ABSTRACT
Purpose: To study the effect of inhaling a beta-agonist (salbutamol) compared to placebo on skiing and cycling performance in well-trained elite athletes. Methods: Three different exercise protocols were used, all with a cross-over double blind placebo-controlled design. Participants inhaled 800 µg salbutamol or a placebo prior to the test, which was repeated on a following day with the participants inhaling the other substance. Fifteen junior elite skiers performed four free-style high intensity sprints (1100 m/work time 3.5–4.5 min). Twelve elite cyclists carried out a short cycling protocol, starting with two 5 min submaximal workloads followed by a maximal intermittent performance test to exhaustion. Another 12 elite cyclists performed the maximal intermittent performance test to exhaustion after a 150 min long submaximal cycling protocol. Results: Group mean time for the ski sprints increased, with no difference between treatment groups. In the short cycling protocol time to exhaustion was 9.1% (95% CI 52–161) lower after inhaling salbutamol compared to placebo and in the long cycling protocol time to exhaustion was 9.1% (95% CI – 121–267) lower after inhaling salbutamol compared to placebo. Blood lactate, heart rate and ventilation increased during submaximal exercise with salbutamol compared to placebo in the short cycling protocol (p < .05). Conclusion: This study could not confirm any positive performance effects from inhaling 800 µg salbutamol compared to placebo in skiing and high-intensity intermittent cycling performance. Instead, time to exhaustion in the maximal intermittent performance test was lower in both cycling protocols.
Highlights
There was no difference in performance time between salbutamol and placebo treatment in real-life applicable repeated ski sprints.
Time to exhaustion in the maximal intermittent performance test was 9.1% lower after inhaling salbutamol compared to placebo, both when performed after 10 and 150 min of submaximal cycling.
Introduction
Beta2-agonists, such as salbutamol (SAL), are used in the treatment of asthma and other respiratory disorders. These substances have also been used by non-asthmatic athletes with the intention to prevent exercise-induced bronchoconstriction. However, the effects on performance in different sports has not been fully determined. In anaerobic type of sports some studies have shown improved short-term anaerobic and strength performances after acute inhalation of World Anti-Doping Agency (WADA) permitted doses of salbutamol (the permitted dose has been lowered from ≤800 µg to ≤600 µg as effective of 2022) (Riiser, Stensrud, Stang, & Andersen, Citation2020). Furthermore, inhalation of supra therapeutic doses of beta2-agonists has consistently been associated with enhancing effects on muscle strength (by 3–10%) and sprint power output (by 2–8%) (Hostrup, Kalsen, Bangsbo, et al., Citation2014), (Kalsen et al., Citation2016). The restriction of permitted doses is in place because supra therapeutic use of beta2-agonists has been shown performance enhancing potential (World-Anti-Doping-Agency, Citation2021). Additionally, some 7–8% of endurance athletes with asthma or other respiratory disorders treated with beta2-agonists are reported to belong to a larger group of 12–15% of medal winners during five Olympic Games (2000–2010) (Fitch, Citation2016).
Therefore it is of special interest to investigate if there are any performance enhancements during more variated prolonged intermittent exercise, with both aerobic and anaerobic demands. Performances lasting more than one minute has been evaluated previously in a review by Riiser et al. (Citation2020), the report concluded that there were no effects of beta2-agonists on VO2max or aerobic performance (Riiser, Stensrud, Stang, & Andersen, Citation2021). There are, however, some reports of enhanced endurance performance after acute inhalation of salbutamol (Andersen & Kanstrup, Citation2009; Collomp et al., Citation2000; Heir & Stemshaug, Citation1995; Hostrup, Kalsen, Auchenberg, Bangsbo, & Backer, Citation2016; Kalsen, Hostrup, Bangsbo, & Backer, Citation2014; Van Baak, De Hon, Hartgens, & Kuipers, Citation2004; van Baak, Mayer, Kempinski, & Hartgens, Citation2000). And, conversely, detrimental effects on running endurance after administration of salbutamol has also been reported (Carlsen, Ingjer, Kirkegaard, & Thyness, Citation1997). However, repeated maximal performance test protocols including shorter rests are scarce. Also, longer performance protocols in healthy elite athletes after acute inhalation of salbutamol are few or lacking. In many endurance sports, athletes perform intermittent intensities, alternating between almost maximal effort and recovery, often at the end of a race, such as in cross-country skiing. Furthermore, in some sports, athletes perform repeated maximal performances during a relatively short period of time, such as in sprint skiing. These examples demand high aerobic but also anaerobic contribution to the overall physical performance.
