ABSTRACT
We aimed to investigate the influence of 4-wk of fish oil (FO) supplementation on markers of muscle damage, inflammation, muscle soreness, and muscle function during acute recovery from eccentric exercise in moderately trained males. Sixteen moderately-trained males ingested 5 g/d of FO (n = 8) or soybean oil (placebo) capsules (n = 8) for 4-wk prior to- and 3-d following an acute eccentric exercise bout. Eccentric exercise consisted of 12 sets of isokinetic knee extension and knee flexion. Indices of muscle damage, soreness, function and inflammation were measured at baseline and during exercise recovery. Eccentric exercise elicited an increase in muscle soreness (p < 0.010) and thigh volume (p < 0.001), and reduced peak isometric torque by 31.7 ± 6.9%, (p < 0.05, 95% CI 10.6–52.8) during 3-d of recovery. Blood omega-3 polyunsaturated fatty acid concentration was 14.9 ± 2.4% higher in FO than PLA (p < 0.01, 95% CI 9.8–20.1). However, FO did not ameliorate the cumulative creatine kinase response (expressed as AUC; p = 0.368), inflammation (p = 0.400), muscle soreness (p > 0.140), or muscle function (p > 0.249) following eccentric exercise. FO supplementation confers no clear benefit in terms of ameliorating the degree of muscle damage, or facilitating the muscle repair process, during acute eccentric exercise recovery. These data suggest that FO supplementation does not provide an effective nutritional strategy to promote exercise recovery, at least in moderately-trained young men.
Abbreviations: ANOVA: Analysis of variance; AUC: Area under curve; CI: Confidence interval; CK: Creatine kinase; CMJ: Countermovement jump; COX: Cyclooxygenase; CRP: C-reactive protein; DHA: Docosahexaenoic acid; DOMS: Delayed-onset muscle soreness; EIMD: Exercise-induced muscle damage; En%: Energy percent; EPA: Eicosapentaenoic acid; FO: Fish oil; IL-6: Interleukin-6; LDH: Lactate dehydrogenase; LOX: Lipoxygenase; Mb: Myoglobin; mTOR: Mechanistic target of rapamycin; PLA: Placebo; ROM: Range of motion; ROS: Reactive oxygen species; SD: Standard deviation; SEM: Standard error of the mean; TNF-α: Tumour necrosis factor alpha; VAS: Visual analogue scale; Ω3-PUFA: Omega-3 polyunsaturated fatty acids; Ω6-PUFA: Omega-6 polyunsaturated fatty acids
Highlights
The anti-inflammatory properties of omega-3 polyunsaturated fatty acids, alongside their propensity to incorporate into the muscle phospholipid membrane underpins the idea that fish oil supplementation may attenuate muscle damage and promote muscle repair following eccentric-based exercise.
Four weeks of high-dose (5 g/d) fish oil supplementation prior to eccentric exercise failed to attenuate the rise in creatine kinase concentration and muscle soreness during acute exercise recovery in physically-active young men.
Future studies are warranted to investigate the efficacy of combining omega-3 polyunsaturated fatty acids with other nutrients (i.e. protein/amino acids) for the promotion of muscle recovery following eccentric-based damaging exercise.
Introduction
Exercise-induced muscle damage (EIMD) is commonly experienced by physically-active individuals, ranging from novice exercisers to highly trained athletes. Despite the adaptive role of muscle strain, local inflammation and the production of reactive oxygen species in facilitating skeletal muscle remodelling and adaptation (Paulsen et al., Citation2012), minimising the detrimental effects of EIMD is critical for athletes to maximise exercise recovery and performance (Owens et al., Citation2019). For instance, success in tournament competition is dependent, at least in part, on the degree of exercise recovery achieved prior to a subsequent match, event or heat, dependent on the sport of interest. Moreover, minimising muscle soreness during the acute 2–3 day period following exercise serves as a key psycho-physiological determinant of adherence to regular physical activity in recreational exercisers (Peake et al., Citation2017). Hence, sport nutrition guidelines regarding nutritional interventions to combat EIMD must be context-specific and tailored to the individual goals of the athlete or exerciser.
EIMD manifests principally from the active lengthening of skeletal muscle fibres during the eccentric (lengthening) phase of muscle contraction (Proske & Morgan, Citation2001). Fewer motor units are recruited than during equivalent isometric or concentric muscle contractions, asserting greater mechanical strain on individual motor units and supportive passive structures including desmin and titin (Allen, Citation2001; Proske & Morgan, Citation2001). Structural damage to myofibres is followed by local inflammation and activation of muscle repair processes (Proske & Morgan, Citation2001). Muscle membrane permeability is increased, initiating an influx of calcium into the sarcoplasm that activates proteolytic pathways and further degrades myofibre structures (Macpherson et al., Citation1996). Cellular components, including creatine kinase (CK), leak into the bloodstream, triggering an inflammatory response characterised by secretion of inflammatory cytokines and neutrophil infiltration into damaged tissues. This inflammatory response ostensibly leads to an increase in reactive oxygen species, muscle swelling, and stiffness (Proske & Morgan, Citation2001).
Multiple studies have investigated the efficacy of nutritional interventions to promote muscle recovery following EIMD, with mixed findings (Rawson et al., Citation2018). Two mechanisms have been proposed to underpin the biological action of Ω3-PUFA ingestion in attenuating EIMD and promoting muscle repair. First, circulating Ω3-PUFA are taken up by the skeletal muscle cell and incorporated into the phospholipid bilayer within 4 wk of supplementation (McGlory et al., Citation2014). Due to the chemical structure of Ω3-PUFA, and specifically the presence of cis-configuration carbon–carbon double bonds in their hydrocarbon backbone, the fluidity of the muscle cell membrane and resilience to strain is increased, thus reducing the mechanical damage experienced for the same applied tension (Jeromson et al., Citation2015). Hence, biological rationale exists that Ω3-PUFA incorporation into the muscle membrane may preserve myofibre structure and ameliorate muscle damage following exercise.
