ABSTRACT
We determined the effects of topically applied (i) isolated menthol cream, (ii) menthol and capsaicin co-application or (iii) placebo cream on exercise tolerance, thermal perception, pain, attentional focus and thermoregulation during exercise in the heat. Ten participants cycled at 70% maximal power output until exhaustion in 35°C and 20% relative humidity after application of (i) 5% isolated menthol, (ii) 5% menthol and 0.025% capsaicin co-application or (iii) placebo cream. Thermo-physiological responses were measured during exercise, with attentional focus and pain determined post-exercise on a 0-to-10 scale. Across the three conditions, time to exhaustion was 13.4 ± 4.8 min, mean ± SD infrared tympanic and skin temperature was 37.2 ± 0.6°C and 35.1 ± 1.2°C, respectively, and heart rate was 152 ± 47 bpm, with no changes between conditions (p > 0.05). Perceived exertion was lower in the isolated menthol vs. all other conditions (p < 0.05, ηp2 = 0.44). Thermal sensation was higher in menthol-capsaicin co-application vs. isolated menthol (p < 0.05, d = 1.1), while sweat rate was higher for capsaicin and menthol co-application compared to menthol (p < 0.05, d = 0.85). The median and interquartile range scores for pain were lower (p < 0.05) in the menthol condition (8, 7-8) compared to both menthol and capsaicin (10, 9-10) and placebo (9, 9-10), which was coupled with a greater distraction (p < 0.05) in the menthol condition (9, 7-10) compared to placebo (6, 5-7). Despite no performance effects for any topical cream application condition, these data reiterate the advantageous perceptual and analgesic role of menthol application and demonstrate no advantage of co-application with capsaicin.
Highlights
Topical application of isolated menthol cream to cold-sensitive areas of the body during exhaustive exercise in the heat, elicited reduced perception of pain and enhanced sensation of cooling.
While this reduction in generally unpleasant feelings (i.e. pain and heat) were coupled with lower RPE scores in the menthol condition and could be considered beneficial, there was no apparent ergogenic effect in an exercise tolerance test.
Co-application of capsaicin and menthol appeared to inhibit the positive sensory effects elicited by menthol.
Isolated menthol can induce changes in cognitive processes related to pain and exertion, while also reducing thermal sensation; however, the decision to use menthol creams must be balanced with the limited performance or thermoregulatory effects reported herein during exercise in hot environments.
Introduction
Menthol is an organic plant compound, which elicits a cooling stimulus in-vivo by binding to transient receptor potential melastatin (TRPM8) cation channel receptors on sensory nerve endings that are located in the skin and oral cavity (Bautista et al., Citation2007). Since these receptors are typically transduced by cold stimuli (Peier et al., Citation2002), oral swilling or topical application confers a cooling effect and reliably elicits a reduction in thermal sensation when exercising in a hot environment (Jeffries & Waldron, Citation2019). This non-thermal cooling sensation appears to be beneficial in a hot environment, with corresponding increases in endurance exercise performance in the heat (Barwood et al., Citation2019; Flood et al., Citation2017; Jeffries et al., Citation2018; Mündel & Jones, Citation2010; Riera et al., Citation2014; Schlader et al., Citation2011). Oral administration of menthol has been reported to have negligible effects on thermo-physiological responses (Flood et al., Citation2017; Jeffries et al., Citation2018); however, some studies examining topical application of menthol have reported enhanced skin vasoconstriction (Kounalakis et al., Citation2010), attenuated cold-induced vasoconstriction (Wang et al., Citation2022), reduced sweating (Barwood et al., Citation2019; Kounalakis et al., Citation2010) or increased core temperature (Gillis et al., Citation2010, Citation2020; Kounalakis et al., Citation2010). Interestingly, despite some notable reports of improved performance following topical menthol application (Barwood et al., Citation2019; Schlader et al., Citation2011) the use of gels, sprays or creams have not been as consistently ergogenic or efficacious in reducing thermal sensation in comparison to oral swilling (Jeffries & Waldron, Citation2019), which might relate to the reported skin irritability (Gillis et al., Citation2010) or the proposed inhibitory effects on avenues of heat dissipation (Kounalakis et al., Citation2010).
We have previously postulated that menthol mouth-rinsing during exercise in the heat might act as a “distracting” stimulus (Jeffries et al., Citation2018), such that the exercising participants’ focus of attention is altered via the menthol-induced cooling sensation. This process could, theoretically, defer the deleterious, and often painful, sensations that might be elicited during exercise (Cook et al., Citation1997). Attentional focus could be driven away from the painful sensations of exercise to the cooling sensations of menthol, thereby creating a “distraction”. Distractions can occur both passively and actively, and some strategies have been reported to redirect attention from painful stimuli (see Brick et al., Citation2014 for review). This is a theoretically feasible mechanism, since the application of menthol can elicit a conscious perceptual change, which is capable of downregulating both thermal sensation and perceived exertion, thereby enhancing exercise tolerance in the heat (Flood et al., Citation2017; Schlader et al., Citation2011). In support of this, reduced pain and discomfort, alongside improved muscle force production, has been observed with topical menthol application (Fritz et al., Citation2020; Johar et al., Citation2012). These findings indicate the ability to elicit perceptual changes, which could feasibly be underpinned by altered cognitive processing. This is particularly important, based on the understanding that external focus of attention can offset fatiguability or enhance performance (Lohse & Sherwood, Citation2011), and that dissociative thoughts during a task might act to distract attention from the painful and unpleasant sensations associated with exhaustive exercise (Baden et al., Citation2004). Manipulation of afferent sensory cues, including pain pathways, is an established way of delaying trait fatiguability (Enoka et al., Citation2021) and, ultimately, offsetting the perceived disruption in whole-body homeostasis that is apparent during arduous physical tasks in extreme environments (St Clair Gibson et al., Citation2018).