The effect of acute inhalation of beta2-agonists on this type of high intensity intermittent exercise has not previously been scientifically evaluated. The mechanism for performance enhancements has been suggested to relate to metabolic advantages like those reported in anaerobic performances (Hostrup et al., Citation2014; Kalsen et al., Citation2014; Kalsen et al., Citation2016). It has also been suggested that there could be a limitation in exercise performance by bronchoconstriction, induced by prolonged exercise in the cold, however, there is insufficient evidence to this claim (Price, Hull, Backer, Hostrup, & Ansley, Citation2014).
Therefore, the aim of this study was to evaluate performance and physiological effects of acute inhalation of a beta2-agonist (800 µg salbutamol) compared to a placebo on elite athletes performing different types of high intensity intermittent exercises, using sport-relevant performance tests. The main outcomes in this study were performance in time to exhaustion and in repeated time trials.
We hypothesized that inhalation of salbutamol would increase time to exhaustion in the maximal intermittent performance test in both long- and short cycling protocols compared to Placebo. We further hypothesized that time in repeated ski sprints would decrease after inhalation of salbutamol compared to placebo.
Methods and materials
Three different performance protocols were carried out in a double-blind cross-over design. Participants inhaled either 800 µg salbutamol (Ventoline®, GlaxoSmithKline, Brentford, UK) or a placebo randomly assigned on the first test occasion (allocation 1:1) prior to testing.
Participants
Fifteen young junior elite skiers, seven females (mean ± SD age 19.1 ± 0.8 years, body mass 63.6 ± 4.2 kg), and eight males (mean ± SD age 19.3 ± 0.7 years, body mass 73.7 ± 3.7 kg), performed the ski sprint protocol. All participants evaluated themselves as either very high elite or elite trained.
Twelve well-trained male elite cyclists (mean ± SD age 41.4 ± 5.5 years, body mass 79.4 ± 4.2 kg, VO2max 59.7 ± 4.3 mL min−1 kg−1), all training daily and regularly competed in cycling competitions for >10 years, were recruited to perform the short cycling protocol (SCP).
Another twelve well-trained male elite cyclists, from the same cohort (mean ± SD age 41.1 ± 6.2 years, body mass 82.7 ± 4.9 kg, VO2max 61.1 ± 5.0 mL min−1 kg−1) took part in the long cycling protocol (LCP).
Finally, a further fifteen well-trained male cyclists from the same cohort (mean ± SD age 43.3 ± 8.1 years, mass 79.6 ± 6.1 kg, VO2max 56.6 ± 5.0 mL min−1 kg−1) participated in a methodological part of the study where the aim was to evaluate test-retest reliability of the maximal intermittent performance test.
None of the participants had previous history of asthma or airway problems. Participants received oral and written information about the aims and contents of the study and of possible risks involved. Thereafter they gave a written informed consent. In addition, before each test, the participants verified their consent filling in a health declaration. All studies were performed in accordance with the Helsinki II declaration and approved by the local ethics committee (KI 2017/1281-31/1).
Ski sprint protocol
The ski sprint protocol was performed on two consecutive days. The day before the test days, participants were asked to visit the laboratory to be informed about the different procedures and to give their written consent. They were also informed that a monetary incentive would be given to ensure each of the sprints was performed as fast as possible. The incentive consisted of a given sum (equal for all participants) minus the amount equivalent to their total race time in seconds (1s = 1 SEK). Lung function tests, including Forced Expired Volume during one second (FEV1.0), Forced Vital Capacity (FVC) and Peak Expiratory Flow (PEF) were performed with a handheld spirometer (Micro 1, CareFusion, Hoechberg, Germany) for familiarization purposes. The lung function tests were repeated before the first sprint, and after the final sprint on each of the two test days. To evaluate subjective breathing symptoms, a 10-point visual analogue scale (Miller & Ferris, Citation1993) (VAS), indicating breathing resistance, mucus and cough, was also performed for familiarization and then repeated on each of the two test days (before the first sprint, after the second sprint and after the final sprint).