Second, Ω3-PUFA ingestion has been proposed to enhance muscle repair by exerting anti-inflammatory properties. The incorporation of Ω3-PUFA into the muscle phospholipid bilayer occurs at the expense of Ω6-PUFA, forming the primary substrate for signalling pathways that regulate eicosanoid production catalysed by cyclooxygenase and lipoxygenase enzymes (Calder, Citation2017). This signalling cascade downregulates the formation of pro-inflammatory lipid mediators (prostaglandin E2/leukotriene B4) and upregulates lipid mediators with low pro-inflammatory potential (prostaglandin E3). Prostaglandin E3 stimulates blood flow and facilitates the repair of damaged tissues that is crucial for muscle remodelling following EIMD.
Preliminary evidence suggests that Ω3-PUFA ingestion may improve indices of muscle recovery, including the attenuation of muscle damage (DiLorenzo et al., Citation2014), inflammation (Tsuchiya et al., Citation2019), oxidative stress (Gray et al., Citation2014), and soreness (Tartibian et al., Citation2009) following EIMD. This improvement in muscle recovery was shown to be associated with the better maintenance of mobility (improved range of motion) (Tsuchiya et al., Citation2016), and muscle function (Jakeman et al., Citation2017). To date, only three studies have investigated the effects of FO supplementation on acute recovery in trained populations (Bloomer et al., Citation2009; Jakeman et al., Citation2017; Philpott et al., Citation2018), yielding conflicting results. Mixed findings are likely attributed to methodological differences regarding participant characteristics (trained vs. untrained), dosing (360 mg/d-6 g/d) and/or duration (single dose-8 wk) of Ω3-PUFA supplementation, exercise protocols (isokinetic dynamometry, downhill run), and functional outcome measurements (MVC, vertical jump) (Anthony et al., Citation2021; Kyriakidou et al., Citation2021; Visconti et al., Citation2021).
This study aimed to investigate the influence of 4-wk of Ω3-PUFA rich fish oil (FO) vs. soybean oil (PLA) supplementation on indices of muscle damage, inflammation, muscle soreness, and muscle function during acute (72-h) recovery following intense lower-limb eccentric exercise in moderately trained, young males. Based on recent findings that 6 g/d of FO supplementation reduced muscle soreness and improved muscle function following eccentric exercise to a greater extent than 2 and 4 g/d of FO supplementation (VanDusseldorp et al., Citation2020), we administered a high dose (5 g/d) FO supplementation regimen. We hypothesised that FO supplementation would attenuate putative markers of muscle damage, inflammation, and muscle soreness during recovery from EIMD, translating to better maintenance of muscle function in moderately trained, young men.
Materials and methods
Subjects
Sixteen moderately trained (3–4 exercise sessions/wk, training history of ≥6y) young males were recruited (Table S1). Prospective participants were excluded if they consumed FO, or any vitamin or mineral supplements, suffered from metabolic conditions, or experienced muscle damage or injury for 6 mo prior to the study. Ethical approval was granted by The University of Stirling Research Ethics Committee (SSREC 12/S/0316).
Experimental design
This study utilised a randomised, double-blinded, parallel design. Participants supplemented their normal diet with 5 g/d of fish oil (n = 8) or soybean oil placebo (n = 8) capsules for 4-wk prior to- and for 3-d following an intense bout of eccentric exercise. Indices of muscle damage, local inflammation, muscle soreness and muscle function were assessed at baseline and during exercise recovery (Figure S1).
Protocol
Participants attended the laboratory on six occasions over a 6-wk period: once for initial familiarisation with experimental procedures, plus five visits for physiological testing. During familiarisation, participants performed peak isometric torque and countermovement jump (CMJ) tasks, as detailed below, until they were able to confidently perform each task and produce consistent results; they were also familiarised with the correct use of the visual analogue scale (VAS) described below. Following familiarisation, participants were randomised into FO or PLA groups (www.randomizer.org). Participants returned to the laboratory for baseline testing and anthropometric measurements. Procedures consisted of blood sampling using the venepuncture technique for the assessment of Ω3-PUFA and CK concentrations, followed by measurements of height and body mass, thigh volume, muscle soreness, and muscle function (using peak isometric torque and CMJ).
Following 4-wk of supplementation, participants returned to the laboratory to perform the exercise protocol (Eccentric exercise protocol) that was designed to elicit muscle damage. Prior to completing this protocol, a baseline blood sample was obtained, and measurements of thigh volume, muscle soreness, and muscle function were conducted. All endpoint measurements were repeated at 1, 2, and 3-d post-eccentric exercise (Figure S1).
Supplementation
Participants consumed visually identical capsules containing 5 g/d of FO or soybean-oil (PLA) for 32-d: 4-wk supplementation period plus 4-d of testing. FO supplements contained 1882 mg EPA and 485 mg DHA per 5 g dose (EPA:DHA ratio 3.8:1) (Select Supplements Inc). The lipid composition of the soybean oil capsules is detailed in Table S2. Compliance was assessed by the quantity of capsules remaining at the end of the test protocol and verified by spot collection for blood Ω3-PUFA concentrations.
Eccentric exercise protocol
Participants were positioned on the isokinetic dynamometer with the seat reclined at 95° and knee folded at the chair edge. First, 3 individual maximal eccentric actions (knee extension and knee flexion) were performed at a velocity of 60°.s−1 with 120-s rest between actions. The greatest eccentric torque produced was used to determine the workload used during the eccentric exercise protocol. this eccentric exercise protocol consisted of 12 sets of isokinetic knee extension and 12 sets of isokinetic knee flexion on the non-dominant leg. A minimum of 60-s rest was permitted between sets. Each set consisted of a pre-set workload based on 120% of peak isokinetic torque performed 10 times/set for 12 sets (Kennedy et al., Citation2017; Paschalis et al., Citation2005; Stupka et al., Citation2001), such that: Workload [per set] = Peak eccentric torque x 90° [ROM] x 1.2 × 10 [repetitions]. Eccentric knee extension was performed over a range of 20–110°, and knee flexion was performed from 0–90° (0° = full extension). Movements were performed at a standardised velocity of 60°.s−1 and 180°.s−1 through the eccentric and concentric phase, respectively.