Capsaicin, also a plant-derived compound and found in most chilli peppers, binds to TRP vanilloid 1 (TRPV1) receptors, which are also found in the mouth and on the skin surface (Caterina, Citation2007). Transduction of these channels creates a spicy or burning sensation, which can lead to irritation or distraction once applied (Yang & Zheng, Citation2017). Whilst capsaicin (Starowicz et al., Citation2007; Vyklický et al., Citation2008) and indeed menthol (Pergolizzi et al., Citation2018), have been primarily used for analgesic purposes, oral ingestion of capsaicin has also been reported to increase endurance time to exhaustion (de Freitas et al., Citation2018; de Freitas et al., Citation2019). However, the ergogenicity of topical capsaicin application is unclear, with Schlader et al. (Citation2011) reporting no ergogenic effect during a perceptually self-regulated time to exhaustion in a temperate environment (∼20°C) following facial 0.025% capsaicin cream application, compared to placebo or 8% menthol conditions. The capsaicin creams also elicited a warmer facial sensation and discomfort. On the other hand, Botonis et al. (Citation2019) found that application of capsaicin cream to four thermally sensitive areas of the body enhanced thermoregulatory processes, such as sweat rate and onset, and skin vasodilation, thereby extending time to reach a set core temperature during cycling exercise. The reasons for these mixed findings are uncertain but, given the sensory adaptations that occur secondary to both capsaicin and menthol application to the skin, it is feasible that their effects on the spinothalamic (i.e. temperature and pain) sensory pathways partly explain their ergogenic effect.
On the basis of early thermal grill experiments (Thunberg, Citation1896), it is understood that simultaneous application of combined innocuous hot and cold stimuli can create illusionary hot pain. More recently, the related concept of hot-cold confusion has been recognised, which is characterised by an inverse thermal sensation (a misperception of temperature loci) when the limb’s skin surface is presented with concurrent opposing temperature stimuli (Arai et al., Citation2021). In these experiments, Arai and colleagues demonstrated how hot-cold confusion is often coupled with increased pain perception vs. singular hot or cold exposure. The induction of an acute pain response is sometimes necessary to elicit analgesic effects, which is the therapeutic basis of menthol or capsaicin creams, with analgesia occurring secondary to nociceptor defunctionalisation (Anand & Bley, Citation2011; Pergolizzi et al., Citation2018). To date, there has been no study of combined topical creams eliciting both hot and cold sensations in thermally cold-sensitive (i.e. trunk, face & thigh; Luo et al., Citation2020) anatomical locations during exhaustive exercise, including the evaluation of the potential effects on thermal sensation, pain perception, attentional focus and thermoregulatory processes during exhaustive exercise in hot environments. We theorised that the concurrent mixed hot-cold stimuli would potentially enhance the analgesic and distractor effects during exercise. However, it is currently unclear whether the sensations elicited during exercise using isolated or combined topical creams are capable of inducing analgesic effects, and if these are facilitative or debilitative to selected cognitive processes underpinning physical performance in the heat. We conducted a study to determine the effects of topically applied (i) isolated menthol cream, (ii) menthol and capsaicin co-application or (iii) placebo cream on the above factors during exercise in the heat. It was hypothesised that both isolated menthol and co-applied creams (menthol and capsaicin) would enhance time to exhaustion vs. the placebo condition and that this would be associated with greater distraction, reduced pain and reduced internal sensory monitoring (i.e. inward association). Based on the equivocal findings, to date, it was unclear whether the creams would influence thermoregulatory processes.
Methods
Study design
A randomised, counter-balanced, placebo-controlled, cross-over design was used to investigate the effects of topical application of menthol and combined menthol capsaicin creams on exercise tolerance, thermal perception, pain, attentional focus and thermoregulation during exhaustive exercise in the heat. An online random number generating software (Urbaniack & Plous, Citation2015) was used to randomise participant conditions. The study consisted of four laboratory visits, with 72-h between each. The first visit consisted of a preliminary incremental ramp test to determine maximal power output on a cycle ergometer (Wmax) in a heat chamber (35 ± 1°C; 20 ± 1% relative humidity, RH). This temperature and humidity were selected for the duration of the study to elicit sufficient heat stress, whilst permitting capacity for evaporative heat exchange via widening of the vapour pressure gradient. In the second, third and fourth visits, the participant conducted their full experimental cross-over trial in the heat chamber by completing a fixed- intensity, time to exhaustion protocol on a cycle ergometer (Monark Exercise AB, Ergomedic 874E, Varberg, Sweden) in one of the three conditions: (i) 5% isolated menthol cream, (ii) 5% menthol and 0.025% capsaicin co-application or (iii) aqueous placebo cream (no menthol or capsaicin). Thermo-physiological responses (heart rate, rating of perceived exertion [RPE], thermal sensation, infrared tympanic temperature [Tc] and mean skin temperature [Msk]) were measured before and during exercise, while attentional focus and pain responses were determined 10-min after exercise via questionnaire. The questions were based upon key attentional focus dimensions and the specific inclusion of a 0–10 pain scale (Brick et al., Citation2014).
Participants
Ten non-acclimated, healthy, and physically active participants (7 male, and 3 female; mean ± SD age = 21 ± 1 years; body mass = 73.2 ± 12.2 kg; Wmax = 255 ± 39 W) provided written, informed consent to take part in the current study. All participants conducted at least three endurance training sessions per week. A sample size of 6 people would be the minimum sample size required to detect an effect size change of f = 0.65 previously reported using menthol spray (Barwood et al., Citation2019) using analysis of variance with a power of 0.8 and alpha of 0.05. We recruited 10 people based on the uncertainty over co-applied topical applications. Participants were instructed to avoid alcohol consumption 48-h prior to any exercise in the study, as well as refraining from dietary supplementation use for the study duration. Participants arrived hydrated for each trial, which was encouraged via pre-trial fluid intake instruction and verified via urine osmolality measurements on arrival at the laboratory (urine osmolality ≤ 700 mOsmol/kg H2O, Osmocheck, Vitech Scientific Ltd, UK). The mean urine osmolality was (189 ± 70 mOsmol/kg H2O). Institutional ethical approval was provided for this research, which was conducted in accordance with the 2016 Helsinki declaration, except for pre-trial registration.
Data collection
Preliminary incremental ramp test
Participants were fitted with a heart rate monitor (Polar T31 HR Monitor) and seated on the cycle ergometer, with the preferred seat/handlebar settings noted for future trials. After a self-paced warm-up for 5-min, the ramp test was started at 60 W and 60 rev/min for all participants and increased by 18 W/min until volitional exhaustion. The test was terminated if there was a continuous drop < 60 rev/min. Criteria for reaching a maximal test were the participant reaching their estimated maximum age-defined heart rate or 20 on the 6–20 RPE scale (Borg, Citation1982). The Wmax was determined as end-power output in the final 30-s of the test using the equation: Wmax = last completed stage (W) + (time of incomplete stage (min) x step increment (W)). This was then used to determine 70%Wmax for the subsequent experimental trials.