The ski sprint protocol was performed outdoors. During the first day, the weather was −5°C and sunny, and on the second day the weather was −10°C with light snowfall. The wind during both days was moderate. The participants used their own skis and equipment on the two days. After arriving at the outdoor ski-stadium, on each day, the participants inhaled either 800 µg of salbutamol or placebo. Thereafter, they carried out a conventional warm up. The participants skied the outdoor forest track (1100 m) four times as fast as possible (approximately 3.5–4.5 min per sprint) in a free-style manner. The starting order was always the same in each sprint with 30 sec intervals between each skier. The skiers started their next sprint exactly 15 min after the start of their previous sprint, with approximal 10–11 min recovery skiing in between. The timing of each sprint was started by one person and stopped at the finish line by another using a shared timing application on a tablet. Fingertip blood samples to determine blood lactate were collected before the first sprint, and after the final sprint, and later analysed using standard laboratory methods (Biosen C-line, EKF Diagnostic, Barleben Germany). Heart rate was continuously monitored during the whole test using Polar RS400 (Polar Electro Oy, Kempele, Finland). Rate of perceived exertion (RPE) according to the Borg scale (Borg & Linderholm, Citation1967) was obtained after the second sprint, and the final sprint.
Cycling protocol tests
The preparations and conditions for the short and long cycling protocols, as well as the methodological study were identical. The participants first visited the lab for familiarization tests and, if they were included, visited the lab on two more occasions to perform either the short- or long cycling protocols or to perform the methodological study. All tests were conducted under standardized laboratory conditions (temperature 20°C, 40% humidity) with a fan in front of the participant for cooling. The participants used the same individual geometric setup, shoes and pedals on all test occasions. Data collection was always performed at the same time of the day to avoid diurnal variations in performance and blood parameters. Participants were instructed to rest the day prior to testing, keep their normal nutritional protocol, and to avoid heavy meals and caffeinated drinks in the hours before the test.
In the familiarization test participants completed two 5 min submaximal workloads on a cycle ergometer (Monark LC6, Monark Exercise AB, Vansbro, Sweden), at RPEs of between 10–12 and 13–15 on the Borg scale, respectively. Thereafter, the participants were fitted with a facemask (Hans-Rudolph 7450 SeriesV2, Kansas, USA) and VO2max was obtained during an incremental exercise test with a starting workload of 3.3 W*kg−1 body mass with 20 W increments every minute until exhaustion. The criteria for reaching VO2max were no further increase in VO2 with increasing workload (levelling off), work time > 5 min, respiratory exchange ratio (RER) > 1.07 and RPE > 17.
Gas composition of expired air oxygen concentration (% O2), carbon dioxide concentration (% CO2), respiratory exchange ratio (RER) and ventilation were measured continuously with an on-line ergo spirometry mixing chamber system (Oxycon Pro, Erich Jaeger GmbH, Hoechberg, Germany). The system was calibrated before each test according to the manufacturer’s instructions. Ambient conditions, volume sensor and gas (15.00 ± 0.01% O2 and 6.00 ± 0.01% CO2, Air Liquid, Kungsängen, Sweden) were used. After the VO2max test, participants performed recovery pedalling on the cycle ergometer for >15 minutes. Thereafter, they performed a familiarization trial of the maximal intermittent performance test. RPE and fingertip blood samples for determining blood lactate were collected after the submaximal workloads, VO2max test and the maximal intermittent performance test. Heart rate was continuously recorded throughout all tests.