Blood sampling and analysis
At the start of each laboratory visit and after an overnight fast, 10 mL of venous blood was collected from a forearm vein into serum-separator vacutainer tubes and left to clot for 30-min. At −4-wk and day 0 (see Figure S1), 200 µL of whole blood was dispensed for blood spot analysis of Ω3-PUFA concentrations using Whatman 903 blood collection cards. All remaining samples were centrifuged at 3500 rpm for 15-min at 4°C and serum was dispensed into Eppendorf tubes and stored at −80°C until further analyses. Enzymatic analysis of serum CK concentrations was performed in duplicate using a semi-automated blood analyser (iLab).
Perceived muscle soreness
A 200 mm horizontal VAS that ranged from “no pain” to “most pain imaginable” was used to quantify perceived muscle soreness of hamstrings and quadriceps in both extended (0°) and flexed (90°) positions. Participants were instructed to mark the appropriate point along the scale that was associated with the level of muscle soreness perceived when 1 kg cm−2 of pressure was applied to the mid-point of the thigh using a custom built spring-loaded algometer. The distance between the “marked” point and the left anchor point was measured and muscle soreness was expressed in mm.
Muscle function
Muscle function was assessed via peak isometric torque performed using an isokinetic dynamometer. The peak isometric torque test consisted of 3 × 5 s contractions of the hamstring (knee flexion) and quadriceps (knee extension) muscles of the dominant (rested, undamaged) and non-dominant (damaged) leg, performed in randomised order. Hamstring and quadriceps peak isometric torque were measured at 30° and 60° knee joint angles, respectively. Participants were instructed to exert maximal force as quickly as possible to enhance reliability (Engel et al., Citation2019). Peak (best of three) torque was recorded at each timepoint.
A vertical jump mat and waist belt was used to assess lower limb power (Takei, Japan). Three bilateral CMJ were completed with 30-s rest between each jump. Mean and peak vertical displacement were recorded to the nearest cm. Thereafter, six unilateral CMJ were performed using each leg. Unilateral jumps were conducted in random order (control or intervention leg) for each participant. Participants were instructed to maintain correct form during each CMJ.
Thigh volume
The measurement of thigh volume was used as an indirect marker of muscle swelling and inflammation. Thigh volume was calculated using the circumference and height of 7 pre-determined regions of the upper leg for both legs measured using anthropometric tape (Wolfe & Chinkes, Citation2005).
Dietary and physical activity control
Participants avoided strenuous exercise and alcohol consumption for 48 h before each laboratory visit. Participants were instructed to maintain their habitual diet, avoiding Ω3-PUFA rich foods, throughout the trial. A 3-d weighed diet diary and activity log were completed prior to supplementation to facilitate compliance (Table S3). Following the exercise protocol, participants received a standardised breakfast containing 29.5 kJ.kg−1 BM energy, of which 60.4 energy percent (En%) was carbohydrate, 24.5 En% fat, and 15.1 En% protein. This meal plan equated to an individualised portion of porridge (cooked with semi-skimmed milk), plain yoghurt, banana, and orange juice.
Statistical analysis
All statistical analysis was performed using IBM SPSS for Windows v27 and α-significance level was set at p < 0.05. Where appropriate, tests for assumption of normality (Shapiro–Wilk), homogeneity of variances (Levene’s test), and sphericity (Mauchley’s test) were conducted prior to analysis. Blood Ω3-PUFA concentrations, peak isometric torque, CMJ, VAS score, and serum CK concentrations were analysed using a mixed-design ANOVA (between subjects factor: FO vs. PLA treatment × within subjects factor: baseline, 1, 2 and 3-d post eccentric exercise time-points). AUC was calculated using the trapezoid method to determine cumulative changes in CK concentrations and muscle soreness over the entire 72 h recovery period, and analysed using independent samples t-tests. All data are presented as means ± SEM with 95% confidence intervals (CI).
Results
No differences in baseline participant characteristics (Table S1) or habitual diet (Table S3) were observed between FO and PLA groups.
Blood Ω3-PUFAConcentrations
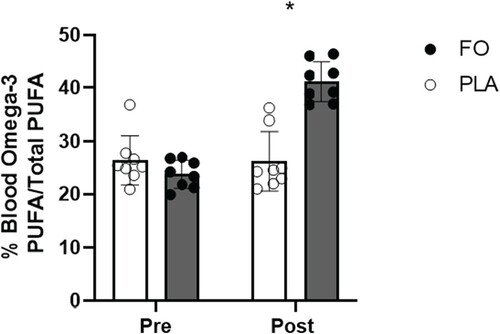
Blood Ω3-PUFA concentrations, expressed as percentage of total PUFA and measured pre-supplementation, did not differ between FO and PLA (p > 0.05). Following 4 wk of supplementation, blood Ω3-PUFA concentrations were higher in FO than PLA (14.9 ± 2.4%, p < 0.001, 95% CI 9.8–20.1) ().