Experimental procedure
Participants were asked to arrive at the laboratory hydrated and rested. A urine osmolality test was conducted each day, with the same criteria for euhydration applied (≤ 700 mOsmol/kg). Participants’ pre-exercise body mass was recorded (Seca 711, Hamburg, Germany) in underwear, after which cycling shorts, sports bra (for females), socks and trainers were worn for the main trial. Participants were then fitted with the same heart rate monitor and skin thermistors, which were secured using micropore tape in the locations: dorsal right middle finger, ventral right forearm, right pectoralis major muscle belly, anterior right thigh, lateral right calf. Skin thermistors were connected to a data logger (Squirrel OM-SQ10, Grant instruments, Cambridge, UK) and weighted mean skin-temperature (Msk) was determined using a four-site equation (Ramanathan, Citation1964). The fifth thermistor was added on the middle finger to monitor the forearm to finger gradient, as an indication of vasomotor tone, with higher values indicating increased vasoconstriction and lower values vasodilation (House & Tipton, Citation2002). The creams were administered to the participants’ skin, prior to entering the heat chamber (35 ± 1°C; 20 ± 6% RH), where resting heart rate, Tc and Msk were recorded. An index of core temperature was measured using an infrared tympanic thermometer (Radiant Tympanic Ear Thermometer, TH889J, Carey Medical, Tredegar, UK). The tip of the thermometer was placed in the left ear for 5-s before recording the reading, with the middle value of three readings taken as the final measurement. The tympanic devices in our laboratory underestimate rectal temperature by 0.8 ± 0.3°C but correlate strongly (R2 = 0.92) while cycling at exercise intensities between 30% and 70% maximal oxygen uptake in environmental temperatures of between 30°C and 35°C at a fixed relative humidity of 40%. We, therefore, consider the infrared tympanic measures to underestimate core temperature but provide a valid index of core temperature change. These data are supported by correlation coefficients ranging from 0.74–0.9, reported among varying populations and during exercise in the heat (Chu & Burnham, Citation1995; Duru et al., Citation2012; Fenemor et al., Citation2020; Towey et al., Citation2017). Furthermore, the test re-test reliability of this tympanic technique within-session is 0.3% (coefficient of variation).
Participants mounted the ergometer at their previously recorded settings and performed exercise at 70% Wmax (179 ± 27 W) at the same cadence as the incremental test (60 rev/min). Two fans were placed directly in front (∼ 1 m) and to their right/rear-hand side (∼ 1 m) to generate an air flow of ∼1 m/s to help convectively cool and mimic an outdoor breeze (Stevens et al., Citation2016). Participants were instructed to exercise to exhaustion by maintaining the set power output for as long as possible, which was determined by a continuous (≥ 20-s) failure to maintain 60 rev/min. The end result was recorded using an automated timer. The participants could not see their elapsed time or feedback of their physiological signals, such as heart rate but could see power output and cadence. The reliability of this protocol in our laboratory (unpublished laboratory data) with recreationally-trained participants is 3.8% (coefficient of variation). The participants’ Msk was measured continuously, while the Tc and heart rate were measured every 5-min. Participants’ body mass was recorded immediately after exiting the chamber and undressing to their underwear, prior to any consumption of fluid, without towel-drying. The change in pre-to-post body mass (kg) was used as an indicator of whole-body sweat loss (WBSL; i.e. kg = L). A total of three identical experimental trials took place in a randomised order for each participant.
Menthol and capsaicin topical cream application
Three different creams were used in this study on three separate visits. Creams were applied 20-min pre-exercise to four thermally cold-sensitive areas of the body: forehead, left pectoralis major, right trapezius, right vastus lateralis (Botonis et al., Citation2019; Luo et al., Citation2020). The precise placements were selected to avoid skin thermistors and adopt the three anatomical positions (plus forehead) reported previously using capsaicin cream during exercise (Botonis et al., Citation2019), whilst maintaining their position in an established cold-sensitive region (Luo et al., Citation2020). The cream was applied to a 25 cm2 area in all locations, excluding the forehead, where a reduced area (16 cm2) was used. Based upon pilot work, 1 g of cream was applied to the forehead, 2 g to the left pectoralis major and 3 g to the right trapezius and right vastus lateralis. The same volume and surface area of cream for each location remained constant in each trial. The volume of cream used (0.32–0.48 g/100 cm2) corresponds to that previously used, which was 0.3 g/100 cm2 (Schlader et al., Citation2011). The menthol cream applied (Dermacool, Pern Consumer Products Ltd., County Durham, UK) was 5% concentration (Jeffries & Waldron, Citation2019), and the capsaicin cream (Rugby Laboratories, Inc. Duluth, Georgia) was 0.025% concentration (Schlader et al., Citation2011). For the co-applied condition, the creams were volume-matched in a 1:1 ratio of menthol and capsaicin (i.e. 0.5 g menthol + 0.5 g capsaicin) and applied to the skin in equally sized (50%/50%) separated sections of the marked area. A non-fragranced aqueous moisturiser was used for the placebo trial (E45 Cream, Crookes Healthcare, Nottingham, UK).
Perceived exertion and focus
A 6–20 point scale (Borg, Citation1982) and a ASHRAE 7-point thermal sensation scale (−3 to +3; ASHRAE, Citation1968) were used to measure RPE and thermal sensation, respectively. These measures were recorded at rest and every 5-min during the trial. An Attentional Focus Rating Scale, in the form of a written questionnaire, was administered 10-min post-exercise. This was based upon key attentional focus dimensions, as well as the specific inclusion of a 0–10 pain scale (Brick et al., Citation2014). In brief, five questions were posed to participants relating to the frequency of their reliance (0–10 scale, with 10 being the most) upon one of the four attentional focus dimensions during exercise: “Active self-regulation”, “Internal sensory monitoring”, “Outward monitoring” and “Distraction”. Examples of thoughts in each dimension were provided to the participants based on previous suggestions (Brick et al., Citation2014). In addition, participants were asked for their general pain response, which was accompanied by a working definition prior to the trial an unpleasant sensory and emotional experience associated with the exertion of the exercise task (Norbury et al., Citation2022). Participants were given time with the questionnaire, full verbal instructions and the opportunity to practice with the questions prior to the trial days.