The maximal intermittent performance test
The maximal intermittent performance test started with two minutes of cycling at 65% of the Wmax (low workload), whereafter the workload was increased to 95% Wmax (high workload) for one minute. The low workload was then repeated. After every 2 min of low workload, a new 1 min high workload was performed, however, increased with 5% of Wmax every other high workload up to 110% of Wmax. This alternating low and high workload continued until exhaustion or cadence falling below 70 rpm. Time to exhaustion (TTE) was noted in seconds from the start of the maximal intermittent performance test until failure.
Methodological study
To test repeatability of TTE in the maximal intermittent performance test, participants repeated the same protocol twice with 7–14 days between tests. The test protocol consisted of two submaximal work rates of 5 min each at RPEs of between 10–12 and 13–15 on the Borg scale, respectively, and thereafter the maximal intermittent performance test was carried out. Oxygen uptake and heart rate were measured continuously. Blood lactate and RPE were collected after each of the two submaximal workloads, and after the maximal intermittent performance protocol.
Short cycling protocol
On two test occasions the participants repeated the same protocol with 7–14 days between tests. At arrival to the laboratory, lung function tests (FEV1.0, FVC and PEF) were performed (Oxycon pro, Jaeger, Germany). Directly thereafter, 800 µg salbutamol or placebo was inhaled. After ∼15 min of warm up, participants repeated the lung function tests. The short cycling protocol (SCP) consisted of two submaximal work rates of 5 min each at RPE 10–12 and 13–15 on the Borg scale, respectively, and thereafter the maximal intermittent performance test. Oxygen uptake and heart rate were measured continuously. Blood lactate and RPE were collected after each of the two submaximal workloads, and after the maximal intermittent performance protocol.
Long cycling protocol
On two test occasions the participants repeated the same protocol with 7–14 days between tests. After arrival to the laboratory an antecubital vein catheter was inserted for collection of blood samples throughout the test. Thereafter 400 µg of salbutamol or placebo was inhaled. Another 400 µg of the same substance was inhaled after 90 min after the start of the prolonged cycling. The long cycling protocol (LCP) consisted of 150 minutes of cycling with starting work rate of 60% of Wmax. At min 30 and 110 of the cycling protocol, the participant performed a 15 min increment (5 min at 65%, then 5 min at 70% and again 5 min at 65% of Wmax) before returning to cycling at 60% of Wmax. Directly after 150 min of prolonged cycling, the maximal intermittent performance test started. Oxygen saturation (SpO2) was monitored by a forehead sensor connected to a Rad-97 pulse oximeter (LNCS TF-I Masimo, Switzerland) attached to the participant with an elastic Velcro-headband.
Heart rate was continuously measured throughout the entire test. Respiratory gases were measured during min 5–10, min 70–75, min 100–105 and min 147–150 of the long cycling protocol and continuously during the maximal intermittent performance test. Venous blood samples were collected before the start, at min 15, 85, 145 and after the maximal intermittent test, the samples were analysed for blood lactate, C-peptide, glucose, and free fatty acids using conventional laboratory methods. RPE according to the Borg-scale was collected at min 15, 60, 120 and 150 during the long cycling protocol, and after the maximal intermittent performance test. FEV1.0, FVC and PEF were measured at min 10, 85 and 145. VAS-values for breathing resistance, mucus and cough were collected before the start, at min 75 and 145 during the long cycling protocol and after the maximal intermittent performance test. Participants received a carbohydrate drink (totally 80 g of carbohydrates mixed with 75 cl water) at 50 and 110 min to prevent hypoglycaemia.
Statistics
Owing to the repeated measure nature of the data in the ski sprint protocol, mixed linear models were undertaken with an interaction term between occasion and treatment as fixed effects, and participant and treatment as random effects. A restricted maximum likelihood estimation was employed due to the small sample size with an unstructured variance covariance structure. Model 1 was unadjusted, whereas in model 2, sex, training status, and period effect (what day the test took place due to differences in weather) were controlled for as fixed effects. The dependent variable was performance time. Main independent variables were occasion (4 occasions for each treatment) and treatment (SAL or PLA). Covariates included sex, training status (highly elite or elite trained) and test day (1 or 2).
