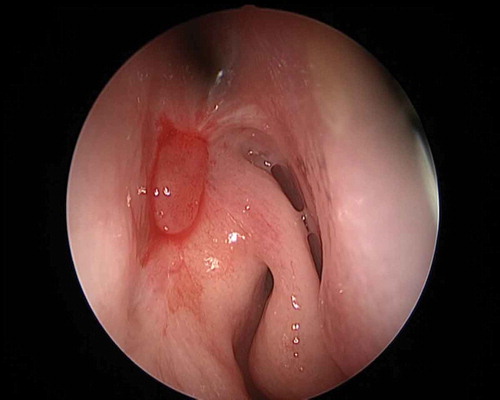
ABSTRACT
Introduction: The utilization of endoscopy in lacrimal surgeries has been increasing in the recent past with increasing indications. With the advent of newer generation of telescopes, the practice and learning of endoscopic lacrimal surgeries is expected to improve further.
Areas covered: This review provides an overview about endoscope assistance in all conditions that involve pathologies of the lacrimal drainage apparatus. The authors have reviewed recently published literature on the techniques and outcomes of endoscopic dacryocystorhinostomy (DCR) in different scenarios such as acute dacryocystitis, pediatric cases, cases with sinonasal anomalies, traumatic nasolacrimal duct obstructions (NLDO) and revision DCRs. Furthermore, promising new avenues such as the 3D endoscopes, variable viewing endoscopes, dacryoendoscopes, navigation-assisted and augmented reality endoscopic lacrimal surgeries have been discussed.
Expert commentary: The aim for clinicians should now move on to addressing lacrimal pathologies with traditionally less favorable outcomes and reducing the failure rates with the technological assistance. As more ophthalmology training programs include structured training modules on endoscopic lacrimal surgery, the popularity, the safety and indications of endoscopic lacrimal surgery by ophthalmologists is expected to increase.
1. Introduction and history
The study of the lacrimal system or ‘Dacryology’ has undergone significant transformation in the past few decades. Historically, the first mention of any form of lacrimal surgery appears to have been in about 1750 BC in one of the oldest recorded set of laws, the King of Babylon’s Code of Hammurabi [Citation1]. Celsus and Galen were the pioneers in treating lacrimal diseases when they treated dacryocystitis using a red-hot cautery iron by passing it through the lacrimal bone into the nose to create a passage to drain the abscess [Citation2,Citation3]. Over the next few millennia though, there seemed to be some amount of intellectual inertia, with no new significant development taking place. The period of Renaissance unfortunately did not contribute much to the advancement of Dacryology. Finally, in 1904, the Italian otolaryngologist, Addeo Toti, published his description of the external dacryocystorhinostomy (DCR) [Citation2,Citation3]. Surprisingly though, endonasal DCR was described by Caldwell in 1893 much before Toti described his external approach [Citation4]. In his surgery, Caldwell used a burr endonasally to create a middle meatal osteotomy after passing a metal probe through a canaliculus, into the lacrimal sac and then into the nostril. This approach, however was complicated with the limited instrumentation available at the time. In addition, other issues such as difficulty in visualization, inadequate soft tissue and bone removal and inability to maintain hemostasis did not make this method popular. It was only in the 1970s with the advent of rigid endoscopes and functional endoscopic sinus surgery, that endoscopic lacrimal surgery became popular once again. Rice, demonstrated the feasibility of endoscopic DCR in cadavers and in 1989, McDonogh and Meiring published the first clinical study on endoscopic DCR [Citation5,Citation6]. Since then, endoscopy has been effectively utilized in the treatment of all conditions that involve the lacrimal drainage apparatus. The purpose of this manuscript is to review the importance of endoscopic techniques in the evaluation and management of lacrimal drainage disorders and also critically review the noteworthy developments and recent updates in endoscopic lacrimal surgery.
2. Pre-operative assessment
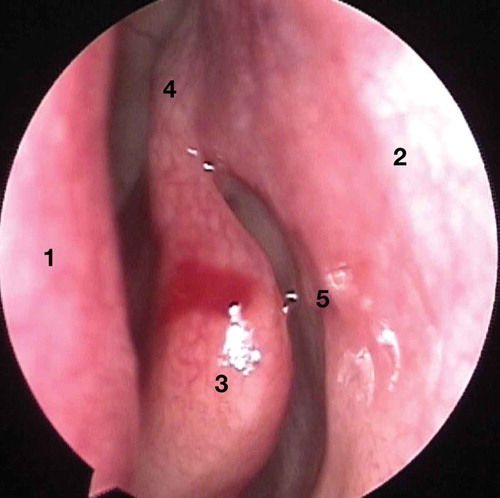
The finer aspects of a detailed assessment of epiphora are beyond the scope of this manuscript. However, once a diagnosis has been arrived upon and a decision taken to perform an endoscopic endonasal lacrimal surgery, it is important to perform a pre-operative surgical assessment of the nasal cavity to identify the surgical landmarks (). This, surprisingly though, is typically not performed by most ophthalmologists, before an external DCR.
Figure 1. The endoscopic view of left nasal cavity. Some important landmarks are nasal septum (1), lateral nasal wall (2), middle turbinate (3), axilla of middle turbinate (4), and maxillary line (5).
