ABSTRACT
To assess the effect of active and passive intra-interval recovery modes in time-efficient high-intensity interval training (HIT) on cardiorespiratory fitness, autonomic function, and endothelial function in sedentary middle-aged men.
Participants (n = 62; age: 49.5 ± 5.8 y; BMI: 29.7 ± 3.7 kg·m−2) completed the assessments of cardiorespiratory fitness, flow-mediated dilation (FMD) and heart rate variability before being randomly allocated to control (CON; n = 14), moderate intensity continuous training (MICT; n = 15), HIT with passive (P-HIT; n-15), or active recovery (A-HIT; n = 15). Participants performed thrice weekly exercise sessions for 12 weeks. MICT completed 50–60 min of continuous cycling at 60–70% heart rate (HR) maximum. HIT completed 30-s work intervals (∼85% HR) interspaced with 2.5 min of active or passive recovery.
All exercise modalities increased oxygen uptake (V̇O2) (MD: ≥ 3.1 ml·kg−1·min−1, 95%CI: 1.5–4.7 ml·kg−1·min−1; P < 0.001), power output (MD: ≥ 26 W, 95%CI: 15–37 W; P < 0.001) and cycle duration (MD: ≥ 62 s, 95%CI: 36–88 s; P < 0.001) at 85% HRM. Significant pre-to-post differences were observed among all exercise groups for FMD (MD: ≥ 3.4%, 95%CI: 0.3–6.5%; P < 0.05), while MICT and P-HIT significantly increased the standard deviation of all NN intervals (SDNN) pre-to-post intervention (MD: ≥ 7 ms, 2–13 ms; P ≤ 0.05).
Time-efficient HIT elicits significant improvements in cardiorespiratory fitness, FMD and autonomic modulation following a thrice weekly 12-week exercise intervention among sedentary middle-aged men. Active recovery between successive high-intensity intervals provided no additional benefit among this deconditioned cohort.
Introduction
Despite advances in medical treatments and lifestyle interventions, cardiovascular disease (CVD) remains the leading cause of mortality in developed nations (Australian Institute of Health and Welfare, Citation2017; Bots et al., Citation2017; de Lucia et al., Citation2019). Males remain at higher risk for adverse events, with 40–60 years of age identified as a transitional stage of disease development (Australian Institute of Health and Welfare, Citation2017; Bots et al., Citation2017). Elevated adiposity, sedentary lifestyle, and adverse lipid profile are established indicators of future cardiovascular risk (Crichton & Alkerwi, Citation2015); however, autonomic nervous system (ANS) dysfunction has become a topic of interest due to the direct mechanistic role in disease pathogenesis (de Lucia et al., Citation2019; Hadaya & Ardell, Citation2020). Regulating all aspects of cardiac function, deregulation of cardiac and vascular adrenergic signalling can result in a hyperactive sympathetic nervous system (SNS) with adverse effects on myocardial and endothelial function (de Lucia et al., Citation2019; Hadaya & Ardell, Citation2020). Therefore, interventions aimed at improving endothelial health, ANS modulation, and cardiovascular function of middle-aged men may decrease future disease burden.
Exercise-based interventions including moderate intensity continuous (MICT) and high-intensity interval training (HIT) have been demonstrated to improve endothelial function (Ramírez-Vélez et al., Citation2019; Ramos et al., Citation2015; Sawyer et al., Citation2016), ANS modulation (Melanson & Freedson, Citation2001; Munk et al., Citation2010) and cardiorespiratory fitness (Gerosa-Neto et al., Citation2019). The observation of comparable or superior improvements in endothelial and vascular function despite reduced exercise time and energy expenditure has made HIT an attractive mode for exercise prescription (Ramírez-Vélez et al., Citation2019; Ramos et al., Citation2015). However, HIT incorporates a broad range of alternating work and rest intervals ranging from 30 to 500-s bouts of aerobic exercise at 85–100% of the maximum rate of oxygen consumption (Gibala et al., Citation2012; MacInnis & Gibala, Citation2017; Ramos et al., Citation2015) with subsequent variations in sessional energy expenditure. Furthermore, when including intra-interval recovery periods, total session time often results in the same (Foster et al., Citation2015), or marginally reduced (Ramírez-Vélez et al., Citation2019; Sawyer et al., Citation2016), time commitments compared to MICT. The potential effectiveness of HIT with substantially reduced total energy expenditure and session time to improve cardiovascular health requires further elucidation.
In addition to varying interval structures, HIT remains relatively undefined regarding intra-interval recovery methods (Buchheit & Laursen, Citation2013a). An oversight given that physiological adaptation is specific to stimulus, and recovery mode might influence metabolic workload during the session (Buchheit & Laursen, Citation2013a). Active recovery, believed to aid metabolite clearance, elevate oxygen consumption, and maximise total session energy expenditure (Buchheit & Laursen, Citation2013a; Germano et al., Citation2019; Kriel et al., Citation2016) is more commonly prescribed (Ramos et al., Citation2015). Alternatively, passive recovery has been demonstrated to increase the mean distance covered and time to voluntary exhaustion in acute exercise bouts (Germano et al., Citation2019; Meyer et al., Citation2012). Limited research has directly compared the training effect of the two recovery modes, but active recovery is reported to elicit superior cardiorespiratory fitness adaptations, albeit among healthy individuals (Abderrahman et al., Citation2018). However, the influence of recovery mode during HIT on sympathetic balance or endothelial function among a deconditioned but apparently healthy population has not been investigated.