A generalized linear mixed model approach was used to estimate a difference in short and long cycling performance variables between salbutamol and placebo, accounting for within-subject correlations arising from the crossover design. Data are presented as mean values (± SD). All variables were tested for normality. All statistical analysis was performed using SPSS v.26 (SPSS Inc, Chicago, Illinois, USA). p-value of <.05 was used for significance.
Results
Ski sprint protocol
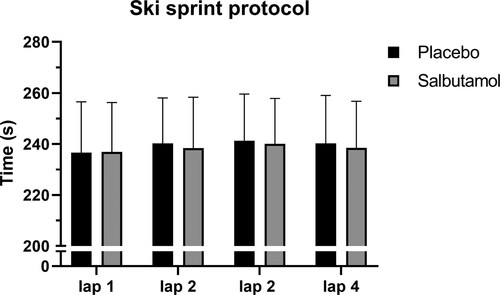
There was a significant increase in total mean performance time in both treatment groups comparing the first skied lap to the last skied lap, from 237 ± 33–240 ± 33 sec with salbutamol and from 237 ± 40–239 ± 37 sec with placebo (see ). There was no significant treatment by occasion effect (Model 1). All these results remained the same after adjusting for covariates, including training status and weather (Model 2). Peak heart rate did not differ between groups at any point during the ski sprint protocol. Blood lactate for salbutamol and placebo groups was 1.64 ± 0.38 and 1.66 ± 0.37 mM before the first sprint, and 11.33 ± 2.69 and 10.26 ± 2.70 mM after the final sprint, respectively. RPE was 17.3 ± 0.9 and 17.4 ± 0.7 directly after the second sprint, and 18.0 ± 0.9 and 18.1 ± 0.6 directly after the final sprint for salbutamol and placebo, respectively. All differences between treatments were not significant (p > .05).
Figure 1. Sprint time for each lap in the ski performance protocol. Presented as mean values for placebo (PLA) and salbutamol (SAL) treatment with error bars indicating 95% CI.
There were no differences between salbutamol treatment and placebo treatment in any of the VAS-indications.
Short cycling protocol
Time to exhaustion in the maximal intermittent performance test was 1044 ± 296 and 1151 ± 294 sec, (difference 9.1%; 95% CI 52–161) for salbutamol and placebo, respectively, see . Peak physiological values (heart rate, oxygen uptake, ventilation, blood lactate, RPE) during maximal intermittent performance test did not differ after inhalation of salbutamol compared to placebo (p > .05) (see ). Results from the submaximal workloads and peak values from the maximal intermittent performance test are presented in . On both submaximal workloads heart rate and blood lactate was significantly higher after salbutamol compared to placebo. On the second submaximal workload ventilation was significantly higher after salbutamol compared to placebo. (p < .05), with no differences for oxygen uptake and RPE on neither of the submaximal workloads (P > 0.05).
Figure 2. Time to exhaustion for the methodological study, the short (SCP) and long (LCP) cycling protocol. Presented as mean values for placebo (PLA) and salbutamol (SAL) treatment with error bars indicating 95% CI.
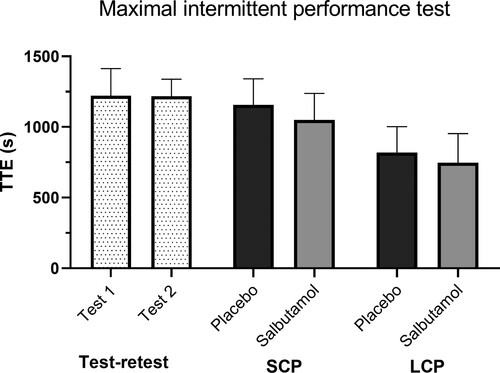
Table 1. Mean ± SD of heart rate, RPE, blood lactate, VO2, RER and ventilation values during the short cycling protocol (SCP) at two submaximal workloads, and peak values from the maximal intermittent performance test (MIP-test).