Statistical analysis
All statistical analyses were completed in SPSS (IBM SPSS statistics Inc, USA). A two-way analysis of variance (ANOVA) was used to determine the effects of the condition (3 conditions), across stages of time on the dependent variables. Stages of time were categorised into rest, 3-min, 6-min and end-point. Greenhouse-Geisser corrections were applied if sphericity was violated. Time to exhaustion, whole-body sweat losses were analysed using a one-way ANOVA, while attentional focus and pain responses were analysed using a Friedman test. Effect sizes are described using partial eta squared (ηp2). Significant differences were accepted at p < 0.05 and parametric data are presented as mean ± standard deviation, while non-parametric data are presented as median and interquartile ranges. When a significant difference was found for main or interaction effects, post-hoc pair-wise comparisons were made incorporating a Bonferroni adjustment. For non-parametric data, a Wilcoxon test was used to determine pairwise differences following the Friedman test. Cohen’s effects sizes (d) were used to describe the magnitude of pairwise differences.
Results
Time to exhaustion and thermo-physiological responses
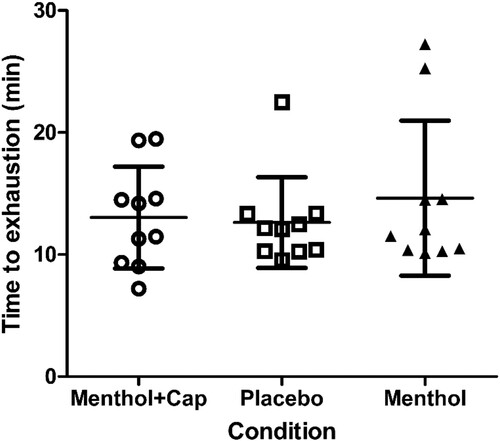
There were no differences in time to exhaustion between the conditions (F(2,18) = 1.3, p = 0.286, ηp2 = 0.13; ) and no trial order effects were found (F(2,18) = 0.5, p = 0.552, ηp2 = 0.053).
Figure 1. Time to exhaustion (mean ± SD; min) during fixed-intensity cycling at 70% maximal incremental test power across three topical cream applications of menthol and capsaicin (Menthol + Cap), placebo and menthol (n = 10).
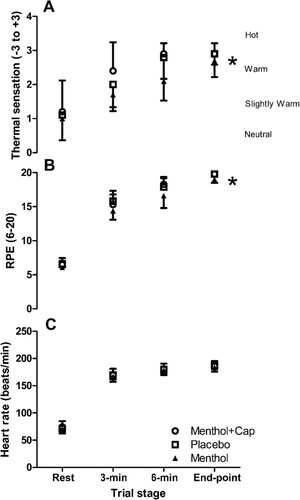
There were no main condition or interaction effects found for the majority of thermo-physiological measures recorded during rest time to exhaustion (p > 0.05), including Tc, Msk, Arm to finger differential and heart rate ( and ). However, there was a main effect of condition for RPE (F(2,18) = 6.98, p = 0.006, ηp2 = 0.44; ), with post-hoc tests demonstrating lower values in the isolated menthol vs. menthol and capsaicin co-application (p = 0.015; d = 1.2) and placebo (p = 0.049, d = 1.6). A main condition effect was also found for thermal sensation (F(2,18) = 4.74, p = 0.022, ηp2 = 0.35; ), with higher values in menthol and capsaicin co-application vs. isolated menthol (p = 0.035, d = 1.1), but no other pairwise differences found (p > 0.05). There were also condition effects for WBSL (F(2,18) = 5.45, p = 0.014, ηp2 = 0.38), with a higher volume lost (p = 0.032, d = 0.1) in the capsaicin and menthol co-application (353 ± 111 mL) compared to the placebo (283 ± 98 mL) condition only. WBSL in the isolated menthol condition was 294 ± 85 mL. When WBSL was expressed as a rate (mL/min), there were also condition effects (F(2,18) = 3.07, p = 0.071, ηp2 = 0.25), which was explained by the higher sweating rates in the menthol and capsaicin co-application compared to menthol alone (28.2 ± 8.9 mL/min vs. 21.9 ± 8.2 mL/min, respectively; p = 0.043, d = 0.85).
Figure 2. Arm to finger temperature differences (A), mean skin (Msk) temperature (B) and Core temperature (C) (mean ± SD) across rest, 3-min, 6-min & end-point of time to exhaustion cycling at 70% maximal incremental test power across three topical cream applications of menthol and capsaicin (Menthol + Cap), placebo and menthol (n = 10).
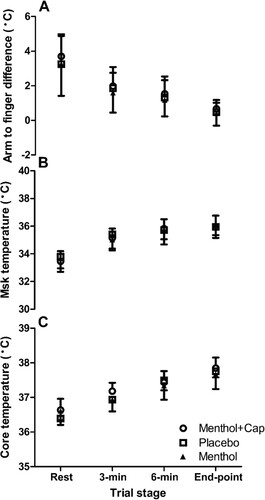
Figure 3. Thermal sensation (A), rating of perceived exertion (RPE), and heart rate (C) (mean ± SD) rest, 3-min, 6-min & end-point of time to exhaustion cycling at 70% maximal incremental test power across three topical cream applications of menthol and capsaicin (Menthol + Cap), placebo and menthol (n = 10). * = condition effect and pairwise difference between menthol + capsaicin vs. menthol for thermal sensation (p < 0.05). In the RPE condition, * = differences between menthol and all other conditions.
Attentional focus and pain responses
There were condition effects for pain scores (χ2 (2) = 16.7, p < 0.001), with post-hoc tests demonstrating a lower score for the menthol condition compared to both menthol and capsaicin (p < 0.007) and placebo (p = 0.006, ).
Table 1. Post-exercise perceptions of pain and attentional focus (median and interquartile range and total range) across three topical cream applications of menthol and capsaicin, placebo and menthol (n = 10).
Active self-regulation was also different between groups (χ2 (2) = 7.2, p = 0.027); with post-hoc testing demonstrating lower values in the menthol and capsaicin condition compared to both placebo (p = 0.024) and menthol (p = 0.024, ).
There were no condition effects for internal sensory monitoring (χ2 (2) = 2.1, p = 0.349) or outward monitoring (χ2 (2) = 5.7, p = 0.058) but there were for distraction (χ2 (2) = 6.5, p = 0.039), with higher values in the menthol condition compared to placebo only (p = 0.012, ).
