Abstract
Nutrient intake before, during, and after training will influence the adaptations that occur in response to the training stimulus. The influence of protein on training adaptations is receiving increasing attention from researchers. Methodological issues should be carefully considered when evaluating evidence of nutritional influence on training adaptations. Evidence suggests that adaptations to training are due to changes in the types and activities of various proteins in response to each exercise bout. Thus, study of the acute metabolic and molecular responses to exercise plus nutrition may provide valuable information about the expected influence on training adaptations. The type of protein, timing of protein ingestion relative to exercise, concurrent ingestion of other nutrients with protein, as well as the type of exercise training performed will impact the adaptations to training with the intake of protein. Protein is an important nutrient for muscle hypertrophy with training, but there is little support for the need for very high (e.g. >2.5–3.0 grams of protein per kilogram of body weight) intakes. Traditionally, endurance athletes have focused on carbohydrate intake, but recently protein has been touted to be critical during and after endurance exercise. There is evidence for and against the importance of protein for endurance exercise and more, well-controlled studies are required to delineate the importance of protein for endurance exercise adaptations. Whereas nutritional manipulations have customarily been focused on preventing protein degradation, muscle damage, and oxidative stress, recent evidence suggests that these processes may be critical for the optimal adaptive response to training.
Introduction
Exercise training results in profound adaptations for all organs and tissues. Genetic information determines the basic boundaries to which adaptations must adhere; however, environmental factors influence the ultimate phenotypic response. Adaptations to training are specific to the nature of the exercise training – that is, mode or type of training, intensity, duration, frequency, and so on (Hildebrandt, Pilegaard, & Neufer, Citation2003; Nader & Esser, Citation2001). Nutrient intake before, during, and after training will influence the adaptations that occur in response to the training stimulus (Hawley, Hargreaves, & Zierath, Citation2006a; Hawley, Tipton, & Millard-Stafford, 2006b). The impact of nutrition may not be as great as that of the training itself; nevertheless, nutritional influences on the final outcome are important. In recent years, the influence of nutrition on training adaptations has received increasing attention, particularly with respect to muscle adaptations.
Protein nutrition is currently an important topic among those seeking to optimize adaptations to training. Athletes have long held protein to be a major part of the diet, especially if increased muscle hypertrophy and strength are major goals. Milo of Croton, winner of many successive Ancient Olympic wrestling titles, is legendary for his feats of strength and power. He was reported to consume 9 kg of meat a day (as well as 8.5 litres of wine). More recently, some athletes in the Berlin Olympics reportedly consumed up to 320 grams of protein per day (Grivetti & Applegate, Citation1997). In recent years, athletes seeking adaptations to increase aerobic power and stamina (Saunders, Citation2007), as well as individuals desiring weight loss (Layman, Citation2003; Layman & Walker, Citation2006), have become increasingly aware of the importance of protein in the diet.
The basis for the phenotypic changes noted with training is through changes in levels or activities of proteins. Adaptations that result in larger muscles or faster marathon times stem from changes in the type, quantity, and/or activity of various enzymes, hormones, and structural proteins. The most obvious example is muscle hypertrophy. Muscle hypertrophy results from increased mass of myofibrillar proteins (e.g. myosin, actin, tropomyosin). Increased muscle mass stems from a net synthesis of muscle myofibrillar proteins.
Net protein balance is the metabolic basis for changes in protein levels and ultimately training adaptations. Changes in protein levels are dependent on changes in the synthesis and breakdown of the proteins. Synthesis and breakdown of proteins are concurrent and constant processes. The difference between synthesis and breakdown rates (i.e. net balance) of a specific protein will determine if amounts of that protein are gained or lost. Net protein synthesis results in increased protein and net protein breakdown results in loss of protein. Thus, cellular adaptations to exercise training result from changes in the magnitude and duration of periods of positive and negative net balance of individual proteins, as well as changes in the activities of those proteins. Exercise and nutritional stimuli both have profound influences on the rates of synthesis and breakdown on several levels.
The influence of protein on training adaptations is receiving increasing attention from researchers. Protein may influence many aspects of training adaptations, particularly in muscle. This review examines the basis for the adaptations to training, primarily in muscle, and the influence of protein nutrition on these adaptations. Recent evidence for the importance of protein on the molecular, metabolic, and whole-body levels – the focus will be on studies in humans – will be discussed.
Methodological considerations
Traditionally, the methods used to assess the influence of nutritional interventions on adaptations to training have involved long-term, longitudinal endpoint studies with large numbers of participants. Individuals, often completely untrained before their participation, undertake the appointed training regime for a matter of weeks to months. Unfortunately, such studies can be somewhat problematic, as the control of all necessary aspects (e.g. diet, rest, other activity, emotional instability) is very difficult. Measurement of many endpoints (e.g. endurance performance, strength increases, lean body mass) can be imprecise. The length of the training and the need for a large number of participants to obtain sufficient statistical power often make the costs prohibitive. Therefore, one potential problem with longitudinal endpoint studies is that the results may not be representative of the actual physiological situation. Many of these studies fail to show a difference between treatment groups, but this lack of measurable difference does not necessarily indicate that no difference exists. The lack of a measurable difference may simply mean that a real difference between groups was not detected because of lack of sufficient control, small numbers of participants, imprecise measurement of endpoints, or the inability to carry out the study for sufficient time. As a result of these difficulties, the results from these studies are often equivocal.
Another approach is to assess trained and untrained individuals in a cross-sectional study. Obviously, the time aspect of this approach is much more favourable. However, an assumption must be made that differences observed between groups is due to the training and not inherent to underlying physiological differences of the selected participants.
Finally, the acute response to exercise and nutritional interventions may be measured and extrapolated to represent the potential for long-term adaptive changes. However, this approach also has its limitations. The measurement of changes in metabolite concentrations in blood, urine, muscle or other tissues is often used to assess metabolic changes that may be related to training. Tissue metabolite concentrations do not allow for changes in flux to be determined and so are limited. In the last 20–30 years, stable isotopic tracers have been used to measure changes in whole-body and muscle protein synthesis and breakdown, as well as net protein balance. Whereas these methods allow for assessment of metabolic flux and so are an improvement over simply measuring metabolites, there are limitations to their use as well. For example, measurement of increased glucose concentrations may be a result of increased release from the liver or decreased uptake by the muscles or other tissues. These limitations have been covered elsewhere and the reader is referred to these excellent reviews (Millward, Citation1994, Citation2001; Millward, Price, Pacy, & Halliday, Citation1991; Rennie, Citation1999; Wagenmakers, Citation1999; Wolfe & George, Citation1993; Wolfe & Sidossis, Citation1993). Nevertheless, measurement of the acute metabolic response to exercise and nutrition may allow for extrapolation to adaptations due to training.
The acute measurement of the response to exercise and nutrition is increasingly acknowledged to be useful for extrapolation of training adaptations. Mounting evidence suggests that adaptations to training are a result of the accumulation of proteins due to each individual exercise bout (Hawley et al., Citation2006b). Deposition or loss of protein following each bout of exercise will be small and the accumulation of these small changes gradually results in changes in protein levels over a period of training. This gradual change in protein may explain why changes in muscle mass or changes in metabolic enzymes with training are often not measurable until after several weeks of training (Kraemer, Fleck, & Evans, 1996). Recent evidence supports this contention. Seynnes and colleagues (Seynnes, de Boer, & Narici, Citation2007) demonstrated measurable increases in muscle mass following less than 3 weeks of resistance exercise training – several weeks earlier than commonly reported (Kraemer et al., Citation1996). Recent studies demonstrate that acute measurement of net muscle protein balance following exercise accurately represent long-term adaptive changes (Hartman et al., Citation2007; Wilkinson et al., Citation2007).