Despite an existing body of research comparing exercise mode and intensity, no consensus has been reached regarding the most effective training prescription for improving autonomic balance and cardiovascular health. The aim of this study was to assess the effect of active and passive intra-interval recovery modes of time-reduced HIT to MICT for effect on cardiorespiratory fitness, autonomic tone, and endothelial responsiveness. It was hypothesised that all exercise conditions would improve cardiovascular health measures, with HIT being more effective than MICT due to superior intensity-based adaptations. Within HIT, we hypothesised a larger effect would be observed among A-HIT group in response to the higher total energy expenditure during training.
Methods
Participants
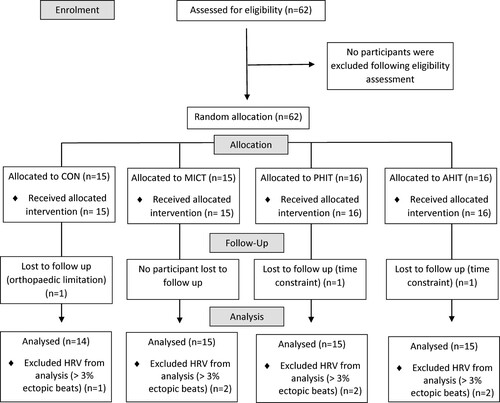
Sixty-two sedentary (<2 d·wk−1 planned exercise in prior 12 months) men (40–60 y) volunteered for this study (). Volunteers were required to consult a physician to confirm their current health status and excluded if they reported resting blood pressure ≥160/100 mmHg, diagnosed CVD, type 2 diabetes, currently receiving pharmacological treatment known to affect heart rate (HR) or HR-regulatory mechanisms, were tobacco smokers (<1 year cessation) or had an orthopaedic limitation precluding exercise participation. Institutional Human Research Ethics Committee approved the study (ref: 2015/044), and participants provided written informed consent before taking part.
Table 1. Participant characteristic at baseline.
Study overview
An overview of the study design is provided in . After screening, participants attended a familiarisation session where testing procedures, maintenance of pre-intervention dietary habits, and avoidance of additional physical activity (PA) were explained. Participants attended the institutional laboratories for measurement of heart rate variability (HRV) and flow-mediated dilation (FMD), which was followed by graded exercise testing (GXT). The Adult Pre-Exercise Screening Tool categorised these sedentary middle-aged males as being at moderate risk for adverse health outcomes (Norton & Norton, Citation2011). As such, 85% of the estimated HR maximum (HR85%) was selected as the termination point for the GXT to assess cardiovascular fitness. Random allocation to a non-exercising control condition (CON; n = 15); moderate intensity continuous training (MICT; n = 15); high-intensity intervals with “passive” recovery (P-HIT; n = 16); or high-intensity intervals with “active” recovery (A-HIT; n = 16) was achieved with a computer-generated sequence in a 1:1:1:1 ratio without stratification (www.randomizer.org) and delivered using sequentially ordered sealed opaque envelopes. Participants in all the exercise conditions completed 12-wk, 3-d·wk−1 fully supervised training programmes. The exercise and CON groups were encouraged to maintain the pre-intervention diet and PA patterns throughout the interventions with no further contact made until post-testing. They returned post-intervention (within 72 h after the final session) to repeat all testing procedures in a standardised manner.
Measures
Heart rate variability
Assessment of HRV was conducted and analysed following standardised procedures (Task Force of the European Society of Cardiology the North American Society of Pacing Electrophysiology, Citation1996). Participants were instructed to avoid strenuous exercise for 48 h prior, and caffeine or alcohol within 12 h of testing. Arriving in a fasted state (∼10 h overnight), participants rested supine for 10 min in a quiet and temperature controlled (22–25°C) room, while a baseline electrocardiogram (ECG) trace (CASE ECG Stress Test System; General Electric Medical Systems, Milwaukee, WI) was acquired to confirm the absence of artefact. Following 2 mins of ventilation familiarisation (ventilation rate matched to an auditory metronome set at 12 breaths·min−1), 5 mins of beat-to-beat ECG data were acquired and saved at a sampling frequency of 1000 Hz using a commercial acquisition device (National Instruments, Austin, TX) with customised software (LabVIEW; National Instruments, Austin, TX). ECG signal was filtered by a high-pass Butterworth 3rd order filter and R-R intervals were computed using additional custom software (LabVIEW; National Instruments, Austin, TX). Recordings were excluded from further analysis if they had frequent atrial or ventricular ectopic beats, which was defined as more than 3% of all heartbeats (MICT n = 2, P-HIT n = 2, A-HIT n = 2, CON n = 1). R-R interval data were then imported into Kubios HRV software (University of Kuopio, Kuopio, Finland). Time-domain parameters of HRV were obtained: mean R-R interval, standard deviation of all normal-to-normal R-R intervals (SDNN), root-mean-square of differences of adjacent normal-to-normal R-R intervals (RMSSD), and percentage of interval differences of successive normal R-R intervals greater than 50 ms (pNN50).