Discussion
The current study investigated the effects of isolated topical menthol and menthol-capsaicin co-application on exercise tolerance, thermal sensation, pain perception, attentional focus and thermoregulatory processes during exhaustive exercise in hot environments. The main findings rejected the primary experimental hypotheses, with no changes in time to exhaustion between conditions, despite descriptively longer trials following isolated menthol cream application (Placebo 12.6 ± 3.7 min; Menthol 14.6 ± 6.4 min; Menthol-Capsaicin co-application 13.0 ± 4.2 min). There were also limited effects on thermo-physiological processes, including heart rate, and body temperature measures. However, in partial support of our hypotheses, an enhanced sensation of cooling and lower RPE was found in the isolated menthol condition, which was accompanied by a lower general pain response compared to both the placebo and menthol-capsaicin co-application. Whilst there were no significant pairwise effects for outward monitoring or internal sensory responses between conditions, distraction was higher in the menthol condition compared to placebo, while active self-regulation was lower following menthol-capsaicin co-application.
Perhaps the most important finding of the current study was the decreased pain response, alongside reduced thermal sensation in the menthol condition but not in the co-application cream condition. Whilst the relationship between pain and thermal sensation was not directly investigated here, it is possible that these responses are coupled, or interact, when participants form their perceptual responses. That is; the feeling of being cooler might explain a reduction in general pain during exercise in the heat. In further support of these findings, the participants in the isolated menthol condition reported a greater frequency of distractions, which would naturally defer feelings of pain induced via muscle contraction or thermal strain, which emanate from internal sources. We had previously suggested a distractor effect using oral rinsing of menthol (Jeffries et al., Citation2018), but did not necessarily anticipate a concomitant analgesic effect. Surprisingly, the effects on pain and distraction did not translate to the dimension of outward monitoring; however, the minimisation of external performance feedback in the laboratory trial could explain the generally low scores in this category. These data indicate that when exercising in the heat under placebo conditions, pain responses were higher and distractions were lower, which can be offset by application of menthol creams in four locations; however, adding capsaicin to the skin abolishes this effect and restores the pain response. The analgesic effect of L-menthol is likely to be related to its interaction with TRPM8 channels, based on its known mediation of acute pain (Liu et al., Citation2013). Whilst the pain-relieving effect of topical L-menthol application has been described among those with musculoskeletal injuries (Taylor et al., Citation2012) or activities that result in muscle soreness (Fritz et al., Citation2020; Johar et al., Citation2012), this is the first to demonstrate a link with exercise in the heat.
The anecdotal feedback from participants during the current study supported the severity of the reported pain response when exposing the skin to capsaicin-menthol cream, especially in the context of thermal challenge. This could be exacerbated by hot-cold confusion, which is associated with an enhanced pain response (Arai et al., Citation2021). Unfortunately, we did not include an isolated capsaicin condition to clearly elucidate the responsible compounds. In contrast, the cooling sensations elicited by isolated menthol appeared to oppose the feelings associated with an immediate homeostatic challenge imposed in a thermally stressful environment. It has been suggested that the use of distraction, in the form of external “dissociative” strategies can be advantageous during exercise performed at lower intensities, typically encompassing manipulation of auditory or visual stimuli (Hutchinson & Sherman, Citation2014; Jones et al., Citation2014). Indeed, the term “distraction” has been eloquently described and incorporated into more recent models of attentional focus (Brick et al., Citation2014) and exists both inwardly and outwardly. Based on the current data, we speculate that the higher distraction scores in the menthol condition were the result of outward dissociative thoughts, caused by the cool stimulus, which might also have reduced emotional pain responses by occupying attentional resources (Brewer & Buman, Citation2006). This is further supported by greater self-regulatory strategies (an inward process) in the co-application condition, which were perhaps necessary in response to greater pain.
The difference in RPE between conditions was anticipated, based on reports using either oral (Flood et al., Citation2017) or topical menthol applications (Schlader et al., Citation2011); however, these RPE responses have not been consistently reported (i.e. Barwood et al., Citation2019). It is unclear why RPE is more responsive to topical menthol application in some studies, but differences in the type and timing of application are inconsistent (see Jeffries & Waldron, Citation2019) and might partly explain these findings. Interestingly, there were no differences in RPE (or any other perceptual scores) between placebo and capsaicin-menthol co-application conditions in the current study. Given that the co-application condition differed only from isolated menthol by the addition of capsaicin to the same anatomical sites, it appears that the hotter sensations inhibited the sensory effects elicited by menthol. It is also possible that the descriptively larger pain response in the co-application condition contributed to this. Indeed, the RPE responses in the isolated menthol condition found here also tended to correspond with the reduced thermal sensation and pain reported by participants. Collectively, these data denote a reduction in “unpleasant” feelings following isolated menthol application, such as pain and temperature perception, culminating in a reduced perception of exertion for the same power output. In the context of fatigue models, it could be argued that the reduced sensation of temperature and pain in the isolated menthol condition feeds the conscious attenuation of RPE, thus lowering the perceived threat to bodily homeostasis during exercise, despite no change in other competing cues, such as the environmental stress or exercise intensity (St Clair Gibson et al., Citation2018). In this instance, perhaps the most surprising result of the current study was the absence of a performance effect, since this should theoretically manifest following manipulation of sensory information. However, the descriptive, non-significant mean differences in time to exhaustion between isolated menthol and capsaicin-menthol co-application (∼11.6%) or placebo (∼14.5%) might explain this performance result. It is worthwhile recognising that two participants (one male one female) appeared to respond more favourably to the menthol cream, who were also two of the highest scoring for “distraction”. This might explain the reason for the higher ergogenic effect but there were no other notable individual features to provide a clear explanation for the responsiveness.