This notion is supported by changes in molecular signalling pathways and gene expression in response to acute exercise (Hawley et al., Citation2006b). The measurement of the response of gene expression and intracellular signalling of transcriptional and translational pathways allow us to determine the molecular response to exercise and nutrition. Recent evidence on the molecular and metabolic levels support the notion that training adaptations occur as protein levels change due to the response to each bout of exercise (Hawley et al., Citation2006b; Tipton & Witard, Citation2007). Exercise and nutrition influence synthesis of proteins at both the transcriptional and translational levels. The response of intracellular signalling pathways for transcription of various metabolic genes may be used as an indicator of the potential for adaptive responses. For these signals to be taken as indicators of adaptation, several assumptions must be made. First and foremost, it is assumed that these signals lead to the physiological result of interest. For example, p70s6 kinase phosphorylation, part of the signalling pathway for translation initiation, is related to increased muscle mass with training (Baar & Esser, Citation1999). Samples must be taken at the most opportune time. If muscle biopsies are taken at 2 and 6 h following exercise, but the response occurs from 30 to 60 min after exercise, then a negative result will be assumed. Therefore, unless the time course of the response is well delineated, interpretation of the results may be difficult. Moreover, the signalling pathways are incredibly complex with many overlapping and redundant aspects (Bolster, Kimball, & Jefferson, 2003; Kimball, Farrell, & Jefferson, 2002; Kimball & Jefferson, Citation2006a; Rennie, Citation2005; Rennie, Wackerhage, Spangenburg, & Booth, 2004). Nevertheless, valuable information regarding adaptations to training may be obtained from measurement of signalling in response to exercise and nutritional manipulations.
Acute response of muscle protein metabolism to exercise and protein ingestion
Exercise has a profound impact on muscle protein metabolism. Both resistance (Biolo, Maggi, Williams, Tipton, & Wolfe, 1995b; Phillips, Tipton, Aarsland, Wolf, & Wolfe, 1997, 1999) and endurance (Carraro, Stuart, Hartl, Rosenblatt, & Wolfe, Citation1990; Tipton, Ferrando, Williams, & Wolfe, Citation1996) exercise result in increases in mixed muscle protein synthesis during recovery. Breakdown of mixed muscle proteins also is increased following resistance exercise (Biolo et al., Citation1995b; Phillips et al., Citation1997, Citation1999). Clearly, breakdown of proteins in response to various types of exercise may have important implications for training adaptations, yet little is known about the response of muscle protein breakdown to endurance exercise. Furthermore, these data stem from measurement of mixed muscle protein – that is, all proteins in the muscle. Endurance and resistance exercise result in fundamentally different adaptations. Even genetically identical twins may have completely different phenotypes depending on the type of training (Rennie, Citation2005). The response of various types of proteins to the exercise stimulus must therefore be different. One would expect the response of myofibrillar proteins to be greater for resistance exercise. However, myofibrillar protein synthesis is increased by both resistance exercise (Moore, Phillips, Babraj, Smith, & Rennie, 2005) and leg-kicking exercise (Miller et al., Citation2005), a form of endurance exercise, albeit neither a traditional nor typically practised form.
Relatively little is known about the response of synthesis of mitochondrial proteins to exercise. It is well known that endurance exercise training results in mitochondrial biogenesis (Holloszy & Coyle, Citation1984; Hoppeler & Fluck, Citation2003), thus it would be predicted that endurance exercise will result in increased mitochondrial protein synthesis. Whereas synthesis rates of these proteins have not been measured, recent studies have reported that mRNA abundance for genes associated with metabolic and stress-related functions are transiently elevated in muscle after a single bout of exercise (Coffey et al., Citation2006a, Citationb; Pilegaard, Ordway, Saltin, & Neufer, Citation2000; Pilegaard et al., Citation2002; Pilegaard, Saltin, & Neufer, Citation2003). Interestingly, resistance (Tang, Hartman, & Phillips, 2006) and sprint (Burgomaster, Heigenhauser, & Gibala, Citation2006; Burgomaster, Hughes, Heigenhauser, Bradwell, & Gibala, Citation2005; Tang et al., Citation2006) exercise also result in increased mitochondrial adaptations similar to what has traditionally been associated with endurance training. In support of this notion, unpublished pilot data from four participants demonstrates that both myofibrillar and mitochondrial protein synthesis rates are increased by a single bout of resistance exercise (K. D. Tipton et al., unpublished results) ().
Figure 1. Synthesis rates (fractional synthetic rate; FSR) of muscle protein subfractions at rest and following intense resistance exercise in four healthy volunteers.
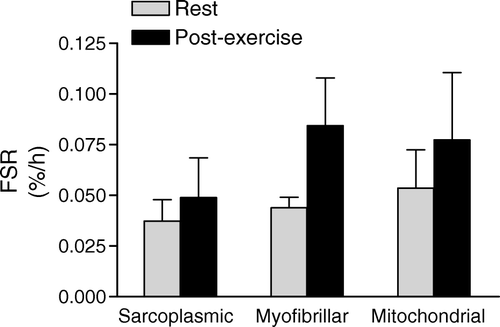
Nutrition may enhance these responses and alter the training adaptations. Provision of amino acids increases the response of muscle protein synthesis to resistance exercise, resulting in positive net muscle protein balance (Biolo, Tipton, Klein, & Wolfe, 1997; Borsheim, Tipton, Wolf, & Wolfe, 2002; Miller, Tipton, Chinkes, Wolf, & Wolfe, 2003; Tang et al., Citation2006; Tipton, Borsheim, Wolf, Sanford, & Wolfe, 2003; Tipton, Ferrando, Phillips, Doyle, & Wolfe, 1999). The impact of exercise plus nutrition on net muscle protein is additive to that at rest (Tipton et al., Citation2003). Similarly, protein ingestion results in an anabolic response following endurance exercise (Levenhagen et al., Citation2002). Presumably, the proteins being synthesized from the amino acids taken up following endurance exercise would be primarily mitochondrial, but this presumption has never been tested. Studies investigating the response of different types of proteins in muscle to different modes of exercise with protein feeding are needed to further delineate the influences on training adaptations and identify the optimal feeding strategies.
The response of muscle protein balance following resistance exercise also may be impacted by the type of protein ingested (Tipton et al., Citation2004; Wilkinson et al., Citation2007), other nutrients ingested along with the protein or amino acids (Borsheim, Aarsland, & Wolfe, Citation2004; Elliot, Cree, Sanford, Wolfe, & Tipton, Citation2006; Koopman et al., Citation2005; Miller et al., Citation2003), the timing of ingestion, and the combination of the timing with the type and form of the amino acids (Levenhagen et al., Citation2001; Rasmussen, Tipton, Miller, Wolf, & Wolfe, 2000; Tipton et al., Citation2001, Citation2007). These results stem primarily from studies on resistance exercise, but the limited data available suggest that resistance and endurance exercise engender similar responses on a mixed muscle protein level (Levenhagen et al., Citation2002). Thus, further investigation is necessary to determine how these factors influence adaptations to different types of training.
Ingestion of an amino acid source, whether it be in food, whole proteins, or free amino acid or hydrolysate – usually small peptide chains from proteins that have been hydrolysed to break some of the peptide bonds – supplements, increases availability of amino acids in blood and muscle. Ingestion of a nutrient that results in hyperaminoacidaemia increases amino acid delivery to the muscle, transport into the muscle cell, and intracellular amino acid availability (Biolo et al., Citation1997). Increased blood flow due to the exercise bout, as well as elevated rates of protein synthesis due to the exercise, would lead to increased amino acid delivery to the muscle and the potential for increased muscle protein synthesis after exercise. Taken together, these factors may explain the additive effect of exercise and amino acids on net muscle protein balance (Biolo et al., Citation1997; Tipton et al., Citation1999; Wolfe & Miller, Citation1999). Increased availability of amino acids is the stimulus for elevation of muscle protein synthesis and net muscle protein synthesis. More specifically, it is the provision of essential amino acids that is responsible for the elevation of synthetic rates (Tipton et al., Citation1999).
The response of muscle protein synthesis and net muscle protein balance to hyperaminoacidaemia may be linked to intracellular amino acid availability (Wolfe, Citation2000, Citation2001; Wolfe and Miller, Citation1999). Alternatively – or in addition to – increased intracellular availability of amino acids, there is evidence linking the stimulation of muscle protein synthesis to the change in arterial amino acid concentrations (Borsheim et al., Citation2002). Similarly, we demonstrated that rapid changes in net balance occur without sustained changes in muscle intracellular concentrations (Tipton et al., Citation2001). Further support comes from a study showing that decreased blood amino acid concentrations resulted in decreased muscle protein synthesis, and restoration of arterial amino acids resulted in restoration of synthesis (Kobayashi et al., Citation2003). These data may be explained by the notion that arterial amino acid concentrations are the key regulating factor for muscle protein synthesis (Bohe, Low, Wolfe, & Rennie, Citation2003).