Indices of muscle damage and inflammation
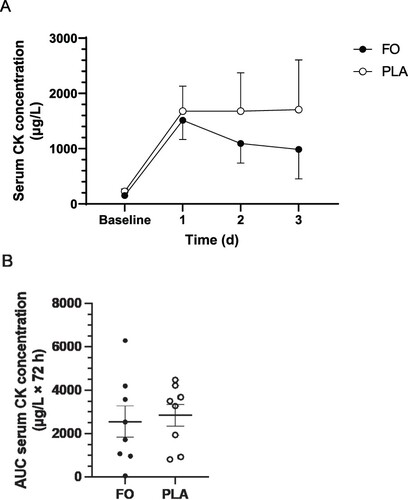
No main effects of time, group, or group × time interaction were observed for serum CK concentration ((A)). Mean values for CK concentration were lower on days 2 and 3 in FO than PLA, however statistical significance was not reached (p > 0.05) ((A)). No differences in CK concentration, expressed as AUC to represent the cumulative muscle damage response during the 72 h recovery period, were observed between FO (2551 ± 724 μg/L × 72 h) and PLA (2856 ± 508 μg/L × 72 h; t(14) = 0.345, p = 0.368) ((B)).
Figure 2. Serum creatine kinase (CK) concentrations (μg/L) measured before (baseline) and 3 days after a single bout of intense eccentric leg exercise (A) and expressed as area under the curve (AUC) (μg/L × 72 h) over the entire 72 h acute recovery period (B). FO, fish oil; PLA, soybean oil placebo. Data expressed as means ± SEM.
A main effect of time was observed for thigh volume in the non-dominant (damaged) leg (F(3,36) = 9.623, p < 0.001), however no main effect of group or group × time interaction effects were observed. No main effects of time, group, or group × time interaction was observed for limb volume in the dominant (rested, undamaged) leg.
Muscle soreness
A main effect of time was observed for quadriceps muscle soreness in the extended (F(1.795, 25.129) = 6.415, p = 0.007) and flexed positions (F(3,42) = 2.960, p = 0.043) of the non-dominant (damaged) leg (). Quadriceps muscle soreness increased on day 2 compared to baseline (19 ± 6 mm, p = 0.034, 95% CI 1–37) in the extended position ((A)). No main effect of group or group × time interaction was observed for quadriceps muscle soreness in both the extended and flexed positions of the non-dominant leg.
Figure 3. Muscle soreness, determined by 200 mm visual analogue scale (VAS) in the extended (A) and flexed (B) positions of the quadricep muscle and extended (C) and flexed (D) positions of the hamstring muscles of the non-dominant (damaged) leg over 72 h of exercise recovery. Muscle soreness (VAS score), expressed as area under the curve (AUC) (mm × 72 h), in the extended (Ext) and flexed (Flex) positions of the quadricep (quad) and hamstring (ham) muscles of the non-dominant leg (E). FO, fish oil; PLA, soybean oil placebo. Data expressed as mean ± SEM (n = 16). *Significant difference (p < 0.05) between timepoints when data for FO and PLA were combined.
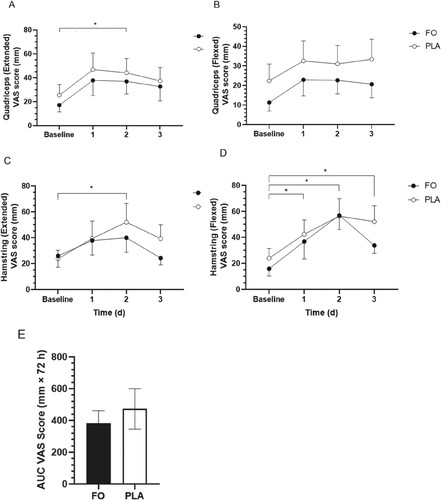
Hamstrings muscle soreness increased (significant main effect of time) in both the extended (F(3,42) = 3.570, p = 0.022) and flexed (F(3,42) = 14.983, p < 0.001) positions of the non-dominant (damaged) leg throughout the recovery period (). Hamstrings muscle soreness was greater on day 2 compared to baseline in the extended position (21 ± 6 mm, p = 0.028, 95% CI 2–40) ((C)), and on days 1 (20 ± 5 mm, p = 0.010, 95% CI 4–35), 2 (37 ± 6 mm, p < 0.001, 95% CI 19–55), and 3 (23 ± 5 mm, p = 0.002, 95% CI 8–38) vs. baseline in the flexed position ((D)) in both groups combined. No main effect of group or group × time interactions were observed for hamstrings muscle soreness in the extended or flexed positions of the non-dominant leg.
No differences in muscle soreness expressed as AUC (global score combining AUC measures for the quadricep and hamstring muscles in both the extended and flexed positions of the non-dominant (damaged) leg throughout the 72 h recovery period) were observed between FO and PLA (91 ± 150 mm × 72 h, t(14) = 0.606, p = 554; (E)). Muscle soreness for quadriceps and hamstrings muscles of the dominant (rested, undamaged) leg, measured in the extended and flexed positions, remained constant throughout the trial.
Muscle function
Peak isometric torque for knee extension (F(3,42) = 5.124, p = 0.004) and knee flexion (F(1.547,21.657) = 15.676, p < 0.001) was reduced following eccentric exercise in the non-dominant (damaged) leg, however no group × time interaction was observed (). Knee extension peak isometric torque was reduced on days 1 (14 ± 4.1%, p = 0.29, 95% CI 1.1–26.1) and 2 (11.7 ± 3.7%, p = 0.043, 95% CI 0.3–23.1) vs. baseline ((A)). At day 3, mean values for knee extension peak torque had returned to baseline in FO, but not PLA. Knee flexion peak torque was reduced on days 1 (29.3 ± 5.6%, p < 0.001, 95% CI 12.0–46.6), 2 (31.7 ± 6.9, p = 0.002, 95% CI 10.6–52.8), and 3 (28.7 ± 7.6%, p = 0.013, 95% CI 5.2–52.1) vs. baseline ((B)). No main effect of group or group × time interaction was observed for knee extension or knee flexion peak torque in the non-dominant (damaged) leg.
