ABSTRACT
Although evidence demonstrates the fundamental role of shear stress in vascular health, predominantly through the release of nitric oxide (NO), the mechanisms by which endothelial cells (EC)s sense and transduce shear are poorly understood. In cultured ECs tyrosine phosphorylation of PECAM-1 has been shown to activate eNOS in response to shear stress. However, in the human skeletal muscle microcirculation PECAM-1 was not activated in response to exercise or passive leg movement. Given this contradiction, this study aimed to assess the effect of exercise on conduit artery PECAM-1 and eNOS activation in humans. Eleven males were randomised to two groups; 30 min of handgrip exercise (n = 6), or a time-control group (n = 5). Protein content of eNOS and PECAM-1, alongside eNOS Ser1177 and PECAM-1 Tyr713 phosphorylation were assessed in ECs obtained from the radial artery pre- and post-intervention. Handgrip exercise resulted in a 5-fold increase in mean shear rate in the exercise group, with no change in the control group (group*time, P < 0.001). There was a 54% increase in eNOS Ser1177 phosphorylation in the exercise group, when compared to control group (group*time, P = 0.016), but no change was reported in PECAM-1 Tyr713 phosphorylation in either group (group*time, P > 0.05). eNOS and PECAM-1 protein content were unchanged (group*time, P > 0.05). Our data show that exercise-induced elevations in conduit artery shear rate increase eNOS Ser1177 phosphorylation but not PECAM-1 Tyr713 phosphorylation. This suggests PECAM-1 phosphorylation may not be involved in the vascular response to acute but prolonged elevations in exercise-induced shear rate in conduit arteries of healthy, active men.
Highlights
Shear stress-mediated nitric oxide (NO) release plays a fundamental role in vascular health and has been proposed as a key-mechanism underpinning exercise-induced vascular adaptation. However, the mechanisms by which endothelial cells (EC)s sense and transduce shear are poorly understood.
PECAM-1 tyrosine phosphorylation has been shown to activate eNOS in response to shear stress in cultured ECs. However, the relationship between PECAM-1 and eNOS has not been shown in-vivo. Therefore, this study assessed the effect of 30 min handgrip exercise on conduit artery EC PECAM-1 and eNOS phosphorylation in humans.
We demonstrated that acute shear rate elevation, mediated by exercise, resulted in a 54% increase in eNOS Ser1177 phosphorylation, but PECAM-1 Tyr713 phosphorylation remained unchanged.
Our data, in addition to previous observations from skeletal muscle suggests that PECAM-1 phosphorylation may not be involved in the vascular response to acute but prolonged elevations in exercise-induced shear rate in humans. Future studies using larger sample sizes should investigate conduit artery PECAM-1 in response to different shear patterns.
Introduction
Endothelial cells (ECs) play a crucial role in controlling vascular tone and homeostasis (Sandoo, Veldhuijzen van Zanten, Metsios, Carroll, & Kitas, Citation2010) and are responsible for the expression of pro- and anti-atherogenic genes (Davignon & Ganz, Citation2004). Many of these important effects are mediated by the release of nitric oxide (NO) in response to hemodynamic stimuli exerted on the luminal surface of ECs by the frictional force of the flowing blood on the ECs (shear stress) (Balligand, Feron, & Dessy, Citation2009; Davies, Citation2009; Green, Hopman, Padilla, Laughlin, & Thijssen, Citation2017).
Although there is clear evidence demonstrating the fundamental role of shear stress in vascular health (Chatzizisis et al., Citation2007), the mechanisms through which ECs sense and transduce shear are less clear, with a number of molecules identified as potential mechanotransduction proteins in-vitro or using animal models (Tzima et al., Citation2005; Wang et al., Citation2016; Wilson, Lee, & McCarron, Citation2016; Xu et al., Citation2018). It has been proposed that changes in fluid shear stress are sensed from the apical surface of ECs, in particular at cell–cell and cell–matrix junctions (Tzima et al., Citation2005), where this mechanical tension is transduced to several biochemical signals, including NO production. Studies on cultured ECs have demonstrated that shear stress is sensed by a mechanosensory complex, which includes platelet endothelial cell adhesion molecule 1 (PECAM-1) (Chatzizisis et al., Citation2007). In cultured cells downregulation of PECAM-1, using a siRNA approach, attenuated the shear-stress-induced phosphorylation of endothelial nitric oxide synthase (eNOS)(Fleming, Fisslthaler, Dixit, & Busse, Citation2005), suggesting that shear stress-mediated activation of eNOS in ECs is modulated by tyrosine phosphorylation of PECAM-1. However, in vivo human studies investigating the activation of PECAM-1 in the skeletal muscle microvasculature did not show increased PECAM-1 phosphorylation in response to increased shear rate, induced by passive leg movement (Gliemann et al., Citation2017); (Gliemann et al., Citation2018) or exercise (Fiorenza, Gliemann, Brandt, & Bangsbo, Citation2020). Although these observations in the skeletal muscle microvasculature are interesting, to date, no studies have investigated the effect of elevated shear rate on PECAM-1 phosphorylation in conduit arteries. Importantly, previous evidence suggests vascular responses to shear rate elevations in conduit arteries and the microcirculation may be distinct and unrelated (Green et al., Citation2004; Tajima et al., 2020). In addition, experiments in the skeletal muscle microvasculature present technical challenges due to potential differences in the size and number of arterioles and unknown perfusion patterns, which make interpretation of data from the microcirculation difficult (Gliemann et al., Citation2018). As such, the disconnect between in vitro and in vivo PECAM-1 response to elevated shear rate may be influenced by the vascular bed investigated (microcirculation), with responses from conduit arteries potentially coming in line with those observed in in-vitro studies.