On the one hand, transiently changing intracellular amino acid concentrations would not appear to support the notion that intracellular amino acid availability regulates muscle protein synthesis and net muscle protein balance (Gibala, Citation2000). However, the intracellular amino acid pool and rates of metabolic processes are in a constant state of flux. Transient changes in intracellular amino acid concentrations may be important, but are not detected without frequent, perhaps even minute-by-minute, biopsy samples. Thus, the two notions of control of muscle protein synthesis are not mutually exclusive. The regulation of muscle protein synthesis and net muscle protein balance by amino acids following exercise may be due to changes in concentrations of arterial amino acids or intracellular amino acid availability, or both.
The increase in muscle protein synthesis in response to amino acid ingestion following exercise may be due to stimulation of the signalling pathways, as well as a mass action effect – that is, provision of substrate for muscle protein synthesis (Rennie, Bohe, Smith, Wackerhage, & Greenhaff, Citation2006; Rennie & Tipton, Citation2000; Tipton & Sharp, Citation2005; Tipton & Witard, Citation2007). The key to stimulation of muscle protein synthesis by protein may be the leucine content. Leucine has received a great deal of attention in recent years with regard to its potential for increasing muscle anabolism (Anthony, Anthony, Kimball, & Jefferson, Citation2001; Kimball & Jefferson, Citation2006b). Recent investigations have examined the potential for leucine to increase muscle hypertrophy through stimulation of signalling pathways. Karlsson et al. (Citation2004) demonstrated that additional stimulation of translation initiation signalling occurs in response to ingestion of branched-chain amino acids following resistance exercise; however, no measurement of muscle protein synthesis was made (Karlsson et al., Citation2004). Recent research has focused on the role of nutrients in signalling and muscle protein synthesis. In support of Karlsson et al. (Citation2004), Koopman and colleagues (Koopman, Pennings, Zorenc, & van Loon, Citation2007) demonstrated that protein ingestion following resistance exercise results in additional stimulation of translational signalling. However, as reported by Koopman et al. (Citation2005), muscle protein synthesis was measured in volunteers following resistance exercise during ingestion of carbohydrates, carbohydrates plus whey protein, and carbohydrates, whey protein plus free leucine. Whereas muscle protein synthesis was greater during ingestion of additional leucine than carbohydrates alone, the increase compared with the trial when whey proteins were ingested was minimal and statistically insignificant (Koopman et al., Citation2005). Similarly, our unpublished data show no difference in net muscle protein synthesis following exercise with whey protein or whey protein plus leucine ingestion (K. D. Tipton et al., unpublished). Thus, additional leucine does not seem to have a major effect, at least in an already anabolic situation following resistance exercise plus protein ingestion. It could be that the signalling pathways are fully activated by resistance exercise and the amino acids, including leucine, in whey proteins (Bolster et al., Citation2003; Kimball et al., Citation2002; Rennie et al., Citation2004; Rennie, Citation2005), thus no further activation from the free leucine is possible. However, no study has examined the impact of leucine alone on muscle protein synthesis following exercise, nor has the impact of leucine alone been compared with wh ey proteins plus leucine. Consequently, it is not possible to make a solid judgement as to the suitability of leucine supplementation for increasing muscle mass in sprint athletes.
Recently, the timing of amino acid and protein intake has been examined and determined to markedly influence the response of net muscle protein balance (Levenhagen et al., Citation2002; Tipton et al., Citation2001, Citation2007). Furthermore, the impact of the timing of ingestion seems to be dependent on the type of amino acid source (i.e. protein or free amino acids), as well as perhaps other nutrients ingested concurrently (Tipton et al., Citation2001, Citation2007). Others have suggested that the timing of intake is critical to the response, such that the optimal response of muscle to intake of proteins or amino acids is immediately (within ∼45 min) following exercise (Cribb & Hayes, Citation2006; Esmarck et al., Citation2001). This concept has been promoted as the “metabolic window” beyond which consumption of protein will not be as effective (Ivy & Portman, Citation2004). However, recent evidence suggests that this “metabolic window” may not be as important as has been suggested. Previously, we demonstrated that the response of muscle to ingestion of an amino acid and carbohydrate solution was similar at 1 and 3 h following resistance exercise (Rasmussen et al., Citation2000). Furthermore, the fact that the response is greater when amino acids and carbohydrates are ingested before exercise (Tipton et al., Citation2001) or similar when proteins are ingested before or after exercise (Tipton et al., Citation2007) suggests that ingestion of these amino acid sources immediately following exercise is, at least, not critical. Taken together, the importance of ingestion of protein or amino acids immediately after exercise may be questioned. Further work on the timing of ingestion in relation to exercise, as well as the combination of timing and concurrent ingestion of other nutrients, is clearly necessary to determine the optimal nutrient strategy to promote muscle adaptations.
Protein for increased muscle mass
Protein is most commonly associated with increasing muscle mass and strength. The importance of protein for muscle is unquestioned, but may often be overblown. The relationship of protein nutrition to increased muscle mass and strength has been addressed in many excellent and rather recent reviews (Phillips, Citation2004, Citation2006; Phillips, Moore, & Tang, 2007; Rennie, Citation2005; Rennie et al., Citation2004; Rennie & Wilkes, Citation2005; Tarnopolsky, Citation1999, Citation2004; Tipton & Sharp, Citation2005; Tipton & Witard, Citation2007; Tipton & Wolfe, Citation2004). Thus, this issue will be summarized here.
The response of muscle to exercise primarily determines the degree of muscle hypertrophy. The intensity, duration, and frequency of the training bouts, as well as the type of training, will have the greatest influence on the metabolic response to exercise. Protein intake may influence this response. The total amount of protein ingested should be sufficient to support the increased anabolism from exercise. Muscle protein synthesis is increased after resistance exercise (Biolo et al., Citation1995b; Phillips et al., Citation1997, Citation1999), Thus, increased protein intake may provide amino acids for the elevated synthesis for muscle growth and perhaps repairing damaged protein. Protein requirements are typically based on nitrogen balance. Well-controlled studies have demonstrated that nitrogen balance is generally greater for athletes than sedentary controls (Lemon, Tarnopolsky, MacDougall, & Atkinson, Citation1992; Tarnopolsky, MacDougall, & Atkinson, 1988; Tarnopolsky et al., Citation1992). Many athletes and coaches advocate and practise consumption of very high protein intakes, from 2.5–3.0 g · kg−1 · day−1 (Phillips, Citation2004) up to 4.0 g · kg−1 · day−1 (Alway, Grumbt, Stray-Gundersen, & Gonyea, Citation1992). The American College of Sports Medicine, American Dietetic Association, and Dieticians of Canada state that protein intake must be >1.6 g · kg−1 · day−1 for gains in muscle mass (American College of Sports Medicine, Citation2000).
On the other hand, many authors maintain that protein needs for active individuals, even those involved in heavy training, are not increased (Millward, Citation2004; Phillips, Citation2004, Citation2006; Rennie & Tipton, Citation2000; Tipton & Witard, Citation2007). This argument is supported by the fact that the efficiency of amino acid utilization is increased by exercise (Todd, Butterfield, & Calloway, Citation1984), perhaps due to increased efficiency of reutilization of amino acids from muscle protein breakdown (Phillips, Citation2006; Phillips et al., Citation1999). On the muscle level, negative net muscle protein balance is greater in resistance-trained than in untrained volunteers in the fasted state (Phillips et al., Citation1999). These data seem to suggest that exercise decreases protein needs. Two recent investigations support this notion. Whole-body nitrogen balance was shown to be greater after resistance training, suggesting that protein retention was improved by the training (Hartman, Moore, & Phillips, 2006; Moore et al., Citation2007). Thus, protein needs are decreased by the anabolic nature of the training. The increased nitrogen retention accompanied an increase in muscle mass and strength, despite a protein intake that many consider to be somewhat moderate (∼1.2–1.5 g · kg−1 · day−1) (Hartman et al., Citation2006; Moore et al., Citation2007) and below that advocated to support increased muscle mass (American College of Sports Medicine, Citation2000; Lemon, Citation1991). These data suggest that the very high levels of protein intake often advocated are unnecessary.