Blood pressure and flow-mediated dilation
Participants were placed in a resting semi-recumbent position for ∼10 min before systolic and diastolic pressure were measured via auscultation. Following blood pressure measurement, Longitudinal B-mode images were obtained for three consecutive 10-s collections using a 10-MHz linear array probe (Terason T3200; Teratech Corp., Burlington, MA) and frame rate of 30 frames·s−1 on the brachial artery area of interest of the right arm, with the probe positioned proximal to the antecubital fossa. Three consecutive blood velocity measurements were then acquired in pulsed-wave mode (4 MHz) for 10 s. Upon completion of pre-occlusion measurements, a pneumatic cuff positioned on the upper arm proximal to the antecubital fossa was inflated using a manual sphygmomanometer to a supra-systolic pressure (∼200 mmHg). The proximal placement was intended to increase potential diameter change among the deconditioned cohort. After 5 min of occlusion, the cuff pressure was rapidly reduced and pulse wave velocity data were acquired for 10 s, followed by B-mode images for 2 min. All images were stored in Digital Imaging and Communications in Medicine (DICOM) format for offline editing and analysis. The FMD analyses consisted of end-diastolic frames being identified and manual edge detection was applied (ImageJ; National Institutes of Health, Bethesda, MD) to identify end-diastolic diameters. Pre-occlusion diameters were determined from the average of 5-s bins while the peak diameter from post-occlusion 5-s bin determined the peak post-occlusion FMD diameter. Data were collected and analysed by the same Sonographer with more than 5 years of experience in the procedure who was blinded to group allocation. Relative FMD was determined via the following equation: (peak end-diastolic diameter – resting end-diastolic diameter)/resting end-diastolic diameter × 100 (Thijssen et al., Citation2011).
Graded exercise testing
The GXT was submaximal in intensity (HR85%) and administered as recommended by Sports Medicine Australia and the American College of Sports Medicine (Fletcher et al., Citation2001). HRmax was estimated using the following equation (205.8 – (0.685 × age)). The GXT was performed on an electronically braked cycle ergometer (CASE ECG Stress Test System; General Electric Medical Systems; Milwaukee, WI). Participants commenced at 50 W and cycled through ascending workloads (25 W·min−1) until HR85% was achieved. During the GXT, pulmonary gas exchange was collected and analysed by a calibrated metabolic cart (TrueOne 2400; ParvoMedics; Salt Lake City, Utah) to permit the computation of peak oxygen uptake at HR85%. Exercise duration (last completed + fraction of last attempted) and final workload were recorded at the termination of the GXT.
Exercise intervention
Exercise training was completed 3 d ·wk−1 for 12-wk in laboratories with supervision by the research team. Participants trained on cycle ergometers (828e; Monark AB; Varburg, Sweden), were fitted with telemetry-based HR monitors (Polar, Vantage, NV), and had a rating of perceived exertion (RPE; 1–10 scale; Borg, Citation1990) recorded. RPE was recorded at the end of each interval in the HIT programmes and 5-min intervals during the MICT session. HR was recorded at the end of individual recovery and interval periods for HIT, and every 10 mins during MICT. Weekly and total session averages were calculated for all training interventions. Participants in the MICT group cycled at 60–70% of estimated HRmax for 50 mins in Wks 1–6, progressing to 60 mins in Wks 7–12. Both HIT modes were identical in programme design in terms of the number and duration of intervals completed in each session. Following a 5-min warm-up at light-moderate intensity (50–60% estimated maximum HR), 30-s intervals were performed at a workload eliciting HR85%, interspersed with 2.5 mins of recovery. Beginning with four intervals in Wks 1–2, participants progressed by one additional interval every 2 weeks, culminating in 10 intervals in week 12. The only distinction between the HIT modes was the “passive” or “active” recovery period between efforts. During recovery, participants of the P-HIT group ceased cycling, conversely, A-HIT continued cycling at 30% of the final GXT workload. Total session energy expenditure due to exercise (MET-min) for MICT, P-HIT, and A-HIT was calculated by accumulating the product of each exercise bout duration (min) and prescribed exercise intensity in metabolic equivalents (METs) determined from the participant’s GXT minus 1 MET (to remove resting metabolism). Average intensity for the exercise sessions (METs) was calculated as the total session energy expenditure due to exercise (MET-min) divided by the total session duration (min) plus 1 MET. Peak exercise intensity was identified as the highest prescribed intensity in METs.
