N. , Fabris , S. , Proietto , J. and Hargreaves , M. 1996 . Pre-exercise glucose ingestion and glucose kinetics during exercise . Journal of Applied Physiology , 81 : 853 – 857 .
- McArdle , W. D. , Katch , F. I. and Katch , V. L. 2006 . Exercise physiology: Energy, nutrition, and human performance , 6th edn. , Baltimore, MD : Williams & Wilkins .
- McClellan , T. M. , Cheung , S. S. and Jacobs , I. 1995 . Variability of time to exhaustion during submaximal exercise . Canadian Journal of Applied Physiology , 20 : 39 – 51 .
- Montain , S. J. , Hopper , M. K. , Coggan , A. R. and Coyle , E. F. 1991 . Exercise metabolism at different time intervals after a meal . Journal of Applied Physiology , 70 : 882 – 888 .
- Palmer , G. S. , Dennis , S. C. , Noakes , T. D. and Hawley , J. A. 1996 . Assessment of the reproducibility of performance testing on an air-braked cycle ergometer . International Journal of Sports Medicine , 17 : 293 – 298 .
- Schabort , E. J. , Bosch , A. N. , Weltan , S. M. and Noakes , T. D. 1999 . The effect of a preexercise meal on time to fatigue during prolonged cycling exercise . Medicine and Science in Sports and Exercise , 31 : 464 – 471 .
- Sherman , W. M. , Brodowicz , G. , Wright , D. A. , Allen , W. K. , Simonsen , J. and Dernbach , A. 1989 . Effects of 4 h pre-exercise carbohydrate feedings on cycling performance . Medicine and Science in Sports and Exercise , 21 : 598 – 604 .
- Shirreffs , S. M. and Maughan , R. J. 1994 . The effect of posture change on blood volume, serum potassium and whole body electrical impedance . European Journal of Applied Physiology , 69 : 461 – 463 .
- Sidossis , L. S. and Wolfe , R. R. 1996 . Glucose and insulin-induced inhibition of fatty acid oxidation: The glucose–fatty acid cycle reversed . American Journal of Physiology , 270 ( 4 Pt 1 ) : E733 – E738 .
- Siu , P. M. , Wong , S. H. , Morris , J. G. , Lam , C. W. , Chung , P. K. and Chung , S. 2004 . Effect of frequency of carbohydrate feedings on recovery and subsequent endurance run . Medicine and Science in Sports and Exercise , 36 : 315 – 323 .
- Soeren , M. H. V. and Graham , T. E. 1998 . Effect of caffeine on metabolism, exercise endurance, and catecholamine responses after withdrawal . Journal of Applied Physiology , 85 : 1493 – 1501 .
- Sparks , M. J. , Selig , S. S. and Febbraio , M. A. 1998 . Pre-exercise carbohydrate ingestion: Effect of the glycemic index on endurance exercise performance . Medicine and Science in Sports and Exercise , 30 : 844 – 849 .
- Thomas , D. E. , Brotherhood , J. R. and Brand , J. C. 1991 . Carbohydrate feeding before exercise: Effect of glycemic index . International Journal of Sports Medicine , 12 : 180 – 186 .
- Thomas , D. E. , Brotherhood , J. R. and Brand-Miller , J. 1994 . Plasma glucose levels after prolonged strenuous exercise correlate inversely with glycemic response to food consumed before exercise . International Journal of Sport Nutrition , 4 : 361 – 373 .
- Thorne , M. J. , Thompson , L. U. and Jenkins , D. J. A. 1983 . Factors affecting starch digestibility and the glycemic response with special reference to legumes . American Journal of Clinical Nutrition , 38 : 481 – 488 .
- Tsintzas , K. , Williams , C. , Boobis , L. and Greenhaff , P. 1996 . Carbohydrate ingestion and single muscle fiber glycogen metabolism during prolonged running in men . Journal of Applied Physiology , 81 : 801 – 809 .
- Wee , S. L. , Williams , C. , Gray , S. and Horabin , J. 1999 . Influence of high and low glycemic index meals on endurance running capacity . Medicine and Science in Sports and Exercise , 31 : 393 – 399 .
- Wee , S. L. , Williams , C. , Tsintzas , K. and Boobis , L. 2005 . Ingestion of a high-glycemic index meal increases muscle glycogen storage at rest but augments its utilization during subsequent exercise . Journal of Applied Physiology , 99 : 707 – 714 .
- Williams , C. , Brewer , J. and Walker , M. 1992 . The effect of a high carbohydrate diet on running performance during a 30-km treadmill time trial . European Journal of Applied Physiology , 65 : 18 – 24 .
- Williams , C. and Serratosa , L. 2006 . Nutrition on match day . Journal of Sports Sciences , 24 : 687 – 697 .
- Wolever , T. M. , Jenkins , D. J. A. , Jenkins , A. L. and Josse , R. G. 1991 . The glycemic index: methodology and clinical implications . American Journal of Clinical Nutrition , 54 : 846 – 854 .
- Wolfe , R. R. , Nadel , E. R. , Shaw , J. H. F. , Stephenson , L. A. and Wolfe , M. H. 1986 . Role of changes in insulin and glucagon in glucose homeostasis in exercise . Metabolism: Clinical and Experimental , 77 : 900 – 907 .
- Wong , H. S. , Chan , O. W. and Chen , Y. J. 2005 . Pre-exercise glycemic index meal: Effect on running performance when carbohydrate-electrolyte solution is consumed during exercise . Medicine and Science in Sports and Exercise , 37 ( 5 ) : 306
- Wong , S. H. and Williams , C. 2000 . Influence of different amounts of carbohydrate on endurance running capacity following short term recovery . International Journal of Sports Medicine , 21 : 444 – 452 .
- Wu , C. L. and Williams , C. 2006 . A low glycemic index meal before exercise improves endurance running capacity in men . International Journal of Sports Nutrition and Exercise Metabolism , 16 : 510 – 527 .