The discrepancy is at least partially due to the limitations of the nitrogen balance technique (Furst & Stehle, Citation2004; Millward, Citation1999; Tipton & Witard, Citation2007; Tipton & Wolfe, Citation2004; Tome & Bos, Citation2000). Briefly, these criticisms include: a lack of sensitivity, since it involves only gross measures of nitrogen intake and excretion (Furst & Stehle, Citation2004); difficulties in precisely quantifying nitrogen losses, which may be particularly important for active individuals (Wolfe, Citation2000); changes in the size of the body urea pool (Millward, Citation1999); mismatches between nitrogen balance and measurable changes in protein mass (Phillips, Citation2004; Tarnopolsky, Citation2004), especially at high intakes (Phillips, Citation2004); poor reproducibility (Young, Citation1986); and accommodation by limitation of other processes at nitrogen balance with low protein intakes (Young, Bier, & Pellett, Citation1989).
Many proponents of very high protein intakes use the high nitrogen balance at high protein intakes as support. Nitrogen accretion has been reported to be as high as 15 g · day−1 (Tarnopolsky et al., Citation1988). These results must be considered rather puzzling, as they are not physiologically possible (Phillips, Citation2004). If nitrogen accretion is ∼15 g · day−1, that would result in muscle gains of approximately 300 g · day−1 or >100 kg in a year. Clearly, this method is not entirely suitable as a basis for estimation of protein needs for increasing muscle mass. As much as 100 years ago, Chittenden (Citation1907) showed that gains in muscle mass and strength are possible on relatively low protein intakes. Furthermore, studies demonstrate that muscle hypertrophy will result when protein intake is ∼1.2–1.5 g · kg−1 · day−1 (Hartman et al., Citation2006; Moore et al., Citation2007). Furthermore, it is clear that very few athletes ingest protein in amounts that would not support muscle hypertrophy (Phillips, Citation2004; Tarnopolsky, Citation2004; Tipton & Witard, Citation2007).
Increased muscle mass and strength can be expected at a wide range of protein intakes and most athletes eat sufficient protein. On the other hand, the amount of protein necessary to optimize muscle hypertrophy has never been determined. Increased muscle mass in response to training and nutrition is clearly highly individual and is dependent on factors other than just the amount of protein to be ingested (Tipton & Witard, Citation2007). The acute response to protein and amino acid ingestion in response to exercise supports this contention. As mentioned above, it is clear that the response of muscle protein synthesis and net muscle protein balance to exercise may be influenced by many factors, including the type of protein or amino acids ingested, the form in which the ingestion takes place, timing of ingestion, concurrent ingestion of other nutrients, and other factors not yet considered or studied (Tipton & Witard, Citation2007). Taken together, it is clear that increased muscle mass resulting from training may not be identical in two situations even given identical protein intakes.
Role of protein for adaptations to endurance exercise
Whereas protein nutrition has received considerable attention due to its role in muscle hypertrophy, the role of protein intake on adaptations to endurance exercise has only recently begun to be investigated. Protein intake during and following endurance exercise has recently been advocated primarily to increase muscle glycogen resynthesis and to reduce muscle damage (Saunders, Citation2007). Protein ingestion following cycling has been shown to increase muscle protein synthesis, resulting in positive net muscle protein balance (Levenhagen et al., Citation2002). Thus, it is clear that protein stimulates muscle protein accretion following endurance exercise. However, since muscle hypertrophy is not the primary adaptation to this type of exercise, it is likely that stimulation of synthesis of proteins other than myofibrillar proteins is the primary response to protein following exercise. Protein intake during and following prolonged endurance exercise results in increased and positive net whole-body protein balance (Koopman et al., Citation2004). However, there was no increase in whole-body protein degradation without the protein, thus the practical implications of these results are not clear.
Protein ingestion during and after endurance exercise has been advocated to ameliorate exercise-induced muscle damage (Luden, Saunders, & Todd, 2007; Romano-Ely, Todd, Saunders, & Laurent, 2006; Saunders, Citation2007; Saunders, Kane, & Todd, 2004; Saunders, Luden, & Herrick, 2007). It is possible that the positive whole-body and muscle net protein synthesis demonstrated following endurance exercise is for repair and remodelling of damaged proteins (Koopman et al., Citation2004; Luden et al., Citation2007; Saunders, Citation2007; Saunders et al., Citation2004; Shimomura et al., Citation2006). However, muscle damage in these studies (Luden et al., Citation2007; Romano-Ely et al., Citation2006; Saunders, Citation2007; Saunders et al., Citation2004, Citation2007) is indicated primarily by increases in plasma creatine kinase, a putative, albeit indirect marker for muscle damage. Whereas these data warrant further investigation for this possible impact of protein ingestion, there are several factors that should be considered before a solid conclusion can be made at this juncture. First, the reported changes typically occur on the order of several hours following protein ingestion (Saunders, Citation2007; Saunders et al., Citation2004; Shimomura et al., Citation2006). Turnover of myofibrillar proteins is relatively slow – on the order of days to weeks (Rennie, Citation2005; Rennie et al., Citation2004). Thus, it is difficult to comprehend how this process may influence changes noted in just a few hours. Second, the reported changes in creatine kinase are minimal (Luden et al., Citation2007; Romano-Ely et al., Citation2006; Saunders, Citation2007; Saunders et al., Citation2004, Citation2007), thus the physiological significance is difficult to determine. Next, blood creatine kinase is considered a poor marker for muscle damage. Creatine kinase concentrations have a poor relationship with muscle damage (Beaton, Allan, Tarnopolsky, Tiidus, & Phillips, Citation2002; Warren, Lowe, & Armstrong, 1999) and loss of muscle function (Nosaka, Chapman, Newton, & Sacco, 2006; Nosaka, Newton, & Sacco, 2002; Warren et al., Citation1999). The mechanism responsible for increased creatine kinase in blood does not appear to be the same as that responsible for damaged muscle or loss of muscle function following intense exercise (Nosaka et al., Citation2002, Citation2006; Warren et al., Citation1999). Finally, there are indications that physiological responses that were previously considered to be negative, such as muscle damage (Shepstone et al., Citation2005), oxidative stress (Jackson, Citation2005), and muscle protein breakdown (see Discussion below), may be part of the adaptive process. Therefore, amelioration of these responses may not be in the best interest of athletes wishing to optimize training responses. This notion should be investigated further.
Role of muscle protein breakdown for training adaptations
The bulk of the data examining the metabolic response to exercise and feeding relates to muscle protein synthesis, with much less information available on the response of muscle protein breakdown. There are methodological and metabolic reasons to help explain this disparity. It is much more difficult to measure muscle protein breakdown, especially in human muscle in vivo (Phillips, Citation2006; Phillips et al., Citation2007; Rennie, Citation2005). More importantly perhaps, the magnitude of the synthetic response to exercise and amino acid ingestion is much greater than that of muscle protein breakdown (Phillips, Citation2006; Phillips et al., Citation2007; Rennie, Citation2005). The difference in the response of muscle protein synthesis to exercise and feeding relative to breakdown may be as much as five-fold (Rennie, Citation2005). Therefore, the emphasis in most investigations of changes in muscle proteins is on the synthetic response.
The response of muscle protein breakdown to exercise is often reported as being detrimental to an appropriate training response, while inhibition of breakdown is assumed to be positive. Nutritional strategies are often designed to globally decrease the response of muscle protein breakdown. The question is, however, is this a wise strategy? Increasingly, data indicate that muscle protein breakdown may be important for accretion of muscle. Muscle protein breakdown is increased following intense resistance exercise (Biolo et al., Citation1995b; Phillips et al., Citation1997, Citation1999). On the other hand, muscle protein breakdown appeared to decline during immobilization-induced muscle atrophy (Gibson et al., Citation1987). Thus, increased degradation of muscle proteins is associated with muscle anabolism, presumably to enhance muscle repair and remodelling.
A recent investigation demonstrated that eccentric, or muscle lengthening, contractions resulted in greater muscle hypertrophy (Shepstone et al., Citation2005), suggesting that these types of contractions increase remodelling of muscle proteins, thereby increasing hypertrophy. Following eccentric exercise in rats, rates of muscle protein degradation increase initially followed by an increase in synthesis (Lowe, Warren, Ingalls, Boorstein, & Armstrong, Citation1995). In humans, Willoughby and colleagues (Willoughby, Taylor, & Taylor, 2003) found a similar pattern of increased muscle protein synthesis and breakdown following muscle lengthening exercise. Increased activity of caspase 3 and mRNA levels of ubiquitin pathway proteins, as well as myosin heavy chain, were elevated in human muscle following eccentric exercise (Willoughby et al., Citation2003). Direct measurement of muscle myofibrillar protein synthesis indicates that synthesis is increased to a greater extent by lengthening than shortening contractions (Moore et al., Citation2005); however, to date, no direct measures of myofibrillar protein breakdown have been made. It is clear that, in the fasted state at least, the response of muscle protein breakdown is qualitatively, if not entirely quantitatively, related to muscle protein synthesis (Phillips et al., Citation1997; Tipton & Wolfe, Citation1998), suggesting that breakdown of proteins is related to enhanced hypertrophy from this type of training. These data suggest, albeit indirectly, that perhaps protein degradation – at least some degradative pathways – may be critical for muscle remodelling and repair. If so, then a global suppression of protein breakdown following exercise would not be desirable.