Statistical analysis
A Priori power analysis was conducted using GPower (G*Power v.3) (Faul et al., Citation2007), for an F Test interaction effect, with significance set at 0.05, power at 0.80, four groups (MICT, P-HIT, A-HIT, and CON), two measurements (pre- and post- training) and correlation among measures based on previous research using FMD (Ghiadoni et al., Citation2012). The calculation resulted in a sample size of 48 participants across four groups to achieve an effect size of 0.25 (medium effect). Additional participants were recruited to allow for an anticipated drop-out of approximately 25%.
Statistical analyses were conducted with SPSS software (version 26.0 SPSS Inc, Chicago, IL) and significance was set at P < 0.05. The assumption of normality was verified using the Shapiro–Wilk W test. Baseline characteristics (), training HR, and rating of perceived exertion () were analysed using one-way analysis of variance (ANOVA). FMD, HRV, and oxygen uptake at 85% HRmax (V̇O2-85%) data were analysed using mixed-model ANOVA with the between factor being group (four levels) and the within subject factor being time (two levels). If a significant group*time interaction was identified, the intervention was deemed to influence response over time and simple main effects of time were evaluated by group using Tukey’s honestly significant difference (Tukey’s HSD). Main effects of time were consulted where group*time interactions did not reach significance, and significant time effects were followed up with Tukey’s HSD. Group data are reported as mean ± standard deviation and differences are reported as mean difference (MD) with 95% confidence intervals (CI).
Table 2. Descriptive training data.
Results
Descriptive characteristics of training
Three participants, one from each of the P-HIT, A-HIT, and CON groups did not complete the study due to time constraints (n = 2) and orthopaedic limitations (n = 1) and were excluded from analysis, yielding final participant numbers of 15 in all exercise conditions and 14 in CON. No differences were observed for baseline characteristics between intervention groups (F3,55 ≤ 1.549; P ≥ 0.182; ). Training data are described in . All participants attended >30 sessions, with mean attendance 32 of the 36 sessions and no differences existed in training compliance between groups (F2,44 = 0.955; P = 0.393). Average sessional HR was higher in MICT (F2,162 = 15.105; P < 0.05) when compared to all other groups (). No differences were observed for average intra-interval recovery HR between P-HIT (115 ± 12 bpm) and A-HIT (117 ± 13 bpm) (T114 = 0.897, P = 0.941) or between peak interval HR and average MICT HR (F2,162 = 2.204; P = 0.114; ). No differences were observed for RPE (F2,170 = 1.517; P = 0.222). Session energy expenditure due to exercise differed by intervention (F2,33 = 250.072; P < 0.001), with MICT being greater than both P-HIT and A-HIT. Peak exercise intensity was higher for P-HIT and A-HIT when compared to MICT (F2,33 = 15.030; P < 0.001; ). Average exercise intensity was different for all intervention groups (F2,33 = 140.368; P < 0.001), being highest in MICT and lowest in P-HIT ().
Cardiorespiratory fitness
A significant group*time interaction was observed for cardiorespiratory fitness (V̇O2-85%) (F3,55 = 5.247, P < 0.001) with pre-to-post increases observed for all exercise groups MICT (MD: 3.8 ml·kg−1·min−1, CI: 2.2–5.4 ml·kg−1·min−1), P-HIT (MD: 5.1 ml·kg−1·min−1, CI: 3.5–6.7 ml·kg−1·min−1) and A-HIT (MD: 3.1 ml·kg−1·min−1, CI: 1.5–4.7 ml·kg−1·min−1). Exercise time to reach HR85% (F3,55 = 6.504; P = 0.001) increased in all exercise groups MICT (MD: 73 s, CI: 47–100 s), P-HIT (MD: 90 s, CI: 63–116 s) and A-HIT (MD: 62 s, CI: 36–88 s). Power output observed at HR85% (F3,55 = 6.919; P < .001) improved in all exercise groups MICT (MD: 30 W, CI: 19–42 W), P-HIT (MD: 39 W, CI: 28–50 W) and A-HIT (MD: 26 W, CI: 15–37 W). No pre-to-post changes were observed among the CON group for any cardiorespiratory fitness measures (P > 0.05) ().
Table 3. Cardiorespiratory fitness, endothelial function and heart rate variability.
Heart rate variability
A significant group*time interaction was identified in the HRV measure SDNN (F3,48 = 4.599, P = 0.007) with pre-to-post differences in the MICT (MD: 14 ms, 9–20 ms) and P-HIT (MD: 7 ms, 2–13 ms) groups (). No differences were observed for A-HIT (P = 0.380) or CON (P = 0.712). No changes were observed for pNN50 (F3,36 = 2.704; P = 0.060) or RMSSD (F3,48 = 2.049; P = 0.119) ().
Flow-mediated dilation
A significant effect for time was identified in FMD (F1,53 = 17.444; P < .001) with significant increases pre-to-post intervention for MICT (MD: 3.4%, CI: 0.3–6.5%), P-HIT (MD: 4.9%, CI: 1.8–8%), and A-HIT (MD: 4%, CI: 0.8–7.4%) (). A significant effect for time was identified in raw changes in post-occlusion vessel diameter pre-to-post intervention (F1,53 = 20.054; P < 0.001) for each exercise group MICT (MD: 0.15 mm, CI: 0.03–0.27 mm), P-HIT (MD: 0.19, CI: 0.07–0.31 mm), and A-HIT (MD: 0.16, CI: 0.03–0.31 mm) ().