Figure 4. Muscle function, expressed as percentage change in peak torque from baseline, in the quadricep (knee extension) (A) and hamstring muscles (knee flexion) (B) of the non-dominant (damaged) leg over 72 h of exercise recovery. Unilateral countermovement jump (CMJ) (cm) on the non-dominant (damaged) leg (C) over 72 h of exercise recovery (n = 16). Data expressed as mean ± SEM (n = 16). FO, fish oil; PLA, placebo. *Significant (p < 0.05) difference between timepoints when data for FO and PLA were combined.
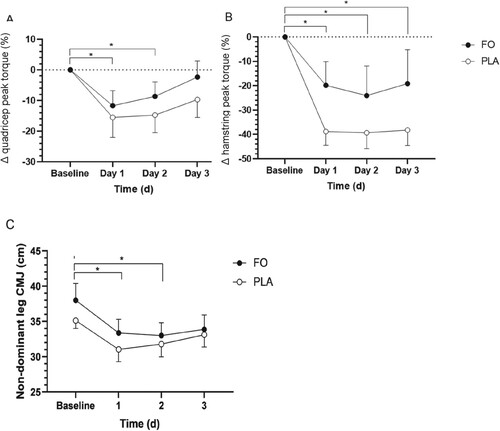
No significant main effect of time, group or group × time interaction was observed for bilateral CMJ. A main effect of time (F(3,42) = 9.438, p < 0.001) was observed for unilateral CMJ for the non-dominant leg, however no group or group × time interaction were observed ((C)). No main effect of time, group, or time × group interaction was observed for CMJ for the dominant (rested, undamaged) leg.
Discussion
This study investigated the influence of a 4-wk high-dose (5 g/d) FO vs. PLA supplementation on indices of EIMD and acute muscle recovery following intense eccentric exercise in moderately trained young males. By design, we observed a marked decrement in quadriceps and hamstring peak isometric torque following eccentric exercise, and an increase in hamstring soreness after 2-d of passive recovery in both groups. Refuting our original hypothesis and based on a single putative biomarker of myofibrillar disruption (i.e. serum CK response), no clear amelioration in the degree of muscle damage experienced following eccentric exercise was observed with FO supplementation. Moreover, no statistical differences in assessments of systemic inflammation, muscle soreness, and muscle function were observed between groups. However, it is conceivable that the small sample size masked the possibility of detecting any effect of FO supplementation on muscle damage and subsequent force loss, as evidenced by the apparent return to baseline for knee extension peak isometric torque in FO but not placebo group. These findings indicate no clear benefit of FO supplementation per se as a nutritional intervention to reduce muscle damage and/or accelerate muscle repair during acute recovery from eccentric exercise in moderately-trained young men.
A mechanism proposed to underpin the protective effect of FO supplementation on EIMD relates to the incorporation of Ω3-PUFA into the muscle phospholipid membrane. Membrane incorporation of EPA and DHA serves to remodel the muscle phospholipid bilayer, resulting in increased membrane fluidity and resilience to physical stress, potentially leading to a reduction in myofibrillar disruption following intense exercise (Chytrova et al., Citation2010). In the present study, 4-wk (5 g/d) of FO supplementation increased whole blood Ω3-PUFA concentrations by ∼74% (). A comparable study administered the same daily dose of FO over a 4-wk period and characterised the timecourse of Ω3-PUFA incorporation into blood and muscle tissues (McGlory et al., Citation2014). While the incorporation of Ω3-PUFA into the red blood cell was saturated after 2-wk of FO supplementation, a pronounced rise in the incorporation of EPA and DHA into the muscle cell was observed between 2 and 4-wk timepoints. Hence, based on these findings, we can assume that Ω3-PUFA were directly incorporated into the muscle phospholipid membrane after 4 wk of FO supplementation in the present study, however a longer supplementation period was necessary to saturate this response. Although mean values for serum CK concentrations were markedly lower in FO than PLA on days 2 (−586 μg/L) and 3 (−719 μg/L) post exercise (), these differences did not reach statistical significance when expressed over time or as cumulative AUC. This observation contrasts with a recent meta-analysis conducted in untrained males that revealed FO supplementation attenuated the rise in various blood biomarkers of muscle damage, including CK, myoglobin, and LDH (Xin & Eshaghi, Citation2021). Taken together, these findings indicate the protective action of Ω3-PUFA in ameliorating EIMD may be specific to untrained individuals who experience a greater severity of myofibrillar disruption given the unaccustomed nature of exercise. Moreover, we acknowledge that our null finding regarding the influence of FO supplementation on EIMD may be attributed to a low participant number (and lack of statistical power), and is limited to a single indirect biomarker of muscle damage and the relatively short 4-wk supplementation period employed in the present study.
The delayed onset of muscle soreness (DOMS) phenomenon in response to eccentric exercise is often associated with a rise in putative blood markers of muscle damage, including CK concentration (Baird et al., Citation2012). This observation reflects a complex sequence of psycho-physiological events that culminate in a decline in muscle functional capacity (Rodenburg et al., Citation1993). Previous studies reported a reduction in muscle soreness in concordance with an attenuation in EIMD following FO supplementation (Tsuchiya et al., Citation2016; Tsuchiya et al., Citation2019). In the present study, mean values for muscle soreness (expressed as AUC over the 3-d recovery period) were lower in FO than PLA ((E)). Moreover, the mean decline in muscle function following EIMD was attenuated with FO supplementation during exercise recovery. However, given that these mean differences failed to reach statistical significance, the present study fails to provide clear evidence that Ω3-PUFA incorporation into the muscle cell membrane exerts a modulatory effect on EIMD, muscle soreness, and muscle function.