A variety of thermoregulatory effects have been reported following isolated capsaicin or menthol applications; however, the current study found a higher WBSL and sweat rate (i.e. sweating response) in the capsaicin-menthol co-application vs. placebo or menthol conditions, respectively. Based on the latent heat of vaporisation (2,426 J/g of sweat loss), this change in whole-body sweating equated to 28 mL/min (∼68 kJ/min of evaporative heat loss) in the co-application condition, as compared to the isolated menthol (21.9 mL/min or ∼53 kJ/min) and placebo conditions (24.4 mL/min or ∼59 kJ/min). Coupled with the progressive rise in Tc across all conditions, these values reflect the likely uncompensable nature of the hot exercise trials and, in the co-application condition, WBSL reached near-maximal values reported among athletes (Barnes et al., Citation2019). The approximate 15 kJ/min increase in evaporative heat loss compared to placebo caused by the addition of capsaicin is not negligible, indicating a significant thermal stimulus for sweating, despite application of only a small amount of low-concentration capsaicin cream. Such is the importance of evaporative heat exchange, in a longer exercise trial, the accumulation of these effects would, theoretically, result in later onset of thermal intolerance or a favourable shift in the threshold of compensability. Indeed, this notion was previously supported, where isolated capsaicin creams applied to similar anatomical areas enhanced the sweating response and improved thermal tolerance (Botonis et al., Citation2019). Therefore, the use of capsaicin with menthol cream appears to enhance at least one major thermoregulatory heat loss avenue (i.e. evaporative), yet evoked a negative change in thermal sensation. This divergent response between thermoregulatory and perceptual processes was previously noted in reference to menthol topical application in the form of a spray (Barwood et al., Citation2019). However, we did not observe a thermoregulatory change from placebo in the isolated menthol condition, but the thermal sensation responses were apparent and similar to those previously reported (Barwood et al., Citation2019). Any divergence effect caused by application of these compounds to the skin could be considered as a potential risk, and not necessarily advantageous in real-world settings (i.e. sport, recreational or occupational), since it may fail to alert individuals to their true level of thermal strain in a timely manner. Therefore, from a practical perspective, the totality of data must be considered, with the decision to use capsaicin creams in hot conditions residing upon the balance of thermoregulatory enhancement vs. perceptual diminishment. The use of more prolonged exercise trials might be necessary in future studies to improve the current understanding of this.
In accordance with our above-reasoning, both the constraints of a controlled laboratory environment and the duration of the current trial limit the translation to a real-world setting and prevent full insight into underlying cognitive processes. For example, in an outdoor environment, there are a range of stimuli available, including variations in ambient conditions, changes in the visual field and many other contextual factors that will place greater demand on decision making and emotional control processes (Scanlon et al., Citation2017). Indeed, this will also logically increase the probability of participants experiencing distraction (of some form), particularly in competitive events with varying scenery and topography. Therefore, it is possible that the potential underlying cognitive processes and pain responses revealed herein are not the same in a more ecologically valid setting. Given the potential importance of the analgesic effects of isolated menthol reported here, it is worthwhile investigating this further in real-world settings. Similarly, the length of the trial was shorter than anticipated among the current participants and is likely to have placed less cognitive demand and, therefore, requirement to adopt coping strategies (Brick et al., Citation2014). A final limitation of the current study was the area covered by the co-applied vs. isolated creams, with the co-applied creams covering approximately half of the initial area. However, the alternative options would be to expand the area of the co-applied creams, which was not always possible (i.e. forehead), and would also access a greater number of skin receptors. Thus, while it is possible that the co-applied condition suffered from limiting the access of menthol cream to all skin areas and thereby weakening its effect, it is likely that the creams mixed and infiltrated larger areas following exercise or sweating onset. The current design was, therefore, most appropriate to answer the current research question.
Conclusion
The current study demonstrated that topical application of isolated menthol cream to thermally sensitive areas of the body during exhaustive exercise in the heat, elicited reduced perception of pain and enhanced sensation of cooling. While this reduction in generally unpleasant feelings (i.e. pain and heat) were coupled lower RPE scores in the menthol condition and could be considered beneficial, there was no apparent ergogenic effect in the exercise tolerance test. However, the co-application of capsaicin and menthol appeared to inhibit the positive sensory effects elicited by menthol. Although capsaicin potentially stimulated greater evaporative heat transfer due to an increased sweating response, this did not manifest in measurements of core (tympanic) or shell temperature. Thus, in trials of this duration, there is no physical, perceptual or cognitive benefit of co-applying menthol and capsaicin creams. These findings suggest that isolated menthol can induce potential changes in cognitive processes related to pain and exertion, while also reiterating its established role in reducing thermal sensation. However, the decision to use menthol creams to elicit seemingly beneficial perceptual or cognitive advantages must be balanced with the limited performance or thermoregulatory effects reported herein during exercise in hot environments.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- ASHRAE. (1968). Handbook of fundamentals. New York: American Society of Heating, Refrigerating and AirConditioning Engineers.
- Anand, P., & Bley, K. (2011). Topical capsaicin for pain management: Therapeutic potential and mechanisms of action of the new high-concentration capsaicin 8% patch. British Journal of Anaesthesia, 107(4), 490–502. https://doi.org/10.1093/bja/aer260
- Arai, K., Matsumuro, M., Hashiguchi, S., Shibata, F., & Kimura, A. (2021). Hot–cold confusion: Inverse thermal sensation when hot and cold stimuli coexist in a thermal localization task. Perception, 50(6), 508–523. https://doi.org/10.1177/03010066211004055
- Baden, D. A., Warwick-Evans, L., & Lakomy, J. (2004). Am I nearly there? The effect of anticipated running distance on perceived exertion and attentional focus. Journal of Sport and Exercise Psychology, 26(2), 215–231. https://doi.org/10.1123/jsep.26.2.215
- Barnes, K. A., Anderson, M. L., Stofan, J. R., Dalrymple, K. J., Reimel, A. J., Roberts, T. J., Randell, R. K., Ungaro, C. T., & Baker, L. B. (2019). Normative data for sweating rate, sweat sodium concentration, and sweat sodium loss in athletes: An update and analysis by sport. Journal of Sports Sciences, 37(20), 2356–2366. https://doi.org/10.1080/02640414.2019.1633159
- Barwood, M. J., Kupusarevic, J., & Goodall, S. (2019). Enhancement of exercise capacity in the heat with repeated menthol-spray application. International Journal of Sports Physiology and Performance, 14(5), 644–649. https://doi.org/10.1123/ijspp.2018-0561
- Bautista, D. M., Siemens, J., Glazer, J. M., Tsuruda, P. R., Basbaum, A. I., Stucky, C. L., Jordt, S. E., & Julius, D. (2007). The menthol receptor TRPM8 is the principal detector of environmental cold. Nature, 448(7150), 204–208. https://doi.org/10.1038/nature05910
- Borg, G. A. V. (1982). Psychophysical bases of perceived exertion. Medicine and Science in Sports and Exercise, 14(5), 377–81.