Support for this notion comes from a recent study from Paul Greenhaff's laboratory. During rehabilitation following immobilization-induced muscle loss, the response of mRNA levels for different protein degradative pathways was not uniform (Jones et al., Citation2004). Whereas mRNA expression of MAFbx and MuRF1, factors associated with protein breakdown, was decreased, calpain 1 and 2 expression was increased, as was that of calpastatin. These seemingly somewhat paradoxical results may be explained by varying roles for the pathways of muscle protein breakdown. Thus, it may be desirable to decrease the rate of breakdown due to some pathways and even overall rates of breakdown with nutritional interventions (Biolo, Williams, Fleming, & Wolfe, 1999; Tipton and Wolfe, Citation2001). However, some degradative pathways may be important for optimal remodelling and repair of muscle during muscle hypertrophy, and inhibition of these pathways could lead to suboptimal muscle adaptation. Interpretation of the results from earlier studies is consistent with this notion. The rate of breakdown of mixed muscle proteins is increased following resistance exercise and related to the increase in muscle protein synthesis (Biolo et al., Citation1995b; Phillips et al., Citation1997). Stimulation of synthetic pathways by exercise results in increased protein breakdown to provide amino acids as substrate for increased synthesis. However, when exogenous amino acids are provided following exercise, the need for increased breakdown to provide amino acids is reduced (Biolo, Fleming, Maggi, & Wolfe, Citation1995a), yet degradative pathways critical for remodelling may still be in operation (). Clearly, further examination of the role of protein breakdown and the response of breakdown to protein ingestion is required. Very little is known about such a potentially interesting and important phenomenon.
Figure 2. Schematic illustration of the proposed relationships between the exercise-induced adaptive response of muscle and muscle protein breakdown, muscle protein synthesis, oxidative stress, and muscle damage.
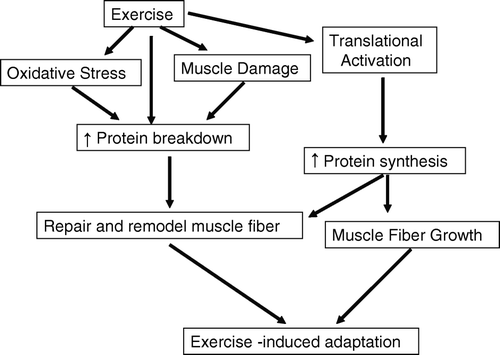
It is clear that nutrition may influence the degradative side of the adaptations to exercise training, but very little information exists on the role of protein, particularly in humans. The mRNA of atrogenic genes may also be modulated by nutrients. Churchley et al. (Citation2007) demonstrated that expression of genes associated with muscle atrophy is reduced in muscle with a low glycogen content. Whereas this result may be viewed as paradoxical, it should be noted that myogenic gene expression was also reduced (Churchley et al., Citation2007); rates of muscle protein breakdown track those of muscle protein synthesis due to amino acid availability (Phillips et al., Citation1997; Tipton & Wolfe, Citation1998).
The influence of protein ingestion on muscle protein breakdown following exercise may be from a direct influence and/or the accompanying rise in insulin. We demonstrated that the normal increase in muscle protein breakdown following resistance exercise is blunted when amino acids are provided (Biolo et al., Citation1997). Subsequently, elevated insulin following exercise was shown to improve net muscle protein balance primarily by reducing the response of muscle protein breakdown (Biolo et al., Citation1999), rather than increasing synthesis. Of course, it is commonly assumed that decreased muscle protein breakdown is the goal if muscle growth is desirable; however, the previous discussion highlights how little is understood about this topic. It is evident that increased muscle protein breakdown is associated with muscle anabolism and thus we should not necessarily strive to diminish the response of breakdown to exercise. Future investigations should focus on the role of different pathways for muscle protein breakdown in adaptation to different types of training and the impact of nutrition on this response.
Conclusions
Protein is an important nutrient with regard to adaptations to training. The nature of the adaptations is determined primarily by the nature of the training regimen. However, nutrient intake may influence these adaptations. Protein intake may influence the acute response to exercise, resulting in alterations in training adaptations. Research to date has focused primarily on the influence of protein on muscle protein synthesis for muscle hypertrophy, while little is known about protein and adaptations to endurance exercise. Furthermore, the role of protein intake on changes in muscle protein breakdown has received relatively little attention. Clearly, there is much to be learned about the role of protein nutrition for adaptive changes to exercise training.
References
- Alway , S. E. , Grumbt , W. H. , Stray-Gundersen , J. and Gonyea , W. J. 1992 . Effects of resistance training on elbow flexors of highly competitive bodybuilders . Journal of Applied Physiology , 72 : 1512 – 1521 .
- American College of Sports Medicine, American Dietetic Association, Dieticians of Canada (2000) . Joint position statement: Nutrition and athletic performance . Medicine and Science in Sports and Exercise , 32 , 2130 – 2145 .
- Anthony , J. C. , Anthony , T. G. , Kimball , S. R. and Jefferson , L. S. 2001 . Signaling pathways involved in translational control of protein synthesis in skeletal muscle by leucine . Journal of Nutrition , 131 : 856S – 860S .
- Baar , K. and Esser , K. 1999 . Phosphorylation of p70(S6k) correlates with increased skeletal muscle mass following resistance exercise . American Journal of Physiology: Cell Physiology , 276 : C120 – C127 .
- Beaton , L. J. , Allan , D. A. , Tarnopolsky , M. A. , Tiidus , P. M. and Phillips , S. M. 2002 . Contraction-induced muscle damage is unaffected by vitamin E supplementation . Medicine and Science in Sports and Exercise , 34 : 798 – 805 .
- Biolo , G. , Fleming , R. Y. , Maggi , S. P. and Wolfe , R. R. 1995a . Transmembrane transport and intracellular kinetics of amino acids in human skeletal muscle . American Journal of Physiology: Endocrinology and Metabolism , 268 : E75 – E84 .
- Biolo , G. , Maggi , S. P. , Williams , B. D. , Tipton , K. D. and Wolfe , R. R. 1995b . Increased rates of muscle protein turnover and amino acid transport after resistance exercise in humans . American Journal of Physiology: Endocrinology and Metabolism , 268 : E514 – E520 .
- Biolo , G. , Tipton , K. D. , Klein , S. and Wolfe , R. R. 1997 . An abundant supply of amino acids enhances the metabolic effect of exercise on muscle protein . American Journal of Physiology: Endocrinology and Metabolism , 273 : E122 – E129 .
- Biolo , G. , Williams , B. D. , Fleming , R. Y. and Wolfe , R. R. 1999 . Insulin action on muscle protein kinetics and amino acid transport during recovery after resistance exercise . Diabetes , 48 : 949 – 957 .
- Bohe , J. , Low , A. , Wolfe , R. R. and Rennie , M. J. 2003 . Human muscle protein synthesis is modulated by extracellular, not intramuscular amino acid availability: A dose–response study . Journal of Physiology , 552 : 315 – 324 .
- Bolster , D. R. , Kimball , S. R. and Jefferson , L. S. 2003 . Translational control mechanisms modulate skeletal muscle gene expression during hypertrophy . Exercise and Sport Science Reviews , 31 : 111 – 116 .
- Borsheim , E. , Aarsland , A. and Wolfe , R. R. 2004 . Effect of an amino acid, protein, and carbohydrate mixture on net muscle protein balance after resistance exercise . International Journal of Sport Nutrition and Exercise Metabolism , 14 : 255 – 271 .
- Borsheim , E. , Tipton , K. D. , Wolf , S. E. and Wolfe , R. R. 2002 . Essential amino acids and muscle protein recovery from resistance exercise . American Journal of Physiology: Endocrinology and Metabolism , 283 : E648 – E657 .