The anti-inflammatory properties of Ω3-PUFA provide another intuitive mechanism to explain, at least in part, previous reports of a beneficial effect of FO supplementation on muscle repair following EIMD in trained (Bloomer et al., Citation2009) and untrained (DiLorenzo et al., Citation2014; Tsuchiya et al., Citation2016) individuals. Dietary Ω3-PUFA exhibit anti-inflammatory properties by replacing potent Ω6-PUFA in the muscle phospholipid bilayer such that EPA and DHA act as the primary substrates in key signalling pathways (COX/LOX) involved in initiating inflammation (Calder, Citation2010, Citation2017). This response initiates an increased production of specialised pro-resolving mediators that exhibit lower inflammatory potential. Consistent with previous studies (Jouris et al., Citation2011; Tartibian et al., Citation2009), in the present study we report no effect of FO supplementation on systemic inflammation, as assessed by the indirect measurement of thigh volume during exercise recovery. Interestingly, Mickleborough et al. (Citation2015) observed no effect of marine-oil lipid supplementation on limb girth, however serum TNF-α concentrations were attenuated for 92 h following EIMD. Hence, while the present study indicates FO supplementation failed to modulate the inflammatory response to EIMD, this observation may be explained by a lack of sensitivity and large variability associated with limb volume measurements as a surrogate marker of inflammation. Moreover, the anti-inflammatory effects of Ω3-PUFA may be offset by a background diet that is rich in Ω6-PUFA given that linoleic acid (Ω6-PUFA) and alpha linolenic acid (Ω3-PUFA) are both metabolised by delta-5-desaturase and delta-6-desaturase (Patterson et al., Citation2012). Hence, any benefit of FO supplementation in dampening the inflammatory response to EIMD may be dependent on the maintenance of a low Ω6-PUFA diet which was not controlled in the present study. The UK population typically consumes an Ω6-PUFA-to-Ω3-PUFA ratio of ∼15-16:1 that exceeds the optimal 1:1 ratio (Simopoulos, Citation2006). Hence, future studies that investigate the impact of FO supplementation on muscle recovery following EIMD should also take into consideration dietary Ω6-PUFA levels.
Recent evidence suggests that nutrient-nutrient interactions are a key factor in determining the efficacy of a nutritional intervention, such as Ω3-PUFA, to promote exercise recovery. Using inferential statistics, Black et al. (Citation2018) observed a very likely beneficial effect of adding FO to a protein-based supplement on muscle soreness that translated into the better maintenance of explosive power in elite rugby players. In the present study, we administered FO-derived Ω3-PUFA in isolated capsules, without the co-ingestion of protein, leucine, or carbohydrate and reported no definitive differences in muscle damage, inflammation, perceived soreness or muscle function between conditions. FO supplementation has previously been shown to activate the mTORC cascade in response to amino acid provision, but not under basal conditions (Smith et al., Citation2011). Thus, it is conceivable that combining Ω3-PUFA with an amino acid source elicits an increased stimulation of mTORC and facilitates the muscle remodelling process following eccentric exercise. Collectively, these studies suggest that FO supplementation may be an effective nutritional strategy to facilitate exercise recovery when consumed alongside a protein and/or amino acid source.
Four principal study limitations should be acknowledged. First, our assessment of muscle damage was based on a single, indirect, biomarker of serum CK concentrations. Due to financial constraints, we did not verify our muscle damage findings with the histological assessment of Z-band streaming. Second, we were unable to directly assess the incorporation of Ω3-PUFA into the muscle phospholipid bilayer following supplementation. We did not obtain muscle biopsies to directly measure muscle cell (or muscle phospholipid bilayer) Ω3-PUFA content due to the potential confounding effect of this invasive procedure on local (muscle damage) and systemic (inflammation) endpoint measurements. Third, the relatively low participant number (n = 16, 8 per group) may have resulted in a lack of statistical power, particularly for less sensitive subjective measurements (i.e. muscle soreness). However, the conduct of post hoc power calculations (GPower version 3.1.9.7) on CK and MVC (knee extension) data sets revealed a 1-ß error probability of 0.5 and 0.6, respectively, that infers a moderate statistical power was achieved in the present study. Finally, while subjects were asked not to consume oily fish during the trial period, no nutritional assessment of omega-3 intake was recorded.
Conclusion
To conclude, in this small-scale laboratory-controlled study, 4-wk of high dose (5 g/d) FO supplementation conferred no clear benefit to physiological indices of muscle damage or muscle repair following eccentric exercise in moderately trained young men. Hence, our preliminary data do not experientially support the practical recommendation for athletic populations to undertake FO supplementation to promote exercise recovery from muscle damaging exercise, at least when administered in isolation rather than in combination with protein or amino acids. Instead, moderately-trained individuals may consider prioritising other evidence-based nutritional interventions to facilitate exercise recovery.
Authors contributions
The study was designed by SDRG and OCW. Data collection was conducted by JM, LM, CC & OCW. Data were analysed by JM, EB & OCW. The manuscript was written by JM, EB and OCW. All authors read and approved the final manuscript.
Ethics approval
The study was approved by the School of Sport Research Ethics Committee (SSREC) at the University of Stirling and the East of Scotland NHS Research Ethics Committee (NHSREC).
Consent to participate
Written informed consent was obtained by all participants prior to data collection.
Consent for publication
All co-authors approved the final version of this manuscript.
Supplemental Material
Download Zip (140.6 KB)Acknowledgements
The authors would like to thank the study participants for their time and dedication to the study. This study received no external funding.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Allen, D. G. (2001). Eccentric muscle damage: mechanisms of early reduction of force. Acta Physiol Scand, 171(3), 311–319.