Therefore, the aim of this study was to assess the effect of exercise-induced shear rate elevations on EC PECAM-1 and eNOS activation in conduit arteries of humans. We hypothesised that shear rate elevations induced by acute exercise would phosphorylate both PECAM-1 and eNOS in conduit artery ECs of healthy males. Given the well-established importance of NO production in vascular health and the role that shear rate plays in exercise-induced functional adaptations (Green et al., Citation2017), clarifying the mechanotransduction pathway responsible for sensing elevations in shear rate associated with exercise, will help us to better understand the mechanisms through which exercise or other interventions benefit the vasculature.
Materials and method
Ethical approval
All participants provided written informed consent prior to the experimental procedures, and the study was approved by the Liverpool North-West NHS Research Ethics Committee (REC 18/NW/0428) and conformed to the Declaration of Helsinki.
Participants
Eleven healthy, active (≥150 min of moderate-intensity or ≥75 min of vigorous-intensity exercise per week), males volunteered to participate in this study (). Participants were randomly assigned to either exercise (n = 6) or time-matched control (n = 5) groups. Participants were <40 years, non-smokers and free of diagnosed cardiovascular disease (CVD), or known CVD risk factors such as diabetes, hypertension, or hypercholesterolemia.
Table 1. Summary of participants’ characteristics.
Study design
Participants attended the cardiovascular lab at Liverpool John Moores University on two occasions: (a) pre-experimental visit and (b) experimental visit, at least 72 h apart. During the pre-experimental visit peak oxygen consumption (VO2peak) was assessed using a maximal graded exercise test on an electromagnetically braked cycle ergometer (Lode Excalibur Sport Cycle Ergometer, The Netherlands) using a gas analysis system (MOXUS Metabolic Cart (AEI Technology, USA)), as previously described (Tryfonos et al., Citation2020b). Briefly, participants started cycling at 60W for 3 min; following this the workload was increased by 35W every 3 min until volitional exhaustion. VO2peak corresponds to the highest value achieved over a 15s recording period. Participants’ handgrip strength was also determined, as the highest of 3 maximum voluntary contraction tests (MVC) (Takei 5420 Grip-D Digital Hand Grip Dynamometer, Japan).
On the experimental visit participants attended the lab, having fasted overnight, and abstained from caffeine, alcohol, and vigorous exercise for 24 h, in accordance with current guidelines (Thijssen et al., Citation2019). Experiments commenced between 7am and 1pm, in a quiet temperature-controlled room (∼22°C). Upon arrival, participants rested in the supine position for at least 10 min to ensure all hemodynamic variables were stabilised. Radial artery catheterisation and EC collection were then performed. Participants subsequently undertook either 30 min of forearm (handgrip) exercise or remained rested for 30 min (control). Haemodynamic and blood flow variables were collected before and during each intervention. EC collection was repeated immediately after the forearm exercise protocol or control period.
Handgrip exercise protocol and radial artery blood flow assessment
Participants in the exercise group performed 30 min of continuous rhythmic handgrip exercise in the catheterised arm at 15% of the predetermined MVC. An automatic metronome (Korg MA30 Metronome 2006, Tokyo, Japan) was used to keep constant pace for the handgrip exercise (30 contractions per minute; 1 contraction per second followed by 1 s relaxation). Blood pressure and heart rate (GE Pro 300V2, Dinamap, Tampa, FL, USA) were measured prior to and every 10 min during the exercise. A 12-MHz multi-frequency linear array probe, attached to high-resolution ultrasound (T3000; Terason, Burlington, MA, USA), was used to simultaneously image diameter and velocity (insonation angle <60°) of the experimental radial artery (15–18 cm proximal to the wrist) prior to and every 10 min during the exercise protocol, as previously described (Tryfonos, Cocks, Mills, Green, & Dawson, Citation2020). Blood pressure, heart rate and radial artery ultrasound-derived variables were also assessed in the time-control group at the same time-points. The same ultrasound and sonographer were used between and within participants. Changes in vascular measures (radial artery diameter, blood velocity, blood flow, shear rate) were calculated in averages of 1 min recordings, before, and every 10 min during each intervention. Recordings were analyzed by the same blinded observer, using custom-designed edge-detection and wall-tracking software (Thijssen et al., Citation2011; Thijssen et al., Citation2019; Woodman et al., Citation2001), to minimise investigator bias.