- Botonis, P. G., Miliotis, P. G., Kounalakis, S. N., Koskolou, M. D., & Geladas, N. D. (2019). Thermoregulatory and cardiovascular effects of capsaicin application on human skin during dynamic exercise to temperate and warm conditions. Physiological Reports, 7(24), e14325. https://doi.org/10.14814/phy2.14325
- Brewer, B. W., & Buman, M. P. (2006). Attetional focus and endurance performance: Review and theoretical integration. Kinesiologia Slovenica, 12(2), 1318–2269. http://www.kinsi.si/
- Brick, N., MacIntyre, T., & Campbell, M. (2014). Attentional focus in endurance activity: New paradigms and future directions. International Review of Sport and Exercise Psychology, 7(1), 106–134. https://doi.org/10.1080/1750984X.2014.885554
- Caterina, M. J. (2007). Transient receptor potential ion channels as participants in thermosensation and thermoregulation. American Journal of Physiology - Regulatory Integrative and Comparative Physiology, 292(1), 64–76. https://doi.org/10.1152/ajpregu.00446.2006
- Chu, A., & Burnham, R. (1995). Reliability and validity of tympanic temperature measurement in persons with high spinal cord injuries. Spinal Cord, 33(8), 476–479. https://doi.org/10.1038/sc.1995.104
- Cook, D. B., O’Connor, P. J., Eubanks, S. A., Smith, J. C., & Lee, M. (1997). Naturally occurring muscle pain during exercise: Assessment and experimental evidence. Medicine and Science in Sports and Exercise, 29(8), 999–1012. https://doi.org/10.1097/00005768-199708000-00004
- de Freitas, M. C., Billaut, F., Panissa, V. L. G., Rossi, F. E., Figueiredo, C., Caperuto, E. C., & Lira, F. S. (2019). Capsaicin supplementation increases time to exhaustion in high-intensity intermittent exercise without modifying metabolic responses in physically active men. European Journal of Applied Physiology, 119(4), 971–979. https://doi.org/10.1007/s00421-019-04086-w
- de Freitas, M. C., Cholewa, J. M., Gobbo, L. A., de Oliveira, J. V. N. S., Lira, F. S., & Rossi, F. E. (2018). Acute capsaicin supplementation improves 1,500-m running time-trial performance and rate of perceived exertion in physically active adults. Journal of Strength and Conditioning Research, 32(2), 572–577. https://doi.org/10.1519/JSC.0000000000002329
- Duru, C. O., Akinbami, F. O., & Orimadegun, A. E. (2012). A comparison of tympanic and rectal temperatures in term Nigerian neonates. BMC Pediatrics, 12(1), 86. https://doi.org/10.1186/1471-2431-12-86
- Enoka, R. M., Almuklass, A. M., Alenazy, M., Alvarez, E., & Duchateau, J. (2021). Distinguishing between fatigue and fatigability in multiple sclerosis. Neurorehabilitation and Neural Repair, 35(11), 960–973. https://doi.org/10.1177/15459683211046257
- Fenemor, S. P., Gill, N. D., Sims, S. T., Beaven, M., & Driller, M. W. (2020). Validity of a tympanic thermometer and thermal imaging camera for measuring core and skin temperature during exercise in the heat. Measurement in Physical Education and Exercise Science, 24(1), 49–55. https://doi.org/10.1080/1091367X.2019.1667361
- Flood, T. R., Waldron, M., & Jeffries, O. (2017). Oral l-menthol reduces thermal sensation, increases work-rate and extends time to exhaustion, in the heat at a fixed rating of perceived exertion. European Journal of Applied Physiology, 117(7), 1501–1512. https://doi.org/10.1007/s00421-017-3645-6
- Fritz, N. B., Calatayud, J., Page, P., Yadav, M., Sidiq, M., & Colado, J. C. (2020). Applying a menthol pain reliever prior to strength training reduces chronic low back pain and increases functional capacity in overweight or obese older adults. Journal of Human Sport and Exercise, 15(4proc), S1224–S1241. https://doi.org/10.14198/jhse.2020.15.Proc4.24
- Gillis, D. J., Capone, S., Nestor, K., & Snell, M. (2020). The influence of menthol dose on human temperature regulation and perception. Journal of Thermal Biology, 92, 102659. https://doi.org/10.1016/j.jtherbio.2020.102659
- Gillis, D. J., House, J. R., & Tipton, M. J. (2010). The influence of menthol on thermoregulation and perception during exercise in warm, humid conditions. European Journal of Applied Physiology, 110(3), 609–618. https://doi.org/10.1007/s00421-010-1533-4
- House, J. R., & Tipton, M. J. (2002). Using skin temperature gradients or skin heat flux measurements to determine thresholds of vasoconstriction and vasodilatation. European Journal of Applied Physiology, 88(1), 141–145. https://doi.org/10.1007/s00421-002-0692-3
- Hutchinson, J. C., & Sherman, T. (2014). The relationship between exercise intensity and preferred music intensity. Sport, Exercise, and Performance Psychology, 3(3), 191–202. https://doi.org/10.1037/spy0000008
- Jeffries, O., Goldsmith, M., & Waldron, M. (2018). l-Menthol mouth rinse or ice slurry ingestion during the latter stages of exercise in the heat provide a novel stimulus to enhance performance despite elevation in mean body temperature. European Journal of Applied Physiology, 118(11), 2435–2442. https://doi.org/10.1007/s00421-018-3970-4
- Jeffries, O., & Waldron, M. (2019). The effects of menthol on exercise performance and thermal sensation: A meta-analysis. Journal of Science and Medicine in Sport, 22(6), 707–715. https://doi.org/10.1016/j.jsams.2018.12.002
- Johar, P., Grover, V., Topp, R. V., & Behm, D. G. (2012). A comparison of topical menthol to ice on pain, evoked tetanic and voluntary force during delayed onset muscle soreness. International Journal of Sports Physical therapy, 7(3), 314–322.