Discussion
The main finding from the present study was that 12 weeks of aerobic exercise improved cardiorespiratory fitness, endothelial function, and autonomic balance regardless of training or recovery mode among sedentary middle-aged men. Submaximal cardiorespiratory fitness and FMD were improved post-intervention in all exercise groups, while MICT and P-HIT improved the time-domain HRV measure of SDNN. Both HIT groups reported comparable improvements in cardiovascular function despite lower total energy expenditure and session time when compared to MICT.
The MICT and P-HIT interventions increased pre-to-post SDNN; however, no change was identified in the A-HIT or CON groups. Our findings support previous research that both continuous and interval aerobic exercise can induce beneficial effects on time-domain measures of HRV (Melanson & Freedson, Citation2001; Munk et al., Citation2010). Time-domain HRV provides non-invasive prognostic information on ANS balance with hyperactive sympathetic influence being associated with future adverse cardiovascular events (Melanson & Freedson, Citation2001; Thayer et al., Citation2010). While SDNN reflects the total variability of both SNS and parasympathetic (PNS) modulation during the collection period, SDNN is primarily mediated by PNS influence during regulated breathing protocols (Shaffer & Ginsberg, Citation2017). Subsequently, the increase in resting autonomic variability post-intervention indicates improved autonomic modulation and reflects a decreased risk of future CVD mortality among the MICT and P-HIT intervention groups. The physiological mechanisms by which aerobic training improves sympathovagal balance are yet to be fully elucidated but have been associated with functional and structural adaptations in the cardiovascular system that result in decreased circulating catecholamine concentrations at rest and during submaximal efforts (Besnier et al., Citation2017). Higher exercise intensity is hypothesised to have a greater relative impact on neurocardiac activity including circulating catecholamine concentrations when compared to lower-intensity exercise (Jacob et al., Citation2004). While not directly measured in the current study, intensity-based changes in catecholamine moderation might explain the significant results elicited by P-HIT in half the total exercise time compared to MICT. Unlike MICT and P-HIT, the pre-to-post intervention change in SDNN for A-HIT was not different from control. It is possible that the transitions in intensity from active recovery to exercise in A-HIT required less neurocardiac accommodation and consequently did not illicit the significant change in autonomic modulation observed in P-HIT and MICT.
All exercise intervention groups improved endothelial function as assessed by FMD (Currie et al., Citation2013; Tjønna et al., Citation2008). Recognised as a clinically valid prognostic assessment of endothelial health (Thijssen et al., Citation2011), the exercise-induced improvements in FMD indicate a reduction in future CVD risk regardless of training mode (Shechter et al., Citation2014). High-intensity exercise has been speculated to facilitate additional vascular adaptation via elevated blood flow, shear stress, and nitric oxide (NO) bioavailability, with repetitive shear stress increasing NO sensitivity (Ramos et al., Citation2015). Both HIT groups had higher peak intensity than MICT and incorporated alternating periods of high and low relative shear stress transitioning from intervals to recovery periods. Training characteristics that may have contributed to comparable pre-to-post changes in FMD elicited HIT despite lower total session duration and average energy expenditure compared to MICT. Therefore, HIT offers an attractive exercise intervention option for time-poor people aiming to improve endothelial function and minimise future disease risk.
The 12-week training intervention improved measures of cardiorespiratory fitness and exercise capacity regardless of prescribed intensity or recovery mode. Low cardiorespiratory fitness, either measured or predicted via exercise testing, is an independent risk factor for all-cause mortality (Kodama et al., Citation2009; Myers et al., Citation2002), and a stronger predictor of future cardiovascular outcomes than total PA levels alone (Lavie et al., Citation2019). While MICT has traditionally been advocated as an exercise intervention to improve cardiorespiratory fitness (Batacan et al., Citation2017; Wilson et al., Citation2016), exercise intensity is generally regarded to be the more critical factor in cardiac output, mitochondrial biogenesis, and the trainability of VO2peak when matched for energy expenditure (Currie et al., Citation2013; MacInnis & Gibala, Citation2017; Tjønna et al., Citation2008). Specifically, HIT has been associated with greater relative improvements in skeletal muscle oxidative capacity (Gibala et al., Citation2012), reduced glycogen utilisation and enhanced peripheral vascular function compared to MICT (Gibala et al., Citation2012). Furthermore, evidence suggests that exercise intensity is the predominant factor in the activation of peroxisome-proliferator activated receptor ƴ coactivator (PGC-1α) and subsequent mitochondrial biogenesis (Gibala et al., Citation2012). The addition of active intra-interval recovery bouts, thereby increasing total energy expenditure compared to passive recovery, was hypothesised to provide additional stimulus and superior adaptation (Foster et al., Citation2015). Although researchers have previously demonstrated A-HIT can induce superior cardiorespiratory fitness improvements compared to passive recovery (Abderrahman et al., Citation2018), no statistical difference was observed for any cardiovascular fitness measure between the HIT groups in this study. Active recovery is associated with improved metabolite clearance and increased efforts in subsequent intervals (Germano et al., Citation2019). However, the peak exercise intervals in both HIT protocols were performed at submaximal intensity (HR85%) and might have been insufficiently intense to observe any anticipated benefit from active recovery. Furthermore, the exercise undertaken during active recovery in A-HIT was probably too low to elicit additional training benefit when compared to P-HIT.