Transradial catheterisation and endothelial cell (EC) collection
A detailed description of the transradial catheterisation and EC collection process used in this study has been described previously (Tryfonos et al., Citation2020b). Briefly, an 18–20-gauge catheter (0.9–1.2 mm diameter, 8–10 cm length) (leadercath, Vygon, UK) was inserted into the radial artery of the right arm, under local anaesthesia (2–4 ml Marcain Polyamp steripack 0.5%, Aspen), by a cardiologist. Two separate flexible J-shaped guide wires (paediatric J-wires, 0.46 mm, 40 cm length, Vygon, UK) were advanced 3–4 cm beyond the tip of the catheter and run back and forth to collect ECs from the inside of the artery (Colombo et al., Citation2002; Feng, Stern, & Pile-Spellman, Citation1999), before (Pre-) and immediately after the (Post-) intervention (within 2–3 min of the end of exercise). The distal portion of each J-shaped guide wire was transferred to ice-cold dissociation buffer (within 10 s of procedure) and taken immediately to the laboratory for processing (see below), where samples were maintained on ice until fixation (duration between sampling and fixation was approx. 10 min), as previous work suggests cold conditions can improve preservation of phosphoproteins by slowing down enzymatic activity (Gündisch et al., Citation2015; Wang et al., Citation2015). Catheters were removed following the post-intervention EC collection.
EC protein expression via immunofluorescence
EC isolation and protein expression measurements were performed as previously described (Donato et al., Citation2007; Pierce et al., Citation2011). ECs were recovered from the dissociation buffer (0.5% BSA, 2 mM EDTA, and 18 U/ml heparin in PBS (pH 7.4)) by centrifugation and washing. Specifically, the dissociation buffer and the two J-shaped wires were centrifuged at 400 RCF, 4°C for 7 min. The supernatant solution was then removed, and the remaining pellet was fixed in 3.7% formaldehyde solution for 10 min. Dulbecco's Phosphate Buffered Saline (DPBS) (Thermo Fisher Scientific, USA) was added, and the pellet resuspended before the sample was centrifuged at 400 RCF, 4°C for 5 min. The supernatant solution was removed, and the pellet was resuspended in DPBS. The wires were then rinsed for 10 min, to gently remove and remaining ECs from the wires. The sample was centrifuged at 400 RCF, 4°C for 6 min. Finally, the supernatant was removed, and the pellet resuspended and evenly spread onto glass slides (VWR, REF number: 631-0705, USA). Slides were dried before being stored at −80°C until further immunofluorescence analysis.
At the time of analysis, cells were rehydrated in DPBS for 5 min. After blocking nonspecific binding sites with 5% donkey serum (1 h) (Sigma Aldrich, USA), cells were incubated for 1 h at room temperature with primary antibodies against eNOS (610297, BD, USA), eNOS phosphorylated at Ser1177 (peNOS Ser1177) (07-428-I, Merck), PECAM-1 (abc24590, Abcam, UK) and PECAM-1 phosphorylated at Tyr713 (BS4666, Bio World, USA). Cells were also stained for vascular-endothelial (VE)-cadherin (NB600-1409, Novus, UK) for positive identification of the endothelial phenotype. Cells were then incubated with appropriate secondary antibodies, in combination with DAPI (4-6-diamidino-2-phenylindole hydrochloride) for assessment of nuclear integrity. Finally, coverslips were mounted in a glycerol and mowiol 4–88 solution in 0.2 m Tris buffer (pH 8.5) with addition of 0.1% DABCO anti-fade medium.
Images were acquired using an inverted confocal microscope (Zeiss LSM-710, Carl Zeiss, Germany) with a 63x 1.3NA oil immersion objective. Positive staining for VE-cadherin coupled with a single intact nucleus was used to reliably select ECs (Casey, Ueda, Wegman-Points, & Pierce, Citation2017). DAPI was excited using the 405 nm line of the diode laser and detected with 371–422 nm emission. Alexa fluor 488 was excited with the 488 nm line of the argon laser and detected with 493–559 nm emission. Alexa Fluor 546 and 633 fluorophores were excited with 543 and 633 nm lines of the helium–neon laser and 548–623 and 638–747 nm emission, respectively. The images were acquired at a resolution of 1024 × 1024 pixels and stored in 24-bit tagged image format file format.
EC image analysis was performed using ImagePro Plus 5.1 (Media Cybernetics Inc, Bethesda, MD, USA) to quantify the intensity of staining (i.e. average pixel intensity). A minimum of 30 ECs were analyzed for each condition. To ensure only the cytosolic fraction was assessed nuclear regions of the ECs, identified through the DAPI stain, were extracted from the rest of the ECs image identified using the VE-cadherin image. The resulting mask was then overlaid onto the corresponding protein of interest image. Mean fluorescence intensity of the protein of interest signal was then quantified within the endothelial cytosolic specific area. Slides were stained and imaged within participants in the same batch using the same microscope and analysis settings, and relative difference between pre- and post-intervention slides was assessed. A single-blinded technician completed all imaging and analysis.
Statistics
The reproducibility of measures for protein content and phosphorylation of eNOS and PECAM-1 was assessed by coefficient of variation between control slides taken at baseline and post-intervention. All statistical analyses were performed using IBM SPSS statistics for Windows, version 26.0. Armonk, NY: IBM Corp. Shapiro–Wilk test and Levene’s test were employed to explore normality and equality of errors variances (pre–post changes) respectively, and all were reasonably equal and normal. As such, radial artery hemodynamic and shear variables as well as EC protein expression were compared via two-way repeated-measures ANOVA to detect differences within and between conditions. Pairwise comparisons were performed when significant main or interaction effects were detected, using Bonferroni test. Data are presented as mean ± SD and alpha significance was set at P≤0.05. All 11 participants completed the protocol; however, an insufficient number of ECs were collected in two participants. As such, EC protein expression data are presented for 9 participants (4 exercise and 5 control for eNOS and eNOS Ser1177 phosphorylation).