- Anthony, R., Macartney, M. J., & Peoples, G. E. (2021). The influence of long-chain omega-3 fatty acids on eccentric exercise-induced delayed muscle soreness: Reported outcomes are compromised by study design issues. International Journal of Sport Nutrition and Exercise Metabolism, 31(2), 143–153. https://doi.org/10.1123/ijsnem.2020-0238
- Baird, M. F., Graham, S. M., Baker, J. S., & Bickerstaff, G. F. (2012). Creatine-kinase- and exercise-related muscle damage implications for muscle performance and recovery. Journal of Nutrition and Metabolism, 1–13. https://doi.org/10.1155/2012/960363
- Black, K. E., Witard, O. C., Baker, D., Healey, P., Lewis, V., Tavares, F., Christensen, S., Pease, T., & Smith, B. (2018). Adding omega-3 fatty acids to a protein-based supplement during pre-season training results in reduced muscle soreness and the better maintenance of explosive power in professional Rugby Union players. European Journal of Sport Science, 18(10), 1357–1367. https://doi.org/10.1080/17461391.2018.1491626
- Bloomer, R. J., Larson, D. E., Fisher-Wellman, K. H., Galpin, A. J., & Schilling, B. K. (2009). Effect of eicosapentaenoic and docosahexaenoic acid on resting and exercise-induced inflammatory and oxidative stress biomarkers: A randomized, placebo controlled, cross-over study. Lipids in Health and Disease, 8(1), 36. https://doi.org/10.1186/1476-511X-8-36
- Calder, P. C. (2010). Omega-3 fatty acids and inflammatory processes. Nutrients, 2(3), 355–374. https://doi.org/10.3390/nu2030355
- Calder, P. C. (2017). Omega-3 fatty acids and inflammatory processes: From molecules to man. Biochemical Society Transactions, 45(5), 1105–1115. https://doi.org/10.1042/BST20160474
- Chytrova, G., Ying, Z., & Gomez-Pinilla, F. (2010). Exercise contributes to the effects of DHA dietary supplementation by acting on membrane-related synaptic systems. Brain Research, 1341, 32–40. https://doi.org/10.1016/j.brainres.2009.05.018
- DiLorenzo, F. M., Drager, C. J., & Rankin, J. W. (2014). Docosahexaenoic acid affects markers of inflammation and muscle damage after eccentric exercise. Journal of Strength and Conditioning Research, 28(10), 2768–2774. https://doi.org/10.1519/JSC.0000000000000617
- Engel, F. A., Faude, O., Kölling, S., Kellmann, M., & Donath, L. (2019). Verbal encouragement and between-day reliability during high-intensity functional strength and endurance performance testing. Frontiers in Physiology, 10, https://doi.org/10.3389/fphys.2019.00460
- Gray, P., Chappell, A., Jenkinson, A. M., Thies, F., & Gray, S. R. (2014). Fish oil supplementation reduces markers of oxidative stress but not muscle soreness after eccentric exercise. International Journal of Sport Nutrition and Exercise Metabolism, 24(2), 206–214. https://doi.org/10.1123/ijsnem.2013-0081
- Jakeman, J. R., Lambrick, D. M., Wooley, B., Babraj, J. A., & Faulkner, J. A. (2017). Effect of an acute dose of omega-3 fish oil following exercise-induced muscle damage. European Journal of Applied Physiology, 117(3), 575–582. https://doi.org/10.1007/s00421-017-3543-y
- Jeromson, S., Gallagher, I., Galloway, S., & Hamilton, D. (2015). Omega-3 fatty acids and skeletal muscle health. Marine Drugs, 13(11), 6977–7004. https://doi.org/10.3390/md13116977
- Jouris, K. B., McDaniel, J. L., & Weiss, E. P. (2011). The effect of omega-3 fatty acid supplementation on the inflammatory response to eccentric strength exercise. Journal of Sports Science & Medicine, 10(3), 432–438. https://pubmed.ncbi.nlm.nih.gov/24150614/
- Kennedy, P., Macgregor, L. J., Barnhill, E., Johnson, C. L., Perrins, M., Hunter, A., Brown, C., van Beek, E. J. R., & Roberts, N. (2017). MR elastography measurement of the effect of passive warmup prior to eccentric exercise on thigh muscle mechanical properties. Journal of Magnetic Resonance Imaging, 46(4), 1115–1127. https://doi.org/10.1002/jmri.25642
- Kyriakidou, Y., Wood, C., Ferrier, C., Dolci, A., & Elliott, B. (2021). The effect of Omega-3 polyunsaturated fatty acid supplementation on exercise-induced muscle damage. Journal of the International Society of Sports Nutrition, 18(1), 9. https://doi.org/10.1186/s12970-020-00405-1
- Macpherson, P. C., Schork, M. A., & Faulkner, J. A. (1996). Contraction-induced injury to single fiber segments from fast and slow muscles of rats by single stretches. American Journal of Physiology-Cell Physiology, 271(5), C1438–C1446. https://doi.org/10.1152/ajpcell.1996.271.5.C1438
- McGlory, C., Galloway, S. D. R., Hamilton, D. L., McClintock, C., Breen, L., Dick, J. R., Bell, J. G., & Tipton, K. D. (2014). Temporal changes in human skeletal muscle and blood lipid composition with fish oil supplementation. Prostaglandins, Leukotrienes and Essential Fatty Acids, 90(6), 199–206. https://doi.org/10.1016/j.plefa.2014.03.001
- Mickleborough, T. D., Sinex, J. A., Platt, D., Chapman, R. F., & Hirt, M. (2015). The effects PCSO-524®, a patented marine oil lipid and omega-3 PUFA blend derived from the New Zealand green lipped mussel (Perna canaliculus), on indirect markers of muscle damage and inflammation after muscle damaging exercise in untrained men: A randomized, placebo controlled trial. Journal of the International Society of Sports Nutrition, 12, https://doi.org/10.1186/s12970-015-0073-z