Long cycling protocol
Time to exhaustion in the maximal intermittent performance test was 742 ± 322 and 815 ± 285 sec (difference 9.1%; 95% CI -121–267) for salbutamol and placebo, respectively, see . Peak physiological values during the maximal intermittent performance test did not differ after inhaling salbutamol compared to placebo (see ). Mean SpO2 during the final minute of the maximal intermittent performance test also did not differ between the salbutamol and placebo treatment group, (97.1 ± 1.4 vs. 97.0 ± 1.1%, respectively) (p > .05). Throughout the long cycling protocol, all physiological and plasma (glucose, free fatty acids, C-peptide, and blood lactate) values did not differ between the salbutamol and placebo groups (p > .05), except for increased ventilation with salbutamol at min 85 and 145, and after the maximal intermittent performance test (p < .05) (see ).
Table 2. Mean ± SD of heart rate, RPE, blood lactate, VO2, RER and ventilation values during the long cycling protocol (LCP) at rest, 10, 85, 105 and 145 min, and peak values from the maximal intermittent performance test (MIP).
There were no differences between treatments (salbutamol versus placebo) for any of the measured lung function parameters (FEV1.0, FVC or PEF) in the three different test protocols (p > .05).
Test-retest
Time to exhaustion in the maximal intermittent performance test was 1237 ± 246 sec, and 1226 ± 155 sec, for test 1 and test 2, respectively (p = .8) CV = 0.11. There were no significant differences in data obtained during the first and second submaximal workloads between test 1 and 2 (data not shown). Nor were there any statistical differences in peak values of heart rate, ventilation, oxygen uptake, blood lactate and RPE between test 1 and test 2.
Discussion
The aim of this study was to evaluate the effects of inhaling an at the time WADA approved amount of the beta2-agonist salbutamol (800 µg – as effective of 2022: ≤600 µg) on physical performance in well-trained athletes using sport-relevant tests. In the ski sprint protocol, mean completion time increased in each sprint from the first to the last, but there was no significant difference between treatment groups. In the short cycling protocol, mean time to exhaustion in the maximal intermittent performance test was significantly decreased (9.1%) after inhaling salbutamol compared to placebo (). The mean time to exhaustion in the maximal intermittent performance test following 150 min in the long cycling protocol was also shorter (9.1%) after inhaling salbutamol compared to placebo. In this case the difference was not statistically significant. Our results of decreases in performance are in line with the results from aerobic performances as reported previously (Riiser et al., Citation2020) and in line with one previous study reporting reduced long time endurance performance (Carlsen et al., Citation1997). There were no differences in performance between treatment groups in the ski sprint protocol. Repeated maximal ski sprints with theoretically higher anaerobic contribution (work time: 4 x ∼ 4 min) (Fitch, Citation2016) yielded no differences in any of the measured parameters. Previously, performance enhancement has been reported in an ergometer sprint (shorter duration ∼60 s), after inhalation of beta2-agonist (Heir & Stemshaug, Citation1995).
In the short cycling protocol, during submaximal workloads, numerous parameters were measured in order to explain any differences in performance between treatment groups (salbutamol compared to placebo) in the maximal intermittent performance test. We found that ventilation, heart rate, and blood lactate were all significantly higher after inhaling salbutamol compared to placebo, with no difference in RPE and oxygen uptake. The increase in blood lactate and elevated heart rate after inhalation of salbutamol compared to placebo suggests a higher anaerobic contribution during the submaximal workloads, something that partly could explain the decreased performance in the following maximal intermittent performance test.
In all three different test protocols, important lung function parameters were measured during both submaximal and maximal exercise, but no effects of the beta2-agonist inhalation were found. Studies have shown that cold air during skiing could induce airway problems during very high ventilation during exhaustive exercise (Heir & Oseid, Citation1994; Larsson, Tornling, Gavhed, Muller-Suur, & Palmberg, Citation1998), but we did not find any differences between treatment groups on subjective reporting of breathing resistance, mucus or cough using the VAS-scale, or in the measured lung function tests at any point during testing. Nor did we find any differences in any of the measured plasma values, besides blood lactate, between treatment groups.