Results
There were no differences in age, VO2peak or handgrip strength between groups. Body mass index (BMI) was in the non-obese range for both groups, but significantly higher in the exercise group compared to control. Values are presented in .
Radial artery haemodynamics
There was a significant interaction effect (group*time) for radial artery mean (P < 0.0001; ) and antegrade shear rate (P < 0.0001; ). Baseline mean (P = 0.576) and antegrade shear rate (P = 0.755) were not different between the groups. Pairwise comparisons demonstrated that mean and antegrade shear rate was respectively increased at 10 (301 ± 316%, P = 0.002; 286 ± 240%, P = 0.002) 20 (365 ± 284%, P < 0.0001; 374 ± 295%, P < 0.0001) and 30 (360 ± 312%, P = 0.001; 329 ± 295% P < 0.0001) minutes during the handgrip exercise, when compared to baseline. In contrast, mean and antegrade shear rate were unchanged from baseline to 10 (P = 0.694, P = 0.849), 20 (P = 0.817, P = 0.970) and 30 min (P = 0.927, P = 0.993) in the control group. Retrograde shear rate displayed a main effect of time (P = 0.035), with no main effect of group (P = 0.315) or interaction (P = 0.095). Blood velocity, and blood flow patterns followed the same trends as mean shear rate (). Finally, radial artery diameter was smaller in the control group compared to exercise group (2.4 vs. 2.9 mm; main effect of group P = 0.015). There was a main effect of time (P = 0.046), however there was no interaction effect (P = 0.205), suggesting that arterial diameter in the control group was typically smaller from the baseline and during the intervention ().
Figure 1. Mean shear rate in the exercise (n = 6) and control groups (n = 5) prior to intervention (Baseline) and at 10, 20 and 30 min. Results are presented as mean ± SD, P < 0.05. *Significantly different from Baseline, †Significantly different from 10 min, #Significantly different from control group.
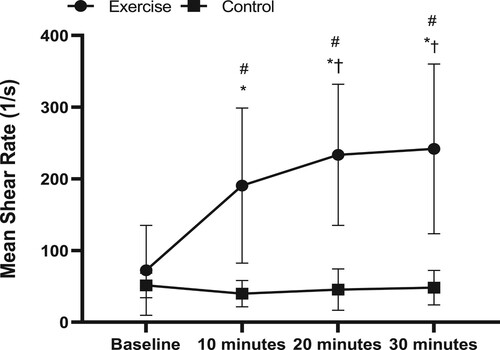
Table 2. Radial artery haemodynamics in the exercise (n = 6) and control groups (n = 5), prior to intervention (Baseline) and at 10, 20 and 30 min.
Heart rate did not change in either group during the trial (group*time, P = 0.877). There was a significant group (P = 0.006), and interaction effect (group*time) for mean arterial pressure (P < 0.0001; ), which increased during the handgrip protocol at 20 (P = 0.009) and 30 (P = 0.005) minutes, compared to baseline. Conversely, mean arterial pressure in the control group was significantly reduced at 30 min compared to baseline (P = 0.031).
Reproducibility of quantitative immunofluorescence
The coefficient of variation within control slides (baseline to post-intervention) for eNOS, eNOS ser1177, PECAM-1 and PECAM-1 Tyr713 were 11.5%, 10.8%, 22.4% and 23.3%, respectively.
EC protein expression and phosphorylation
eNOS content was unchanged in either group following the intervention (group*time, P = 0.557; (A)). In contrast, a significant interaction effect was observed in eNOS Ser1177 phosphorylation (P = 0.016). Pairwise comparisons showed increased eNOS Ser1177 phosphorylation in the exercise group (∼54% increase, P = 0.009), whereas no change was observed in the control group (P = 0.499; (B)). In addition, eNOS Ser1177 phosphorylation was higher in the exercise group than in the control group following intervention (P = 0.016; (C)).
Figure 2. eNOS, eNOS Ser1177 phosphorylation (peNOS), PECAM-1 and PECAM-1 Tyr713 phosphorylation (pPECAM-1) in the exercise and control groups pre- and post-intervention. A, Representative confocal microscopy images of radial artery endothelial cells (EC)s stained for eNOS (a, b) and peNOS Ser1177 (c, d) pre (a, c) and post (b, d) handgrip exercise, images are taken from the same participant from the exercise group. B, Representative confocal microscopy images of radial artery EC stained for PECAM-1 (a, b) and pPECAM-1 Tyr713 (c, d) pre (a, c) and post (b, d) handgrip exercise, images are taken from the same participant from the exercise group. Mean fluorescence intensity of eNOS; C, peNOS Ser1177; D, PECAM-1; E, and pPECAM-1 Tyr713; F is summarised. The mean fluorescence intensity pre intervention was assigned a value of 1, and the relative intensity post intervention was calculated. Results are mean ± SD, *Significantly different from Pre (P < 0.05).