A strength of the present study was the exploration of time-efficient HIT with two popular modes of intra-interval recovery methods in comparison to conventionally prescribed MICT. The findings add to the literature exploring HIT as a health intervention and can be used to inform future exercise prescription. A limitation in the current repeated measures study is the use of some procedures that vary from previously validated FMD methods (Shechter et al., Citation2014; Thijssen et al., Citation2011), including cuff placement, manual detection software, and no assessment of shear stress. These limitations might prevent the magnitude of the current values being directly compared to previously published data. The lack of data on participants’ training preferences and perceptions about the effects of training (e.g. muscle soreness, feelings of fatigue, and mental health) is an additional limitation when attempting to evaluate differences between the training modes. While these factors might be useful to inform prescription practices by incorporating individual preferences and training comfort, they are unlikely to have influenced the physiological changes evaluated in this study. Finally, the medication usage of participants was not recorded during this study. Although screening was conducted for diagnosed cardio-metabolic conditions and exclusion criteria included taking medication known to influence HR, it is possible that some participants might have been taking other medications during this study.
Perspectives
In summary, 12-weeks of time-efficient HIT improved submaximal cardiorespiratory fitness, endothelial compliance, and autonomic modulation to a similar extent as conventionally prescribed MICT in sedentary middle-aged men. The inclusion of active recovery periods between successive HIT bouts performed at an intensity of HR85% provided no additional benefit for cardiorespiratory fitness or endothelial compliance and did not induce a significant pre-to-post change in time-domain HRV despite increased total workload (Buchheit & Laursen, Citation2013b) and altered physiological response (Buchheit & Laursen, Citation2013b; Tschakert & Hofmann, Citation2013). These novel results demonstrate the efficacy of time-efficient HIT to improve risk factors associated with CVD and suggest that HIT can be used with or without active recovery as exercise prescriptions for this classically inactive and time-poor cohort.
Acknowledgements
The results of this study do not constitute an endorsement of any product by the authors and no have no conflicts of interest relevant to the project to disclose.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Abderrahman, A., Rhibi, F., Ouerghi, N., Hackney, A., Saeidi, A., & Zouhal, H. (2018). Effects of recovery mode during high intensity interval training on glucoregulatory hormones and glucose metabolism in response to maximal exercise. Journal of Athletic Enhancement, 7(3), 292. https://doi.org/10.4172/2324-9080.1000292
- Australian Institute of Health and Welfare. (2017). Trends in cardiovascular death. (Cat. no: AUS 216):24.
- Batacan, R. B., Duncan, M. J., Dalbo, V. J., Tucker, P. S., & Fenning, A. S. (2017). Effects of high-intensity interval training on cardiometabolic health: A systematic review and meta-analysis of intervention studies. British Journal of Sports Medicine, 51(6), 494–503. https://doi.org/10.1136/bjsports-2015-095841
- Besnier, F., Labrunee, M., Pathak, A., Pavy-Le Traon, A., Galès, C., Sénard, J.-M., & Guiraud, T. (2017). Exercise training-induced modification in autonomic nervous system: An update for cardiac patients. Annals of Physical and Rehabilitation Medicine, 60(1), 27–35. https://doi.org/10.1016/j.rehab.2016.07.002
- Borg, G. (1990). Psychophysical scaling with applications in physical work and the perception of exertion. Scandinavian Journal of Work, Environment & Health, 16, 55–58. https://doi.org/10.5271/sjweh.1815
- Bots, S. H., Peters, S. A., & Woodward, M. (2017). Sex differences in coronary heart disease and stroke mortality: A global assessment of the effect of ageing between 1980 and 2010. BMJ Global Health, 2(2), e000298. https://doi.org/10.1136/bmjgh-2017-000298
- Buchheit, M., & Laursen, P. B. (2013a). High-intensity interval training, solutions to the programming puzzle. Part I: Cardiopulmonary emphasis. Sports Medicine, 43(5), 313–338. https://doi.org/10.1007/s40279-013-0029-x
- Buchheit, M., & Laursen, P. B. (2013b). High-intensity interval training, solutions to the programming puzzle. Part II: Anaerobic energy, neuromuscular load and practical applications. Sports Medicine, 43(10), 927–954. https://doi.org/10.1007/s40279-013-0066-5
- Crichton, G. E., & Alkerwi, A. (2015). Physical activity, sedentary behavior time and lipid levels in the observation of cardiovascular risk factors in Luxembourg study. Lipids in Health and Disease, 14(1), 1–9. https://doi.org/10.1186/s12944-015-0085-3
- Currie, K. D., Dubberley, J. B., McKelvie, R. S., & MacDonald, M. J. (2013). Low-volume, high-intensity interval training in patients with CAD. Medicine & Science in Sports & Exercise, 45(8), 1436–1442. https://doi.org/10.1249/MSS.0b013e31828bbbd4