- Burgomaster , K. A. , Heigenhauser , G. J. and Gibala , M. J. 2006 . Effect of short-term sprint interval training on human skeletal muscle carbohydrate metabolism during exercise and time-trial performance . Journal of Applied Physiology , 100 : 2041 – 2047 .
- Burgomaster , K. A. , Hughes , S. C. , Heigenhauser , G. J. , Bradwell , S. N. and Gibala , M. J. 2005 . Six sessions of sprint interval training increases muscle oxidative potential and cycle endurance capacity in humans . Journal of Applied Physiology , 98 : 1985 – 1990 .
- Carraro , F. , Stuart , C. A. , Hartl , W. H. , Rosenblatt , J. and Wolfe , R. R. 1990 . Effect of exercise and recovery on muscle protein synthesis in human subjects . American Journal of Physiology: Endocrinology and Metabolism , 259 : E470 – E476 .
- Chittenden , R. H. 1907 . The nutrition of man , London : Heinemann .
- Churchley , E. G. , Coffey , V. G. , Pedersen , D. J. , Shield , A. , Carey , K. A. Cameron-Smith , D. 2007 . Influence of preexercise muscle glycogen content on transcriptional activity of metabolic and myogenic genes in well-trained humans . Journal of Applied Physiology , 102 : 1604 – 1611 .
- Coffey , V. G. , Shield , A. , Canny , B. J. , Carey , K. A. , Cameron-Smith , D. and Hawley , J. A. 2006a . Interaction of contractile activity and training history on mRNA abundance in skeletal muscle from trained athletes . American Journal of Physiology: Endocrinology and Metabolism , 290 : E849 – E855 .
- Coffey , V. G. , Zhong , Z. , Shield , A. , Canny , B. J. , Chibalin , A. V. Zierath , J. R. 2006b . Early signaling responses to divergent exercise stimuli in skeletal muscle from well-trained humans . FASEB Journal , 20 : 190 – 192 .
- Cribb , P. J. and Hayes , A. 2006 . Effects of supplement timing and resistance exercise on skeletal muscle hypertrophy . Medicine and Science in Sports and Exercise , 38 : 1918 – 1925 .
- Elliot , T. A. , Cree , M. G. , Sanford , A. P. , Wolfe , R. R. and Tipton , K. D. 2006 . Milk ingestion stimulates net muscle protein synthesis following resistance exercise . Medicine and Science in Sports and Exercise , 38 : 667 – 674 .
- Esmarck , B. , Andersen , J. L. , Olsen , S. , Richter , E. A. , Mizuno , M. and Kjaer , M. 2001 . Timing of postexercise protein intake is important for muscle hypertrophy with resistance training in elderly humans . Journal of Physiology , 535 : 301 – 311 .
- Furst , P. and Stehle , P. 2004 . What are the essential elements needed for the determination of amino acid requirements in humans? . Journal of Nutrition , 134 : 1558S – 1565S .
- Gibala , M. J. 2000 . Nutritional supplementation and resistance exercise: What is the evidence for enhanced skeletal muscle hypertrophy? . Canadian Journal of Applied Physiology , 25 : 524 – 535 .
- Gibson , J. N. , Halliday , D. , Morrison , W. L. , Stoward , P. J. , Hornsby , G. A. Watt , P. W. 1987 . Decrease in human quadriceps muscle protein turnover consequent upon leg immobilization . Clinical Science (London) , 72 : 503 – 509 .
- Grivetti , L. E. and Applegate , E. A. 1997 . From Olympia to Atlanta: A cultural-historical perspective on diet and athletic training . Journal of Nutrition , 127 : 860S – 868S .
- Hartman , J. W. , Moore , D. R. and Phillips , S. M. 2006 . Resistance training reduces whole-body protein turnover and improves net protein retention in untrained young males . Applied Physiology, Nutrition and Metabolism , 31 : 557 – 564 .
- Hartman , J. W. , Tang , J. E. , Wilkinson , S. B. , Tarnopolsky , M. A. , Lawrence , R. L. Fullerton , A. V. 2007 . Consumption of fat-free fluid milk after resistance exercise promotes greater lean mass accretion than does consumption of soy or carbohydrate in young, novice, male weightlifters . American Journal of Clinical Nutrition , 86 : 373 – 381 .
- Hawley , J. A. , Hargreaves , M. and Zierath , J. R. 2006a . Signalling mechanisms in skeletal muscle: Role in substrate selection and muscle adaptation . Essays in Biochemistry , 42 : 1 – 12 .
- Hawley , J. A. , Tipton , K. D. and Millard-Stafford , M. L. 2006b . Promoting training adaptations through nutritional interventions . Journal of Sports Sciences , 24 : 1 – 13 .
- Hildebrandt , A. L. , Pilegaard , H. and Neufer , P. D. 2003 . Differential transcriptional activation of select metabolic genes in response to variations in exercise intensity and duration . American Journal of Physiology: Endocrinology and Metabolism , 285 : E1021 – E1027 .
- Holloszy , J. O. and Coyle , E. F. 1984 . Adaptations of skeletal muscle to endurance exercise and their metabolic consequences . Journal of Applied Physiology , 56 : 831 – 838 .
- Hoppeler , H. and Fluck , M. 2003 . Plasticity of skeletal muscle mitochondria: Structure and function . Medicine and Science in Sports and Exercise , 35 : 95 – 104 .
- Ivy , J. L. and Portman , R. 2004 . Nutrient timing: The future of sports nutrition , North Bergen, NJ : Basic Health Publications .
- Jackson , M. J. 2005 . Reactive oxygen species and redox-regulation of skeletal muscle adaptations to exercise . Philosophical Transactions of the Royal Society of London B , 360 : 2285 – 2291 .
- Jones , S. W. , Hill , R. J. , Krasney , P. A. , O'Conner , B. , Peirce , N. and Greenhaff , P. L. 2004 . Disuse atrophy and exercise rehabilitation in humans profoundly affects the expression of genes associated with the regulation of skeletal muscle mass . FASEB Journal , 18 : 1025 – 1027 .
- Karlsson , H. K. , Nilsson , P. A. , Nilsson , J. , Chibalin , A. V. , Zierath , J. R. and Blomstrand , E. 2004 . Branched-chain amino acids increase p70S6k phosphorylation in human skeletal muscle after resistance exercise . American Journal of Physiology: Endocrinology and Metabolism , 287 : E1 – E7 .
- Kimball , S. R. , Farrell , P. A. and Jefferson , L. S. 2002 . Invited review: Role of insulin in translational control of protein synthesis in skeletal muscle by amino acids or exercise . Journal of Applied Physiology , 93 : 1168 – 1180 .
- Kimball , S. R. and Jefferson , L. S. 2006a . New functions for amino acids: Effects on gene transcription and translation . American Journal of Clinical Nutrition , 83 : 500S – 507S .
- Kimball , S. R. and Jefferson , L. S. 2006b . Signaling pathways and molecular mechanisms through which branched-chain amino acids mediate translational control of protein synthesis . Journal of Nutrition , 136 : 227S – 231S .
- Kobayashi , H. , Borsheim , E. , Anthony , T. G. , Traber , D. L. , Badalamenti , J. Kimball , S. R. 2003 . Reduced amino acid availability inhibits muscle protein synthesis and decreases activity of initiation factor eIF2B . American Journal of Physiology: Endocrinology and Metabolism , 284 : E488 – E498 .
- Koopman , R. , Pannemans , D. L. , Jeukendrup , A. E. , Gijsen , A. P. , Senden , J. M. Halliday , D. 2004 . Combined ingestion of protein and carbohydrate improves protein balance during ultra-endurance exercise . American Journal of Physiology: Endocrinology and Metabolism , 287 : E712 – E720 .
- Koopman , R. , Pennings , B. , Zorenc , A. H. and van Loon , L. J. 2007 . Protein ingestion further augments S6K1 phosphorylation in skeletal muscle following resistance type exercise in males . Journal of Nutrition , 137 : 1880 – 1886 .
- Koopman , R. , Wagenmakers , A. J. , Manders , R. J. , Zorenc , A. H. , Senden , J. M. Gorselink , M. 2005 . Combined ingestion of protein and free leucine with carbohydrate increases postexercise muscle protein synthesis in vivo in male subjects . American Journal of Physiology: Endocrinology and Metabolism , 288 : E645 – E653 .