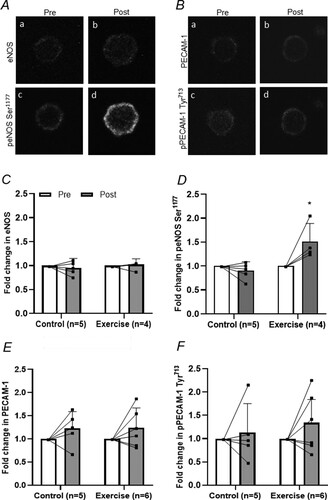
PECAM-1 was unchanged in either group following the intervention (group*time, P = 0.968) ((D)). PECAM-1 Tyr713 phosphorylation was also unchanged in either group following intervention (group*time, P = 0.579; (E)).
Discussion
This study demonstrated that elevated shear rate induced by a single bout of handgrip exercise stimulates eNOS Ser1177 phosphorylation but does not lead to Tyr713 phosphorylation of PECAM-1 in ECs obtained from the radial artery of healthy, active males. Therefore, this study provides further evidence to suggest that PECAM-1 may not be activated by acute but prolonged elevations in shear rate in humans.
The major outcome of the present study was that PECAM-1 Tyr713 phosphorylation was not elevated in human ECs following a 5-fold increase in shear rate induced by a prolonged (30 min) acute exercise bout. This is the first study to investigate conduit artery endothelial PECAM-1 phosphorylation in response to exercise. However, this observation is in line with two recent studies reporting no change in PECAM-1 Tyr713 phosphorylation in the skeletal muscle microcirculation, following hyperaemia generated by passive leg movement (Gliemann et al., Citation2017) and exercise (Fiorenza et al., Citation2020). Together these studies suggest that endothelial PECAM-1 is not activated in response to acute but prolonged (10-50 min) elevations in shear rate, in humans. This contrasts with observation from cultured ECs, where the application of fluid shear stress (12 dynes cm–2 equals to 1.2 Pa) elicits marked tyrosine phosphorylation of PECAM-1 (Fleming et al., Citation2005). Importantly, decreasing EC expression of PECAM-1, using siRNA oligonucleotides or ECs isolated from PECAM-1 knockout mice, blunted eNOS Ser1177 phosphorylation and attenuated cyclic GMP levels in response to shear stress (Fleming et al., Citation2005). Collectively, this suggests an inconsistency between in vitro and in vivo human responses, which raises questions about the role of PECAM-1 in shear rate mediated NO production in humans. The discrepancy between in vivo and in vitro models may be caused by differences in the magnitude, exposure time and the pattern of shear stimulus between cultured ECs and ECs in the in vivo setting (Gliemann et al., Citation2018). It is worth noting that, although blood viscosity was not measured in this study and thus shear stress cannot be accurately calculated, we obtained viscosity from previously published data in healthy young males (Tremblay et al., Citation2020). Utilising their methods, we calculated that shear stress in our radial artery model during handgrip exercise was lower (approx. 40%) than that presented in Fleming et al. (Citation2005) (0.86 ± 0.46 Pa vs. 1.2 Pa, respectively), but was in line with the values reported by Dammers et al. (Citation2003) in human brachial artery at rest (0.48 ± 0.15 Pa). Importantly, shear rate in Fleming et al. (Citation2005) was continuous, whereas in human conduit arteries shear rate is pulsative. Our team have previously demonstrated that distinct patterns of shear in humans induce different changes in endothelial function in vivo (Green et al., Citation2017; Thijssen et al., Citation2009). In addition, to distinct shear patterns reported in in vitro vs. in vivo studies, exercise elicits other responses including cyclical stretch and elevated reactive oxygen species, which are known to result in different vascular responses (Birukov, Citation2009), perhaps through additional transduction pathways.
Interestingly, using muscle arterioles isolated from PECAM-1 knockout mice, Bagi et al. (Citation2005) observed that the initial dilation of vessels (up to 120 s) in response to fluid shear stress was impaired compared to wild type animals, but the second phase of dilation, when shear stress increases had reached steady-state, was similar in the two groups. Similarly, in human umbilical vein endothelial cells sudden change in shear stress, but not steady flow, resulted in dissociation of eNOS from PECAM-1, an important step in eNOS activation (Dusserre et al., Citation2004). Together these studies suggest that PECAM-1 is involved in the initial response to shear stress and/ or high temporal gradients of shear stress, whereas other mechanosensory pathways may be required to respond to a steady-state increase in shear stress. In the current study and that of Gliemann et al. (Citation2017) and Fiorenza et al. (Citation2020), change in PECAM-1 Tyr713 phosphorylation was primarily investigated following a sustained continuous increase in shear rate (30 min of continuous handgrip exercise, 50 min of continuous cycling (Fiorenza et al., Citation2020) or 20 min of continuous passive leg movement (Gliemann et al., Citation2017)), therefore it is possible that any change in PECAM-1 Tyr713 phosphorylation was missed. However, Fiorenza et al. (Citation2020) also showed no change in PECAM-1 phosphorylation in response to two high intensity interval training protocols (18 × 5s maximal sprints and 6 × 20s maximal efforts on a cycle ergometer), which would be assumed to create rapid changes in shear rate. As such, future studies in human conduit arteries should examine PECAM-1 phosphorylation status at different stages of shear stimulus (initial vs. prolonged) and in response to different patterns of shear stimulus (rapid vs. steady increases). Such studies should also use proteomics approaches to investigate whether additional phosphorylation sites and/or post-translational modifications participate in the exercise-induced activation of mechanosensor proteins in humans.