- Owens, D. J., Twist, C., Cobley, J. N., Howatson, G., & Close, G. L. (2019). Exercise-induced muscle damage: What is it, what causes it and what are the nutritional solutions? European Journal of Sport Science, 19(1), 71–85. https://doi.org/10.1080/17461391.2018.1505957
- Paschalis, V., Koutedakis, Y., Baltzopoulos, V., Mougios, V., Jamurtas, A. Z., & Giakas, G. (2005). Short vs. long length of rectus femoris during eccentric exercise in relation to muscle damage in healthy males. Clinical Biomechanics, 20(6), 617–622. https://doi.org/10.1016/j.clinbiomech.2005.02.011
- Patterson, E., Wall, R., Fitzgerald, G. F., Ross, R. P., & Stanton, C. (2012). Health implications of high dietary omega-6 polyunsaturated fatty acids. Journal of Nutrition and Metabolism, 2012, 1–16. https://doi.org/10.1155/2012/539426
- Paulsen, G., Mikkelsen, U. R., Raastad, T., & Peake, J. M. (2012). Leucocytes, cytokines and satellite cells: What role do they play in muscle damage and regeneration following eccentric exercise? Exercise Immunology Review, 18, 42–97. https://pubmed.ncbi.nlm.nih.gov/22876722/
- Peake, J. M., Neubauer, O., Della Gatta, P. A., & Nosaka, K. (2017). Muscle damage and inflammation during recovery from exercise. Journal of Applied Physiology, 122(3), 559–570. https://doi.org/10.1152/japplphysiol.00971.2016
- Philpott, J. D., Donnelly, C., Walshe, I. H., MacKinley, E. E., Dick, J., Galloway, S. D. R., Tipton, K. D., & Witard, O. C. (2018). Adding fish oil to whey protein, leucine, and carbohydrate over a six-week supplementation period attenuates muscle soreness following eccentric exercise in competitive soccer players. International Journal of Sport Nutrition and Exercise Metabolism, 28(1), 26–36. https://doi.org/10.1123/ijsnem.2017-0161
- Proske, U., & Morgan, D. L. (2001). Muscle damage from eccentric exercise: Mechanism, mechanical signs, adaptation and clinical applications. The Journal of Physiology, 537(2), 333–345. https://doi.org/10.1111/j.1469-7793.2001.00333.x
- Rawson, E. S., Miles, M. P., & Larson-Meyer, D. E. (2018). Dietary supplements for health, adaptation, and recovery in athletes. International Journal of Sport Nutrition and Exercise Metabolism, 28(2), 188–199. https://doi.org/10.1123/ijsnem.2017-0340. Epub 2018 Feb 19.
- Rodenburg, J. B., Bar, P. R., & De Boer, R. W. (1993). Relations between muscle soreness and biochemical and functional outcomes of eccentric exercise. Journal of Applied Physiology, 74(6), 2976–2983. https://doi.org/10.1152/jappl.1993.74.6.2976
- Simopoulos, A. P. (2006). Evolutionary aspects of diet, the omega-6/omega-3 ratio and genetic variation: Nutritional implications for chronic diseases. Biomedicine & Pharmacotherapy, 60(9), 502–507. https://doi.org/10.1016/j.biopha.2006.07.080
- Smith, G. I., Atherton, P., Reeds, D. N., Mohammed, B. S., Rankin, D., Rennie, M. J., & Mittendorfer, B. (2011). Omega-3 polyunsaturated fatty acids augment the muscle protein anabolic response to hyperinsulinaemia–hyperaminoacidaemia in healthy young and middle-aged men and women. Clinical Science, 121(6), 267–278. https://doi.org/10.1042/CS20100597
- Stupka, N., Tarnopolsky, M. A., Yardley, N. J., & Phillips, S. M. (2001). Cellular adaptation to repeated eccentric exercise-induced muscle damage. Journal of Applied Physiology, 91(4), 1669–1678. https://doi.org/10.1152/jappl.2001.91.4.1669
- Tartibian, B., Maleki, B. H., & Abbasi, A. (2009). The effects of ingestion of omega-3 fatty acids on perceived pain and external symptoms of delayed onset muscle soreness in untrained men. Clinical Journal of Sport Medicine, 19(2), 115–119. https://doi.org/10.1097/JSM.0b013e31819b51b3
- Tsuchiya, Y., Yanagimoto, K., Nakazato, K., Hayamizu, K., & Ochi, E. (2016). Eicosapentaenoic and docosahexaenoic acids-rich fish oil supplementation attenuates strength loss and limited joint range of motion after eccentric contractions: A randomized, double-blind, placebo-controlled, parallel-group trial. European Journal of Applied Physiology, 116(6), 1179–1188. https://doi.org/10.1007/s00421-016-3373-3
- Tsuchiya, Y., Yanagimoto, K., Ueda, H., & Ochi, E. (2019). Supplementation of eicosapentaenoic acid-rich fish oil attenuates muscle stiffness after eccentric contractions of human elbow flexors. Journal of the International Society of Sports Nutrition, 16(1), https://doi.org/10.1186/s12970-019-0283-x
- VanDusseldorp, T. A., Escobar, K. A., Johnson, K. E., Stratton, M. T., Moriarty, T., Kerksick, C. M., Mangine, G. T., Holmes, A. J., Lee, M., Endito, M. R., & Mermier, C. M. (2020). Impact of varying dosages of fish oil on recovery and soreness following eccentric exercise. Nutrients, 12(8), 2246. https://doi.org/10.3390/nu12082246
- Visconti, L. M., Cotter, J. A., Schick, E. E., Daniels, N., Viray, F. E., Purcell, C. A., Brotman, C. B. R., Ruhman, K. E., & Escobar, K. A. (2021). Impact of varying doses of omega-3 supplementation on muscle damage and recovery after eccentric resistance exercise. Metabolism Open, 12, 100133. https://doi.org/10.1016/j.metop.2021.100133
- Wolfe, R. R., & Chinkes, D. L. (2005). Isotope tracers in metabolic research: Principles and practice of kinetic analysis (2nd ed.). Wiley-Liss.
- Xin, G., & Eshaghi, H. (2021). Effect of omega-3 fatty acids supplementation on indirect blood markers of exercise-induced muscle damage: Systematic review and meta-analysis of randomized controlled trials. Food Science & Nutrition, 9, 6429–6442. https://doi.org/10.1002/fsn3.2598