A weakness of the study is that salbutamol concentration in the plasma was not measured. However, 800 µg of salbutamol was inhaled in the manner described by the manufacturer and had clear effects on circulation and ventilation during submaximal exercise. Another weakness is that only men were tested in the cycling tests. However, there were no differences between sexes in the ski-performance.
There are generally some difficulties in measuring cycling performance as time to exhaustion in terms of reliability. To minimize measurement error participants performed a practice trial that preceded the test trials. Participants were highly trained male cyclist, previously shown to have higher reliability when it comes to these type of performance tests (Hopkins, Schabort, & Hawley, Citation2001). Furthermore, this study reports data from a methodological trial with no group differences in time to exhaustion between test occasions in the maximal intermittent performance cycling protocol.
The results from the sport-like test protocols in this study show no beneficial, rather a negative, effect on performance (time to exhaustion and repeated time trial) after acute inhalation of 800 µg salbutamol in well-trained athletes. Whether our overall conclusion from these results also holds true for higher amounts of beta2-agonists (>800 µg) or the use of the drug under a prolonged time (weeks and months) cannot be determined from this study. The positive association between performance (medal winning) in world class Olympic athletes (Fitch, Citation2016) and treatment with beta2-agonist for asthma or other respiratory problems, may be explained by other reasons. For instance, many Olympic disciplines relies mainly on anaerobic components, where beta2-agonists have shown acute performance-enhancing effect (Andersen et al., Citation2021). Furthermore, long time use of beta2-agonist has been sparsely investigated. It has been discussed previously that consistent usage over time might lead to favourable muscle adaptations and leaner body composition, and thus indirectly enhance physical performance (Andersen et al., Citation2021; Hostrup, Jessen, Backer, Bangsbo, & Jacobson, Citation2021). More studies are warranted to fully determine possible effects of beta2-agonists on performance.
Conclusions
This study could not confirm any positive performance effects from inhaling 800 µg of the beta2-agonist salbutamol during high intensity intermittent exercise in skiing or cycling, neither after shorter nor longer exercise protocols. Inhalation of salbutamol at high therapeutic doses do not seem to enhance real-life applicable repeated ski sprint performance. There may even be a detrimental effect during shorter intermittent cycling exercise under standard laboratory conditions.
Acknowledgements
The authors would like to thank all the participants.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Andersen, A. B., Jacobson, G. A., Bejder, J., Premilovac, D., Richards, S. M., Rasmussen, J. J., et al. (2021). An abductive inference approach to assess the performance-enhancing effects of drugs included on the World Anti-Doping Agency prohibited list. Sports Medicine, 51(7), 1–24.
- Andersen, K., & Kanstrup, I.-L. (2009). Effects Of acute oral administration Of 4 Mg salbutamol On exercise performance In Non-asthmatic elite athletes. Journal of Exercise Physiology Online, 12(1), 36–49.
- Borg, G., & Linderholm, H. (1967). Perceived exertion and pulse rate during graded exercise in various age groups. Acta Medica Scandinavica, 181(S472), 194–206.
- Carlsen, K. H., Ingjer, F., Kirkegaard, H., & Thyness, B. (1997). The effect of inhaled salbutamol and salmeterol on lung fuction and endurance performance in healthy well-trained athletes. Scandinavian Journal of Medicine & Science in Sports, 7(3), 160–165.
- Collomp, K., Candau, R., Lasne, F., Labsy, Z., Prefaut, C., & De Ceaurriz, J. (2000). Effects of short-term oral salbutamol administration on exercise endurance and metabolism. Journal of Applied Physiology, 89(2), 430–436.
- Fitch, K. (2016). The World Anti-Doping code: Can you have asthma and still be an elite athlete? Breathe, 12(2), 148–158.
- Heir, T., & Oseid, S. (1994). Self-reported asthma and exercise-induced asthma symptoms in high-level competitive cross-country skiers. Scandinavian Journal of Medicine & Science in Sports, 4(2), 128–133.