Our data demonstrate that a single bout of handgrip exercise resulted in a ∼54% increase in eNOS phosphorylation at Ser1177, with no effect on eNOS content in human ECs. This finding of elevated eNOS Ser1177 phosphorylation post-exercise agrees with previous work showing a similar increase in eNOS phosphorylation (57%) following 2 h of handgrip exercise in human ECs collected from the brachial artery (Casey et al., Citation2017). Park et al. (Citation2019) have also reported similar outcomes in response to 1 h of handgrip exercise in ECs obtained from the radial artery, but the baseline cell collection occurred during cuff occlusion (no blood flow and therefore shear rate). As such, the current study adds physiological relevance as eNOS Ser1177 phosphorylation was shown to be elevated when shear rate was increased from resting, physiologically normal, values. Indeed, Hambrecht et al. (Citation2003) showed that repeated episodic exposure to elevated shear rate, such as occurs in response to exercise training, resulted in upregulation of eNOS both its protein content and Ser1177 phosphorylation in ex vivo human arteries.
Limitations
This study examined the activation of PECAM-1 and eNOS in ECs collected from healthy, active males. Considering the well-established benefits of oestrogen on endothelial function (Green et al., Citation2016; Hellsten & Gliemann, Citation2018), including post-exercise vascular responses (Hellsten & Gliemann, Citation2018), it is possible that different mechanotransduction pathways may be apparent between sexes. As such, we only studied males to limit any influence of sex hormones on vascular responses. We included participants aged 18–40 years. Although all participants were active, individuals in their thirties may have had different responses compared to younger participants. This study examined the activation of PECAM-1 only at the Tyr713 residue. Previous research has identified other Tyr residues (633, 686, and 690) which may play a role in PECAM-1 activation in response to different stimuli (Newman, Citation1999). As such, future research should investigate the potential that phosphorylation of other Tyr residues takes place in response to elevated shear rate. However, Osawa, Masuda, Harada, Lopes, and Fujiwara (Citation1997) demonstrated that the Tyr 713 residue is likely to be the key site for EC activation in response to fluid shear stress. The Pre-intervention EC sample was collected immediately after the catherisation procedure. Although ECs were collected 3–4 cm proximal to the site of catherisation it cannot be ruled out that acute trauma may have affected baseline measurements. However, no difference in eNOS or PECAM-1 phosphorylation were observed in the control group. We also acknowledge that the analysis of protein content and enzyme phosphorylation status using immunofluorescence microscopy is semi-quantitative in nature and, as such, only allows for analysis of relative differences to be assessed. Although the reproducibility of eNOS and eNOS Ser1177 measures between control slides were similar to those presented previously (Colombo et al., Citation2002) the coefficient of variation between control slides was higher for PECAM-1 (22.4%) and PECAM-1 Tyr713 (23.3%). As such, it is possible that our assay was not sensitive enough to detect small changes in PECAM-1 Tyr713 phosphorylation that occurred in response to exercise. However, assessing variability between control slides is not optimal, as it is unclear to what extent the variability comes from time differences or measurement variability. In addition, the onerous and invasive nature of the experiment affected recruitment. This resulted in a small sample size, which may have impacted effect size in some variables. To further support the above, previous studies employing the same technique of collecting arterial ECs pre–post handgrip exercise have used similar sample sizes, n = 13 (Casey et al., Citation2017), and n = 7 (Park et al., Citation2019) respectively. Interestingly, when individual data for PECAM-1 Tyr713 phosphorylation is examined (), a bimodal response may be observed following exercise. We believe that our preliminary observations are novel, interesting and add further clarity to the literature and therefore warrant further exploration in future larger studies.
To conclude, this is the first study to assess changes in PECAM-1 activation in response to exercise-induced increases in shear rate in conduit arteries of humans. This finding suggests that although 30 min of handgrip exercise resulted in a 5-fold increase in radial artery shear stress, and eNOS phosphorylation at Ser1177, PECAM-1 Tyr713 phosphorylation was not increased. As such, this study suggests that PECAM-1 activation may not be involved in the vascular response to acute but prolonged elevations in shear rate. Given the variability in PECAM measures (content and phosphorylation) the study is limited by investigating only a small number of participants (n = 5–6 subjects/group). However, we believe that when combined with recent data in the skeletal muscle microcirculation, also showing a lack of PECAM-1 phosphorylation in response to prolonged elevations in shear rate, our conclusions are valid. Given previous observations regarding the role of PECAM-1 in regulating eNOS activation in response to the onset of shear and rapid changes in shear but not steady elevation, future studies using larger sample sizes should investigate conduit artery PECAM-1 phosphorylation in response to different shear pattern in humans.
Acknowledgements
The authors are grateful to the study volunteers for their participation. We would like to thank Rafaella Rodighiero, Sophie Holder, Katie Whytock and Conner Ward for their help during the data collection.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Bagi, Z., Frangos, J. A., Yeh, J.-C., Charles R, W., Kaley, G., & Koller, A. (2005). PECAM-1 mediates NO-dependent dilation of arterioles to high temporal gradients of shear stress. Arteriosclerosis, Thrombosis, and Vascular Biology, 25(8), 1590–1595.