- de Lucia, C., Piedepalumbo, M., Paolisso, G., & Koch, W. J. (2019). Sympathetic nervous system in age-related cardiovascular dysfunction: Pathophysiology and therapeutic perspective. The International Journal of Biochemistry & Cell Biology, 108, 29–33. https://doi.org/10.1016/j.biocel.2019.01.004
- Faul, F., Erdfelder, E., Lang, A.-G., & Buchner, A. (2007). G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods, 39(2), 175–191. https://doi.org/10.3758/BF03193146
- Fletcher, G. F., Balady, G. J., Amsterdam, E. A., Chaitman, B., Eckel, R., Fleg, J., Froelicher, V. F., Leon, A. S., Piña, I. L., Rodney, R., Simons-Morton, D. A., Williams, M. A., & Bazzarre, T. (2001). Exercise standards for testing and training a statement for healthcare professionals from the American Heart Association. Circulation, 104(14), 1694–1740. https://doi.org/10.1161/hc3901.095960
- Foster, C., Farland, C. V., Guidotti, F., Harbin, M., Roberts, B., Schuette, J., Tuuri, A., Doberstein, S. T., & Porcari, J. P. (2015). The effects of high intensity interval training vs steady state training on aerobic and anaerobic capacity. Journal of Sports Science & Medicine, 14(4), 747.
- Germano, M. D., Sindorf, M. A., Crisp, A. H., Braz, T. V., Brigatto, F. A., Nunes, A. G., Verlengia, R., Moreno, M. A., Aoki, M. S., & Lopes, C. R. (2019). Effect of different recoveries during HIIT sessions on metabolic and cardiorespiratory responses and sprint performance in healthy Men. Journal of Strength and Conditioning Research. https://doi.org/10.1519/JSC.0000000000003423
- Gerosa-Neto, J., Panissa, V. L. G., Monteiro, P. A., Inoue, D. S., Ribeiro, J. P. J., Figueiredo, C., Zagatto, A. M., Little, J. P., & Lira, F. S. (2019). High- or moderate-intensity training promotes change in cardiorespiratory fitness, but not visceral fat, in obese men: A randomised trial of equal energy expenditure exercise. Respiratory Physiology & Neurobiology, 266, 150–155. https://doi.org/10.1016/j.resp.2019.05.009
- Ghiadoni, L., Faita, F., Salvetti, M., Cordiano, C., Biggi, A., Puato, M., Di Monaco, A., De Siati, L., Volpe, M., Ambrosio, G., Gemignani, V., Muiesan, M. L., Taddei, S., Lanza, G. A., & Cosentino, F. (2012). Assessment of flow-mediated dilation reproducibility: A nationwide multicenter study. Journal of Hypertension, 30(7), 1399–1405. https://doi.org/10.1097/HJH.0b013e328353f222
- Gibala, M. J., Little, J. P., MacDonald, M. J., & Hawley, J. A. (2012). Physiological adaptations to low-volume, high-intensity interval training in health and disease. The Journal of Physiology, 590(5), 1077–1084. https://doi.org/10.1113/jphysiol.2011.224725
- Hadaya, J., & Ardell, J. L. (2020). Autonomic modulation for cardiovascular disease. Frontiers in Physiology, 11, 1653. https://doi.org/10.3389/fphys.2020.617459
- Jacob, C., Zouhal, H., Prioux, J., Gratas-Delamarche, A., Bentue-Ferrer, D., & Delamarche, P. (2004). Effect of the intensity of training on catecholamine responses to supramaximal exercise in endurance-trained men. European Journal of Applied Physiology, 91(1), 35–40. https://doi.org/10.1007/s00421-003-1002-4
- Kodama, S., Saito, K., Tanaka, S., Maki, M., Yachi, Y., Asumi, M., Sugawara, A., Totsuka, K., Shimano, H., Ohashi, Y., Yamada, N., & Sone, H. (2009). Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: A meta-analysis. JAMA, 301(19), 2024–2035. https://doi.org/10.1001/jama.2009.681
- Kriel, Y., Kerherve, H. A., Askew, C. D., & Solomon, C. (2016). The effect of active versus passive recovery periods during high intensity intermittent exercise on local tissue oxygenation in 18–30 year old sedentary Men. PLoS ONE, 11(9), e0163733. https://doi.org/10.1371/journal.pone.0163733
- Lavie, C. J., Ozemek, C., Carbone, S., Katzmarzyk, P. T., & Blair, S. N. (2019). Sedentary behavior, exercise, and cardiovascular health. Circulation Research, 124(5), 799–815. https://doi.org/10.1161/CIRCRESAHA.118.312669
- MacInnis, M. J., & Gibala, M. J. (2017). Physiological adaptations to interval training and the role of exercise intensity. The Journal of Physiology, 595(9), 2915–2930. https://doi.org/10.1113/JP273196
- Melanson, E. L., & Freedson, P. S. (2001). The effect of endurance training on resting heart rate variability in sedentary adult males. European Journal of Applied Physiology, 85(5), 442–449. https://doi.org/10.1007/s004210100479
- Meyer, P., Normandin, E., Gayda, M., Billon, G., Guiraud, T., Bosquet, L., Fortier, A., Juneau, M., White, M., & Nigam, A. (2012). High-intensity interval exercise in chronic heart failure: Protocol optimization. Journal of Cardiac Failure, 18(2), 126–133. https://doi.org/10.1016/j.cardfail.2011.10.010
- Munk, P. S., Butt, N., & Larsen, A. I. (2010). High-intensity interval exercise training improves heart rate variability in patients following percutaneous coronary intervention for angina pectoris. International Journal of Cardiology, 145(2), 312–314. https://doi.org/10.1016/j.ijcard.2009.11.015
- Myers, J., Prakash, M., Froelicher, V., Do, D., Partington, S., & Atwood, J. E. (2002). Exercise capacity and mortality among men referred for exercise testing. New England Journal of Medicine, 346(11), 793–801. https://doi.org/10.1056/NEJMoa011858
- Norton, K., & Norton, L. (2011). Pre-exercise screening. Guide to the Australian adult pre-exercise screening system exercise and sports science Australia.