- Heir, T., & Stemshaug, H. (1995). Salbutamol and high-intensity treadmill running in nonasthmatic highly conditioned athletes. Scandinavian Journal of Medicine & Science in Sports, 5(4), 231–236.
- Hopkins, W. G., Schabort, E. J., & Hawley, J. A. (2001). Reliability of power in physical performance tests. Sports Medicine, 31(3), 211–234.
- Hostrup, M., Jessen, S., Backer, V., Bangsbo, J., & Jacobson, G. A. (2021). Beta2-adrenergic agonists can enhance intense performance and muscle strength in healthy individuals. Allergy, 76(7), 2318–2319.
- Hostrup, M., Kalsen, A., Auchenberg, M., Bangsbo, J., & Backer, V. (2016). Effects of acute and 2-week administration of oral salbutamol on exercise performance and muscle strength in athletes. Scandinavian Journal of Medicine & Science in Sports, 26(1), 8–16.
- Hostrup, M., Kalsen, A., Bangsbo, J., Hemmersbach, P., Karlsson, S., & Backer, V. (2014). High-dose inhaled terbutaline increases muscle strength and enhances maximal sprint performance in trained men. European Journal of Applied Physiology, 114(12), 2499–2508.
- Hostrup, M., Kalsen, A., Ørtenblad, N., Juel, C., Mørch, K., Rzeppa, S., et al. (2014). Β2-adrenergic stimulation enhances Ca2 + release and contractile properties of skeletal muscles, and counteracts exercise-induced reductions in Na+–K+-ATPase vmax in trained men. The Journal of Physiology, 592(24), 5445–5459.
- Kalsen, A., Hostrup, M., Bangsbo, J., & Backer, V. (2014). Combined inhalation of beta2-agonists improves swim ergometer sprint performance but not high-intensity swim performance. Scandinavian Journal of Medicine & Science in Sports, 24(5), 814–822.
- Kalsen, A., Hostrup, M., Söderlund, K., Karlsson, S., Backer, V., & Bangsbo, J. (2016). Inhaled Beta2-agonist increases power output and glycolysis during sprinting in Men. Medicine and Science in Sports and Exercise, 48(1), 39–48.
- Larsson, K., Tornling, G., Gavhed, D., Muller-Suur, C., & Palmberg, L. (1998). Inhalation of cold air increases the number of inflammatory cells in the lungs in healthy subjects. European Respiratory Journal, 12(4), 825–830.
- Miller, M. D., & Ferris, D. G. (1993). Measurement of subjective phenomena in primary care research: The visual analogue scale. Family Practice Research Journal, 13(1), 15–24.
- Price, O. J., Hull, J. H., Backer, V., Hostrup, M., & Ansley, L. (2014). The impact of exercise-induced bronchoconstriction on athletic performance: A systematic review. Sports Medicine, 44(12), 1749–1761.
- Riiser, A., Stensrud, T., Stang, J., & Andersen, L. B. (2020). Can β2-agonists have an ergogenic effect on strength, sprint or power performance? Systematic review and meta-analysis of RCTs. British Journal of Sports Medicine, 54(22), 1351–1359.
- Riiser, A., Stensrud, T., Stang, J., & Andersen, L. B. (2021). Aerobic performance among healthy (non-asthmatic) adults using beta2-agonists: A systematic review and meta-analysis of randomised controlled trials. British Journal of Sports Medicine, 55(17), 975–983.
- Van Baak, M., De Hon, O., Hartgens, F., & Kuipers, H. (2004). Inhaled salbutamol and endurance cycling performance in non-asthmatic athletes. International Journal of Sports Medicine, 25(07), 533–538.
- van Baak, M. A., Mayer, L., Kempinski, R., & Hartgens, F. (2000). Effect of salbutamol on muscle strength and endurance performance in nonasthmatic men. Medicine and Science in Sports and Exercise, 32(7), 1300–1306.
- World-Anti-Doping-Agency. (2021). WADA statement on the salbutamol threshold/decision limit: WADA; Available from: https://www.wada-ama.org/sites/default/files/note_15may.pdf.