- Kraemer , W. J. , Fleck , S. J. and Evans , W. J. 1996 . Strength and power training: Physiological mechanisms of adaptation . Exercise and Sport Science Reviews , 24 : 363 – 397 .
- Layman , D. K. 2003 . The role of leucine in weight loss diets and glucose homeostasis . Journal of Nutrition , 133 : 261S – 267S .
- Layman , D. K. and Walker , D. A. 2006 . Potential importance of leucine in treatment of obesity and the metabolic syndrome . Journal of Nutrition , 136 : 319S – 323S .
- Lemon , P. W. 1991 . Protein and amino acid needs of the strength athlete . International Journal of Sport Nutrition , 1 : 127 – 145 .
- Lemon , P. W. , Tarnopolsky , M. A. , MacDougall , J. D. and Atkinson , S. A. 1992 . Protein requirements and muscle mass/strength changes during intensive training in novice bodybuilders . Journal of Applied Physiology , 73 : 767 – 775 .
- Levenhagen , D. K. , Carr , C. , Carlson , M. G. , Maron , D. J. , Borel , M. J. and Flakoll , P. J. 2002 . Postexercise protein intake enhances whole-body and leg protein accretion in humans . Medicine and Science in Sports and Exercise , 34 : 828 – 837 .
- Levenhagen , D. K. , Gresham , J. D. , Carlson , M. G. , Maron , D. J. , Borel , M. J. and Flakoll , P. J. 2001 . Postexercise nutrient intake timing in humans is critical to recovery of leg glucose and protein homeostasis . American Journal of Physiology: Endocrinology and Metabolism , 280 : E982 – E993 .
- Lowe , D. A. , Warren , G. L. , Ingalls , C. P. , Boorstein , D. B. and Armstrong , R. B. 1995 . Muscle function and protein metabolism after initiation of eccentric contraction-induced injury . Journal of Applied Physiology , 79 : 1260 – 1270 .
- Luden , N. D. , Saunders , M. J. and Todd , M. K. 2007 . Postexercise carbohydrate–protein–antioxidant ingestion decreases plasma creatine kinase and muscle soreness . International Journal of Sports Nutrition and Exercise Metabolism , 17 : 109 – 123 .
- Miller , B. F. , Olesen , J. L. , Hansen , M. , Dossing , S. , Crameri , R. M. Welling , R. J. 2005 . Coordinated collagen and muscle protein synthesis in human patella tendon and quadriceps muscle after exercise . Journal of Physiology , 567 : 1021 – 1033 .
- Miller , S. L. , Tipton , K. D. , Chinkes , D. L. , Wolf , S. E. and Wolfe , R. R. 2003 . Independent and combined effects of amino acids and glucose after resistance exercise . Medicine and Science in Sports and Exercise , 35 : 449 – 455 .
- Millward , D. J. 1994 . Appendix: Protein turnover measurement by the leucine oxidation–nitrogen excretion end-product method . Clinical Science (London) , 86 : 116 – 118 .
- Millward , D. J. (1999) . Inherent difficulties in defining amino acid requirements . In Committee on Military Nutrition Research FaNBIoM The role of protein and amino acids in sustaining and enhancing performance (pp. 169 – 216 ). Washington, DC : National Academy Press .
- Millward , D. J. 2001 . Methodological considerations . Proceedings of the Nutrition Society , 60 : 3 – 5 .
- Millward , D. J. 2004 . Protein and amino acid requirements of athletes . Journal of Sports Sciences , 22 : 143 – 144 .
- Millward , D. J. , Price , G. M. , Pacy , P. J. and Halliday , D. 1991 . Whole-body protein and amino acid turnover in man: What can we measure with confidence? . Proceedings of the Nutrition Society , 50 : 197 – 216 .
- Moore , D. R. , Del Bel , N. C. , Nizi , K. I. , Hartman , J. W. , Tang , J. E. Armstrong , D. 2007 . Resistance training reduces fasted- and fed-state leucine turnover and increases dietary nitrogen retention in previously untrained young men . Journal of Nutrition , 137 : 985 – 991 .
- Moore , D. R. , Phillips , S. M. , Babraj , J. A. , Smith , K. and Rennie , M. J. 2005 . Myofibrillar and collagen protein synthesis in human skeletal muscle in young men after maximal shortening and lengthening contractions . American Journal of Physiology: Endocrinology and Metabolism , 288 : E1153 – E1159 .
- Nader , G. A. and Esser , K. A. 2001 . Intracellular signaling specificity in skeletal muscle in response to different modes of exercise . Journal of Applied Physiology , 90 : 1936 – 1942 .
- Nosaka , K. , Chapman , D. , Newton , M. and Sacco , P. 2006 . Is isometric strength loss immediately after eccentric exercise related to changes in indirect markers of muscle damage? Applied Physiology . Nutrition and Metabolism , 31 : 313 – 319 .
- Nosaka , K. , Newton , M. and Sacco , P. 2002 . Delayed-onset muscle soreness does not reflect the magnitude of eccentric exercise-induced muscle damage . Scandinavian Journal of Science and Medicine in Sports , 12 : 337 – 346 .
- Phillips , S. M. 2004 . Protein requirements and supplementation in strength sports . Nutrition , 20 : 689 – 695 .
- Phillips , S. M. 2006 . Dietary protein for athletes: From requirements to metabolic advantage. Applied Physiology . Nutrition and Metabolism , 31 : 647 – 654 .
- Phillips , S. M. , Moore , D. R. and Tang , J. E. 2007 . A critical examination of dietary protein requirements, benefits, and excesses in athletes . International Journal of Sports Nutrition and Exercise Metabolism , 17 : S58 – S76 .
- Phillips , S. M. , Tipton , K. D. , Aarsland , A. , Wolf , S. E. and Wolfe , R. R. 1997 . Mixed muscle protein synthesis and breakdown following resistance exercise in humans . American Journal of Physiology: Endocrinology and Metabolism , 273 : E99 – E107 .
- Phillips , S. M. , Tipton , K. D. , Ferrando , A. A. and Wolfe , R. R. 1999 . Resistance training reduces the acute exercise-induced increase in muscle protein turnover . American Journal of Physiology: Endocrinology and Metabolism , 276 : E118 – E124 .
- Pilegaard , H. , Keller , C. , Steensberg , A. , Helge , J. W. , Pedersen , B. K. Saltin , B. 2002 . Influence of pre-exercise muscle glycogen content on exercise-induced transcriptional regulation of metabolic genes . Journal of Physiology , 541 : 261 – 271 .
- Pilegaard , H. , Ordway , G. A. , Saltin , B. and Neufer , P. D. 2000 . Transcriptional regulation of gene expression in human skeletal muscle during recovery from exercise . American Journal of Physiology: Endocrinology and Metabolism , 279 : E806 – E814 .
- Pilegaard , H. , Saltin , B. and Neufer , P. D. 2003 . Exercise induces transient transcriptional activation of the PGC-1alpha gene in human skeletal muscle . Journal of Physiology , 546 : 851 – 858 .
- Rasmussen , B. B. , Tipton , K. D. , Miller , S. L. , Wolf , S. E. and Wolfe , R. R. 2000 . An oral essential amino acid–carbohydrate supplement enhances muscle protein anabolism after resistance exercise . Journal of Applied Physiology , 88 : 386 – 392 .
- Rennie , M. J. 1999 . An introduction to the use of tracers in nutrition and metabolism . Proceedings of the Nutrition Society , 58 : 935 – 944 .
- Rennie , M. J. 2005 . Body maintenance and repair: How food and exercise keep the musculoskeletal system in good shape . Experimental Physiology , 90 : 427 – 436 .
- Rennie , M. J. , Bohe , J. , Smith , K. , Wackerhage , H. and Greenhaff , P. 2006 . Branched-chain amino acids as fuels and anabolic signals in human muscle . Journal of Nutrition , 136 : 264S – 268S .
- Rennie , M. J. and Tipton , K. D. 2000 . Protein and amino acid metabolism during and after exercise and the effects of nutrition . Annual Review of Nutrition , 20 : 457 – 483 .
- Rennie , M. J. , Wackerhage , H. , Spangenburg , E. E. and Booth , F. W. 2004 . Control of the size of the human muscle mass . Annual Review of Physiology , 66 : 799 – 828 .
- Rennie , M. J. and Wilkes , E. A. 2005 . Maintenance of the musculoskeletal mass by control of protein turnover: The concept of anabolic resistance and its relevance to the transplant recipient . Annals of Transplantation , 10 : 31 – 34 .