- Ramírez-Vélez, R., Hernández-Quiñones, P. A., Tordecilla-Sanders, A., Álvarez, C., Ramírez-Campillo, R., Izquierdo, M., Correa-Bautista, J. E., Garcia-Hermoso, A., & Garcia, R. G. (2019). Effectiveness of HIIT compared to moderate continuous training in improving vascular parameters in inactive adults. Lipids in Health and Disease, 18(1), 1–10. https://doi.org/10.1186/s12944-019-0981-z
- Ramos, J. S., Dalleck, L. C., Tjonna, A. E., Beetham, K. S., & Coombes, J. S. (2015). The impact of high-intensity interval training versus moderate-intensity continuous training on vascular function: A systematic review and meta-analysis. Sports Medicine, 45(5), 679–692. https://doi.org/10.1007/s40279-015-0321-z
- Sawyer, B. J., Tucker, W. J., Bhammar, D. M., Ryder, J. R., Sweazea, K. L., & Gaesser, G. A. (2016). Effects of high-intensity interval training and moderate-intensity continuous training on endothelial function and cardiometabolic risk markers in obese adults. Journal of Applied Physiology, 121(1), 279–288. https://doi.org/10.1152/japplphysiol.00024.2016
- Shaffer, F., & Ginsberg, J. (2017). An overview of heart rate variability metrics and norms. Frontiers in Public Health, 5, 258. https://doi.org/10.3389/fpubh.2017.00258
- Shechter, M., Shechter, A., Koren-Morag, N., Feinberg, M. S., & Hiersch, L. (2014). Usefulness of brachial artery flow-mediated dilation to predict long-term cardiovascular events in subjects without heart disease. The American Journal of Cardiology, 113(1), 162–167. https://doi.org/10.1016/j.amjcard.2013.08.051
- Task Force of the European Society of Cardiology the North American Society of Pacing Electrophysiology. (1996). Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Circulation, 93(5), 1043–1065. https://doi.org/10.1161/01.CIR.93.5.1043
- Thayer, J. F., Yamamoto, S. S., & Brosschot, J. F. (2010). The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. International Journal of Cardiology, 141(2), 122–131. https://doi.org/10.1016/j.ijcard.2009.09.543
- Thijssen, D. H., Black, M. A., Pyke, K. E., Padilla, J., Atkinson, G., Harris, R. A., Parker, B., Widlansky, M. E., Tschakovsky, M. E., & Green, D. J. (2011). Assessment of flow-mediated dilation in humans: A methodological and physiological guideline. American Journal of Physiology-Heart and Circulatory Physiology, 300(1), H2–H12. https://doi.org/10.1152/ajpheart.00471.2010
- Tjønna, A. E., Lee, S. J., Rognmo, Ø., Stølen, T. O., Bye, A., Haram, P. M., Loennechen, J. P., Al-Share, Q. Y., Skogvoll, E., Slørdahl, S. A., Kemi, O. J., Najjar, S. M., & Wisløff, U. (2008). Aerobic interval training versus continuous moderate exercise as a treatment for the metabolic syndrome: A pilot study. Circulation, 118(4), 346–354. https://doi.org/10.1161/CIRCULATIONAHA.108.772822
- Tschakert, G., & Hofmann, P. (2013). High-intensity intermittent exercise: Methodological and physiological aspects. International Journal of Sports Physiology and Performance, 8(6), 600–610. https://doi.org/10.1123/ijspp.8.6.600
- Wilson, M. G., Ellison, G. M., & Cable, N. T. (2016). Basic science behind the cardiovascular benefits of exercise. British Journal of Sports Medicine, 50(2), 93–99. https://doi.org/10.1136/bjsports-2014-306596rep