- Jones, L., Karageorghis, C. I., & Ekkekakis, P. (2014). Can high-intensity exercise be more pleasant? Attentional dissociation using music and video. Journal of Sport and Exercise Psychology, 36(5), 528–541. https://doi.org/10.1123/jsep.2013-0251
- Kounalakis, S. N., Botonis, P. G., Koskolou, M. D., & Geladas, N. D. (2010). The effect of menthol application to the skin on sweating rate response during exercise in swimmers and controls. European Journal of Applied Physiology, 109(2), 183–189. https://doi.org/10.1007/s00421-009-1345-6
- Liu, B., Fan, L., Balakrishna, S., Sui, A., Morris, J. B., & Jordt, S. E. (2013). TRPM8 is the principal mediator of menthol-induced analgesia of acute and inflammatory pain. Pain, 154(10), 2169–2177. https://doi.org/10.1016/j.pain.2013.06.043
- Lohse, K. R., & Sherwood, D. E. (2011). Defining the focus of attention: Effects of attention on perceived exertion and fatigue. Frontiers in Psychology, 2, 332. https://doi.org/10.3389/fpsyg.2011.00332
- Luo, M., Wang, Z., Zhang, H., Arens, E., Filingeri, D., Jin, L., Ghahramani, A., Chen, W., He, Y., & Si, B. (2020). High-density thermal sensitivity maps of the human body. Building and Environment, 167, 106435. https://doi.org/10.1016/j.buildenv.2019.106435
- Mündel, T., & Jones, D. A. (2010). The effects of swilling an l(-)-menthol solution during exercise in the heat. European Journal of Applied Physiology, 109(1), 59–65. https://doi.org/10.1007/s00421-009-1180-9
- Norbury, R., Smith, S. A., Burnley, M., Judge, M., & Mauger, A. R. (2022). The effect of hypertonic saline evoked muscle pain on neurophysiological changes and exercise performance in the contralateral limb. Experimental Brain Research, 240(5), 1423–1434. https://doi.org/10.1007/s00221-022-06342-6
- Peier, A. M., Moqrich, A., Hergarden, A. C., Reeve, A. J., Andersson, D. A., Story, G. M., Earley, T. J., Dragoni, I., McIntyre, P., Bevan, S., & Patapoutian, A. (2002). A TRP channel that senses cold stimuli and menthol. Cell, 108(5), 705–715. https://doi.org/10.1016/S0092-8674(02)00652-9
- Pergolizzi, J. v., Taylor, R., LeQuang, J. A., & Raffa, R. B. (2018). The role and mechanism of action of menthol in topical analgesic products. Journal of Clinical Pharmacy and Therapeutics, 43(3), 313–319. https://doi.org/10.1111/jcpt.12679
- Ramanathan, N. L. (1964). A new weighting system for mean surface temperature of the human body. Journal of Applied Physiology, 19(3), 531–533. https://doi.org/10.1152/jappl.1964.19.3.531
- Riera, F., Trong, T. T., Sinnapah, S., & Hue, O. (2014). Physical and perceptual cooling with beverages to increase cycle performance in a tropical climate. PLoS ONE, 9(8), e103718. https://doi.org/10.1371/journal.pone.0103718
- Scanlon, J. E. M., Townsend, K. A., Cormier, D. L., Kuziek, J. W. P., & Mathewson, K. E. (2017). Taking off the training wheels: Measuring auditory P3 during outdoor cycling using an active wet EEG system. Brain Research, 53, 50–61. https://doi.org/10.1016/j.brainres.2017.12.010
- Schlader, Z. J., Simmons, S. E., Stannard, S. R., & Mündel, T. (2011). The independent roles of temperature and thermal perception in the control of human thermoregulatory behavior. Physiology and Behavior, 103(2), 217–224. https://doi.org/10.1016/j.physbeh.2011.02.002
- St Clair Gibson, A., Swart, J., & Tucker, R. (2018). The interaction of psychological and physiological homeostatic drives and role of general control principles in the regulation of physiological systems, exercise and the fatigue process–the integrative governor theory. European Journal of Sport Science, 18(1), 25–36. https://doi.org/10.1080/17461391.2017.1321688
- Starowicz, K., Maione, S., Cristino, L., Palazzo, E., Marabese, I., Rossi, F., de Novellis, V., & di Marzoe, V. (2007). Tonic endovanilloid facilitation of glutamate release in brainstem descending antinociceptive pathways. The Journal of Neuroscience, 27(50), 13739–13749. https://doi.org/10.1523/JNEUROSCI.3258-07.2007
- Stevens, C. J., Thoseby, B., Sculley, D. V., Callister, R., Taylor, L., & Dascombe, B. J. (2016). Running performance and thermal sensation in the heat are improved with menthol mouth rinse but not ice slurry ingestion. Scandinavian Journal of Medicine & Science in Sports, 26(10), 1209–1216. https://doi.org/10.1111/sms.12555
- Taylor, R., Tong, G., Raffa, R. B., Gharibo, C., Pappagallo, M., Sinclair, N. R., Fleischer, C., & Tabor, A. (2012). A randomized, double-blind comparison shows the addition of oxygenated glycerol triesters to topical mentholated cream for the treatment of acute musculoskeletal pain demonstrates incremental benefit over time. Pain Practice, 12(8), 610–619. https://doi.org/10.1111/j.1533-2500.2012.00529.x
- Thunberg, T. (1896). Förnimmelserna vid till samma ställe lokasirerad, samtidigtb pagaende köld och värmeretning. Uppsala Läkkfören.Föhr, 2, 489–495.
- Towey, C., Easton, C., Simpson, R., & Pedlar, C. (2017). Conventional and novel body temperature measurement during rest and exercise induced hyperthermia. Journal of Thermal Biology, 63, 124–130. https://doi.org/10.1016/j.jtherbio.2016.11.010
- Urbaniack, G. C., & Plous, S. (2015). Research Randomizer - Mathematical software - swMATH. https://www.swmath.org/software/24912.
- Vyklický, L., Nováková-Toušová, K., Benedikt, J., Samad, A., Touška, F., & Vlachova, V. (2008). Calcium-dependent desensitization of vanilloid receptor TRPV1: A mechanism possibly involved in analgesia induced by topical application of capsaicin. Physiological Research, 57(3), 59–68. https://doi.org/10.33549/physiolres.931478
- Wang, G., Zhang, T., Wang, A., & Hurr, C. (2022). Topical analgesic containing methyl salicylate and l-menthol accelerates heat loss during skin cooling for exercise-induced hyperthermia. Frontiers in Physiology, 13, 945969. https://doi.org/10.3389/fphys.2022.945969
- Yang, F., & Zheng, J. (2017). Understand spiciness: Mechanism of TRPV1 channel activation by capsaicin. Protein & Cell, 8(3), 169–177. https://doi.org/10.1007/s13238-016-0353-7