- Romano-Ely , B. C. , Todd , M. K. , Saunders , M. J. and Laurent , T. S. 2006 . Effect of an isocaloric carbohydrate–protein–antioxidant drink on cycling performance . Medicine and Science in Sports and Exercise , 38 : 1608 – 1616 .
- Saunders , M. J. 2007 . Coingestion of carbohydrate and protein during endurance exercise: Influence on performance and recovery . Journal of Sports Sciences , 17 : S87 – S103 .
- Saunders , M. J. , Kane , M. D. and Todd , M. K. 2004 . Effects of a carbohydrate–protein beverage on cycling endurance and muscle damage . Medicine and Science in Sports and Exercise , 36 : 1233 – 1238 .
- Saunders , M. J. , Luden , N. D. and Herrick , J. E. 2007 . Consumption of an oral carbohydrate–protein gel improves cycling endurance and prevents postexercise muscle damage . Journal of Strength and Conditioning Research , 21 : 678 – 684 .
- Seynnes , O. R. , de Boer , M. and Narici , M. V. 2007 . Early skeletal muscle hypertrophy and architectural changes in response to high-intensity resistance training . Journal of Applied Physiology , 102 : 368 – 373 .
- Shepstone , T. N. , Tang , J. E. , Dallaire , S. , Schuenke , M. D. , Staron , R. S. and Phillips , S. M. 2005 . Short-term high- vs. low-velocity isokinetic lengthening training results in greater hypertrophy of the elbow flexors in young men . Journal of Applied Physiology , 98 : 1768 – 1776 .
- Shimomura , Y. , Yamamoto , Y. , Bajotto , G. , Sato , J. , Murakami , T. Shimomura , N. 2006 . Nutraceutical effects of branched-chain amino acids on skeletal muscle . Journal of Nutrition , 136 : 529S – 532S .
- Tang , J. E. , Hartman , J. W. and Phillips , S. M. 2006 . Increased muscle oxidative potential following resistance training induced fibre hypertrophy in young men . Applied Physiology, Nutrition and Metabolism , 31 : 495 – 501 .
- Tarnopolsky , M. A. 1999 . Protein and physical performance . Current Opinion in Clinical Nutrition and Metabolic Care , 2 : 533 – 537 .
- Tarnopolsky , M. 2004 . Protein requirements for endurance athletes . Nutrition , 20 : 662 – 668 .
- Tarnopolsky , M. A. , Atkinson , S. A. , MacDougall , J. D. , Chesley , A. , Phillips , S. and Schwarcz , H. P. 1992 . Evaluation of protein requirements for trained strength athletes . Journal of Applied Physiology , 73 : 1986 – 1995 .
- Tarnopolsky , M. A. , MacDougall , J. D. and Atkinson , S. A. 1988 . Influence of protein intake and training status on nitrogen balance and lean body mass . Journal of Applied Physiology , 64 : 187 – 193 .
- Tipton , K. D. , Borsheim , E. , Wolf , S. E. , Sanford , A. P. and Wolfe , R. R. 2003 . Acute response of net muscle protein balance reflects 24-h balance after exercise and amino acid ingestion . American Journal of Physiology: Endocrinology and Metabolism , 284 : E76 – E89 .
- Tipton , K. D. , Elliott , T. A. , Cree , M. G. , Aarsland , A. A. , Sanford , A. P. and Wolfe , R. R. 2007 . Stimulation of net muscle protein synthesis by whey protein ingestion before and after exercise . American Journal of Physiology: Endocrinology and Metabolism , 292 : E71 – E76 .
- Tipton , K. D. , Elliott , T. A. , Cree , M. G. , Wolf , S. E. , Sanford , A. P. and Wolfe , R. R. 2004 . Ingestion of casein and whey proteins results in muscle anabolism after resistance exercise . Medicine and Science in Sports and Exercise , 36 : 2073 – 2081 .
- Tipton , K. D. , Ferrando , A. A. , Phillips , S. M. , Doyle , D. Jr and Wolfe , R. R. 1999 . Postexercise net protein synthesis in human muscle from orally administered amino acids . American Journal of Physiology: Endocrinology and Metabolism , 276 : E628 – E634 .
- Tipton , K. D. , Ferrando , A. A. , Williams , B. D. and Wolfe , R. R. 1996 . Muscle protein metabolism in female swimmers after a combination of resistance and endurance exercise . Journal of Applied Physiology , 81 : 2034 – 2038 .
- Tipton , K. D. , Rasmussen , B. B. , Miller , S. L. , Wolf , S. E. , Owens-Stovall , S. K. Petrini , B. E. 2001 . Timing of amino acid–carbohydrate ingestion alters anabolic response of muscle to resistance exercise . American Journal of Physiology: Endocrinology and Metabolism , 281 : E197 – E206 .
- Tipton , K. D. and Sharp , C. P. 2005 . The response of intracellular signaling and muscle protein metabolism to nutrition and exercise . European Journal of Sport Science , 5 : 107 – 121 .
- Tipton , K. D. and Witard , O. C. 2007 . Protein requirements and recommendations for athletes: Relevance of ivory tower arguments for practical recommendations . Clinics in Sports Medicine , 26 : 17 – 36 .
- Tipton , K. D. and Wolfe , R. R. 1998 . Exercise-induced changes in protein metabolism . Acta Physiologica Scandinavica , 162 : 377 – 387 .
- Tipton , K. D. and Wolfe , R. R. 2001 . Exercise, protein metabolism, and muscle growth . International Journal of Sports Nutrition and Exercise Metabolism , 11 : 109 – 132 .
- Tipton , K. D. and Wolfe , R. R. 2004 . Protein and amino acids for athletes . Journal of Sports Sciences , 22 : 65 – 79 .
- Todd , K. S. , Butterfield , G. E. and Calloway , D. H. 1984 . Nitrogen balance in men with adequate and deficient energy intake at three levels of work . Journal of Nutrition , 114 : 2107 – 2118 .
- Tome , D. and Bos , C. 2000 . Dietary protein and nitrogen utilization . Journal of Nutrition , 130 : 1868S – 1873S .
- Wagenmakers , A. J. 1999 . Tracers to investigate protein and amino acid metabolism in human subjects . Proceedings of the Nutrition Society , 58 : 987 – 1000 .
- Warren , G. L. , Lowe , D. A. and Armstrong , R. B. 1999 . Measurement tools used in the study of eccentric contraction-induced injury . Sports Medicine , 27 : 43 – 59 .
- Wilkinson , S. B. , Tarnopolsky , M. A. , Macdonald , M. J. , MacDonald , J. R. , Armstrong , D. and Phillips , S. M. 2007 . Consumption of fluid skim milk promotes greater muscle protein accretion after resistance exercise than does consumption of an isonitrogenous and isoenergetic soy-protein beverage . American Journal of Clinical Nutrition , 85 : 1031 – 1040 .
- Willoughby , D. S. , Taylor , M. and Taylor , L. 2003 . Glucocorticoid receptor and ubiquitin expression after repeated eccentric exercise . Medicine and Science in Sports and Exercise , 35 : 2023 – 2031 .
- Wolfe , R. R. 2000 . Protein supplements and exercise . American Journal of Clinical Nutrition , 72 : 551S – 557S .
- Wolfe , R. R. 2001 . Effects of amino acid intake on anabolic processes . Canadian Journal of Applied Physiology , 26 ( suppl. ) : S220 – S227 .
- Wolfe , R. R. and George , S. 1993 . Stable isotopic tracers as metabolic probes in exercise . Exercise and Sport Science Reviews , 21 : 1 – 31 .
- Wolfe , R. R. and Miller , S. 1999 . Amino acid availability controls muscle protein metabolism . Diabetes, Nutrition and Metabolism , 5 : 322 – 328 .
- Wolfe , R. R. and Sidossis , L. S. 1993 . Isotopic measurement of substrate flux and cycling . International Journal of Obesity and Related Metabolic Disorders , 17 ( suppl, 3 ) : S10 – S13 .
- Young , V. R. 1986 . Nutritional balance studies: Indicators of human requirements or of adaptive mechanisms? . Journal of Nutrition , 116 : 700 – 703 .
- Young , V. R. , Bier , D. M. and Pellett , P. L. 1989 . A theoretical basis for increasing current estimates of the amino acid requirements in adult man, with experimental support . American Journal of Clinical Nutrition , 50 : 80 – 92 .