- Balligand, J. L., Feron, O., & Dessy, C. (2009). eNOS activation by physical forces: From short-term regulation of contraction to chronic remodeling of cardiovascular tissues. Physiological Reviews, 89(2), 481–534.
- Birukov, K. G. (2009). Cyclic stretch, reactive oxygen species, and vascular remodeling. Antioxidants & Redox Signaling, 11(7), 1651–1667.
- Casey, D. P., Ueda, K., Wegman-Points, L., & Pierce, G. L. (2017). Muscle contraction induced arterial shear stress increases endothelial nitric oxide synthase phosphorylation in humans. American Journal of Physiology-Heart and Circulatory Physiology, 313(4), H854–h859.
- Chatzizisis, Y. S., Coskun, A. U., Jonas, M., Edelman, E. R., Feldman, C. L., & Stone, P. H. (2007). Role of endothelial shear stress in the natural history of coronary atherosclerosis and vascular remodeling: Molecular, cellular, and vascular behavior. Journal of the American College of Cardiology, 49(25), 2379–2393.
- Colombo, P. C., Ashton, A. W., Celaj, S., Talreja, A., Banchs, J. E., Dubois, N. B., … Le Jemtel, T. H. (2002). Biopsy coupled to quantitative immunofluorescence: A new method to study the human vascular endothelium. Journal of Applied Physiology (1985), 92(3), 1331–1338. doi:10.1152/japplphysiol.00680.2001
- Dammers, R., Stifft, F., Tordoir, J. H. M., Hameleers, J. M., Hoeks, A. P. G., & Kitslaar, J. E. H. M. (2003). Shear stress depends on vascular territory: Comparison between common carotid and brachial artery. Journal of Applied Physiology (1985), 94(2), 485–489. doi:10.1152/japplphysiol.00823.2002
- Davies, P. F. (2009). Hemodynamic shear stress and the endothelium in cardiovascular pathophysiology. Nature Clinical Practice Cardiovascular Medicine, 6(1), 16–26.
- Davignon, J., & Ganz, P. (2004). Role of endothelial dysfunction in atherosclerosis. Circulation, 109(23 Suppl 1), Iii27–Iii32. doi:10.1161/01.CIR.0000131515.03336.f8
- Donato, A. J., Eskurza, I., Silver, A. E., Levy, A. S., Pierce, G. L., Gates, P. E., & Seals, D. R. (2007). Direct evidence of endothelial oxidative stress with aging in humans: Relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappaB. Circulation Research, 100(11), 1659–1666.
- Dusserre, N., L’Heureux, N., Bell, K. S., Stevens, H. Y., Yeh, J., Otte, L. A., … Frangos, J. A. (2004). PECAM-1 interacts with nitric oxide synthase in human endothelial cells: Implication for flow-induced nitric oxide synthase activation. Arteriosclerosis, Thrombosis, and Vascular Biology, 24(10), 1796–1802.
- Feng, L., Stern, D. M., & Pile-Spellman, J. (1999). Human endothelium: Endovascular biopsy and molecular analysis. Radiology, 212(3), 655–664.
- Fiorenza, M., Gliemann, L., Brandt, N., & Bangsbo, J. (2020). Hormetic modulation of angiogenic factors by exercise-induced mechanical and metabolic stress in human skeletal muscle. American Journal of Physiology-Heart and Circulatory Physiology, 319(4), H824–h834.
- Fleming, I., Fisslthaler, B., Dixit, M., & Busse, R. (2005). Role of PECAM-1 in the shear-stress-induced activation of Akt and the endothelial nitric oxide synthase (eNOS) in endothelial cells. Journal of Cell Science, 118(Pt 18), 4103–4111.
- Gliemann, L., Rytter, N., Lindskrog, M., Slingsby, M. H. L., Åkerström, T., Sylow, L., … Hellsten, Y. (2017). Endothelial mechanotransduction proteins and vascular function are altered by dietary sucrose supplementation in healthy young male subjects. The Journal of Physiology, 595(16), 5557–5571.
- Gliemann, L., Rytter, N., Piil, P., Nilton, J., Lind, T., Nyberg, M., … Hellsten, Y. (2018). The endothelial mechanotransduction protein platelet endothelial cell adhesion molecule-1 is influenced by aging and exercise training in human skeletal muscle. Frontiers in Physiology, 9, 1807.
- Green, D. J., Hopkins, N. D., Jones, H., Thijssen, D. H. J., Eijsvogels, T. M. H., & Yeap, B. B. (2016). Sex differences in vascular endothelial function and health in humans: Impacts of exercise. Experimental Physiology, 101(2), 230–242.
- Green, D. J., Hopman, M. T. E., Padilla, J., Laughlin, M. H., & Thijssen, D. H. J. (2017). Vascular adaptation to exercise in humans: Role of hemodynamic stimuli. Physiological Reviews, 97(2), 495–528.
- Green, D J, Maiorana, A, O'driscoll, G, & Taylor, R. (2004). Effect of exercise training on endothelium-derived nitric oxide function in humans. The Journal of Physiology, 561(1), 1–25.
- Gündisch, S., Annaratone, L., Beese, C., Drecol, E., Marchiò, C., Quaglino, E., … Bussolati, G. (2015). Critical roles of specimen type and temperature before and during fixation in the detection of phosphoproteins in breast cancer tissues. Laboratory Investigation, 95(5), 561–571.
- Hambrecht, R., Adams, V., Erbs, S., Linke, A., Kränkel, N., Shu, Y., … Schuler, G. (2003). Regular physical activity improves endothelial function in patients with coronary artery disease by increasing phosphorylation of endothelial nitric oxide synthase. Circulation, 107(25), 3152–3158.
- Hellsten, Y., & Gliemann, L. (2018). Limb vascular function in women – effects of female sex hormones and physical activity. Translational Sports Medicine, 1(1), 14–24.
- Newman, P. J. (1999). Switched at birth: A new family for PECAM-1. Journal of Clinical Investigation, 103(1), 5–9.
- Osawa, M., Masuda, M., Harada, N., Lopes, R. B., & Fujiwara, K. (1997). Tyrosine phosphorylation of platelet endothelial cell adhesion molecule-1 (PECAM-1, CD31) in mechanically stimulated vascular endothelial cells. European Journal of Cell Biology, 72(3), 229–237.
- Park, S.-K., La Salle, D. T., Cerbie, J., Cho, J. M., Bledsoe, A., Nelson, A., … Symons, J. D. (2019). Elevated arterial shear rate increases indexes of endothelial cell autophagy and nitric oxide synthase activation in humans. American Journal of Physiology-Heart and Circulatory Physiology, 316(1), H106–H112.
- Pierce, G. L., Donato, A. J., LaRocca, T. J., Eskurza, I., Silver, A. E., & Seals, D. R. (2011). Habitually exercising older men do not demonstrate age-associated vascular endothelial oxidative stress. Aging Cell, 10(6), 1032–1037.
- Sandoo, A., Veldhuijzen van Zanten, J. J. C. S., Metsios, G. S., Carroll, D., & Kitas, G. D. (2010). The endothelium and its role in regulating vascular tone. The Open Cardiovascular Medicine Journal, 4, 302–312.
- Thijssen, D. H. J., Bruno, R. M., van Mil, A. C. C. M., Holder, S. M., Faita, F., Greyling, A., … Ghiadoni, L. (2019). Expert consensus and evidence-based recommendations for the assessment of flow-mediated dilation in humans. European Heart Journal, 40(30), 2534–2547.
- Thijssen, D. H. J., Black, M. A., Pyke, K. E., Padilla, J., Atkinson, G., Harris, R. A., … Green, D. J. (2011). Assessment of flow-mediated dilation in humans: A methodological and physiological guideline. American Journal of Physiology-Heart and Circulatory Physiology, 300(1), H2–H12.
- Thijssen, D. H. J., Dawson, E. A., Black, M. A., Hopman, M. T. E., Cable, N. T., & Green, D. J. (2009). Brachial artery blood flow responses to different modalities of lower limb exercise. Medicine & Science in Sports & Exercise, 41(5), 1072–1079.
- Tremblay, J. C., Ainslie, P. N., Turner, R., Gatterer, H., Schlittler, M., Woyke, S., … Siebenmann, C. (2020). Endothelial function and shear stress in hypobaric hypoxia: Time course and impact of plasma volume expansion in men. American Journal of Physiology-Heart and Circulatory Physiology, 319(5), H980–H994.
- Tryfonos, A., Cocks, M., Mills, J., Green, D. J., & Dawson, E. A. (2020a). Exercise-induced vasodilation is not impaired following radial artery catheterization in coronary artery disease patients. Journal of Applied Physiology (1985), 128(2), 422–428. doi:10.1152/japplphysiol.00695.2019
- Tryfonos, A., Cocks, M., Rasoul, D., Mills, J., Green, D. J., & Dawson, E. A. (2020b). Impact of catheterization on shear-mediated arterial dilation in healthy young men. European Journal of Applied Physiology, 120(11), 2525–2532.
- Tzima, E., Irani-Tehrani, M., Kiosses, W. B., Dejana, E., Schultz, D. A., Engelhardt, B., … Schwartz, M. A. (2005). A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature, 437(7057), 426–431.
- Wang, S., Chennupati, R., Kaur, H., Iring, A., Wettschureck, N., & Offermanns, S. (2016). Endothelial cation channel PIEZO1 controls blood pressure by mediating flow-induced ATP release. Journal of Clinical Investigation, 126(12), 4527–4536.
- Wang, Y., Zhang, Y., Hu, W., Xie, S., Gong, C.-X., Iqbal, K., & Liu, F. (2015). Rapid alteration of protein phosphorylation during postmortem: Implication in the study of protein phosphorylation. Scientific Reports, 5(1), 1–12.
- Wilson, C., Lee, M. D., & McCarron, J. G. (2016). Acetylcholine released by endothelial cells facilitates flow-mediated dilatation. The Journal of Physiology, 594(24), 7267–7307.
- Woodman, R. J., Playford, D. A., Watts, G. F., Cheetham, C., Reed, C., Taylor, R. R., … Green, D. (2001). Improved analysis of brachial artery ultrasound using a novel edge-detection software system. Journal of Applied Physiology (1985), 91(2), 929–937. doi:10.1152/jappl.2001.91.2.929
- Xu, J., Mathur, J., Vessières, E., Hammack, S., Nonomura, K., Favre, J., … Patapoutian, A. (2018). GPR68 senses flow and Is essential for vascular physiology. Cell, 173(3), 762–775.e16. e716.