Usually, a nasal endoscopy viewing system consists of rigid endoscope, a camera head, a fiber optic cable, a light source and a camera image processing system along with a viewing screen. The commonly used nasal endoscopes today are of two diameters: 2.7 mm for pediatric use or office endoscopies and 4 mm for routine adult surgeries. They have working length of 18 cm and come in a wide range of angulations: 0°, 30°, 45°, 70°, and 90° for different viewing purposes [Citation7]. Pre-operative nasal endoscopy is important because it helps in identification of anatomical variations and assessment of co-morbidities before surgery. As mentioned earlier, despite having many advantages, not all ophthalmologists perform an endoscopic examination of the nose prior to an external DCR. Some of the findings that may potentially affect the surgical procedure itself and its outcomes include nasal septal deviation, paradoxical middle turbinate, concha bullosa, large bulla ethmoidalis, prominent uncinate process, thick frontal process of maxilla, thick lacrimal bone, posterior or high position of the lacrimal sac and sac in ethmoid sinus syndrome [Citation8–Citation12].
3. Dacryocystorhinostomy
With the increasing understanding of nasal anatomy, the advent of rigid nasal endoscopes and high definition cameras; fiber-optic light sources and increasing experience of oculoplastic surgeons; endoscopic endonasal dacryocystorhinostomy (En-DCR) is being recognized as an equally effective route of surgery when compared to the external approach [Citation8,Citation12,Citation13]. The use of diamond burr for superior osteotomy in En-DCR helps in achieving adequate bone clearance and complete exposure of lacrimal sac fundus, and the use of this high-speed drill or any other powered instrument like the ultrasound is referred to as ‘powered endoscopic DCR’ ().
Figure 2. Powered endoscopic DCR: an irrigating-suction diamond burr being used for superior osteotomy and complete exposure of lacrimal sac until the fundus of the sac.
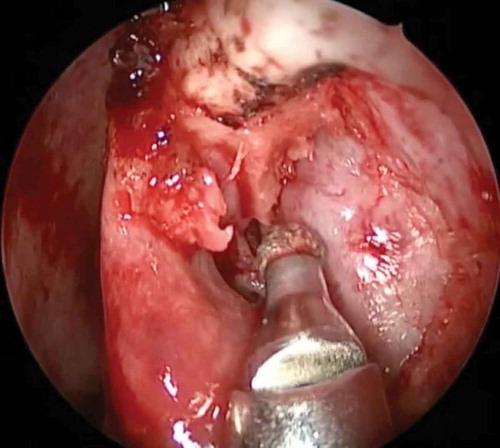
Some of the initial challenges for oculoplastic surgeons are unfamiliar nasal anatomy which is often variable; newer instruments, lack of binocularity with nasal endoscopes, achieving dexterity and instruments maneuvering within the confines of nasal cavity [Citation14]. The use of a trans-canalicular light source and observing it through the nasal endoscope allows a quick and easy way to find the location of lacrimal sac on the lateral nasal wall for the uninitiated ophthalmologist. The brightest light point indicates the location of common canaliculus and the surrounding few millimeters area would correspond to where the lacrimal sac is located. Although it is easy, it does not help in identifying the entire intranasal surface marking of lacrimal sac in relation to the lateral nasal wall and therefore the precise knowledge of the important nasal structures is essential. Previously it was thought that up to 20% of the lacrimal sac lies above the level of middle turbinate, therefore until the late 1990s the idea of creating a superior osteotomy was not popular. A landmark study by Wormald described that the significant part of the sac lies above the level of common canaliculus and major portion of lacrimal sac is situated above the level of the axilla of middle turbinate on the lateral nasal wall [Citation13]. It was hence identified as an important step to remove the superior thick frontal process of maxilla during En-DCR to ensure the complete exposure of sac. This not only has implications in En-DCR but even in external DCR, when often times, surgeons do not remove bone above the level of the common canaliculus, thus highlighting the importance of endoscopy.
3.1. Endoscopic vs. external dacryocystorhinostomy: which has a better outcome?
While the final answer to this question remains to be conclusively answered, there has always been a perception that endoscopic DCR has inferior success rates compared to external DCR. However, recent literature suggests that there may not be any difference, if any, at all. Huang et al. [Citation15] reviewed the literature exhaustively in an attempt to compare outcomes. The review identified 3582 studies and 355 papers were reviewed after screening. Overall, external DCR had slightly better success rates than endoscopic DCR. They, however, acknowledged that initially, endoscopic DCR was performed with laser but over the years, the preferred surgical technique had shifted to mechanical bone removal or the use of powered drills and the outcomes of these cases (non-laser) were comparable to external DCR whereas laser-assisted DCR had poorer when compared separately [Citation15]. Lee et al. [Citation16] reviewed 14 different studies (1680 patients and 1724 DCRs total) that compared primary external versus endoscopic DCR (5 of which were laser-assisted). In this review, no statistically significant difference was found between the 2 techniques in terms of full success (OR: 1.28; 95% CI, 0.85–1.95; P = 0.24.) In contrast to the review by Huang et al., subgroup analysis within this study showed that the success rates were not significantly different between external versus laser En-DCR (P = 0.08) as well as for external versus non-laser En-DCR (P = 0.40). Dolman and colleagues [Citation17] reported successful outcomes in 90.2% of external DCR patients and 89.9% of endonasal DCR patients. Partial success was achieved in 2% of external DCR and 4% of endonasal DCR patients. Surgical failure was noted in 7.8% of the external DCRs and 7% of endonasal DCRs. They summarized that no statistically significant difference in surgical outcomes was found between the 2 different approaches.
Apart from the outcomes, one of the major patient concerns about the external approach is the risk of scarring. Huang et al also reported that among 554 external DCR procedures performed, it was found that 10.8% of patients reported an unacceptable scar [Citation15]. Proponents of the endonasal endoscopic technique cite the obvious benefits of no skin incision and precluding a scar [Citation15,Citation18]. The other often cited advantages of endoscopic DCR include being less surgically invasive, a shorter operative time, preservation of the lacrimal pump and faster postoperative rehabilitation [Citation19].
3.2. Acute dacryocystitis and/or lacrimal abscess
Acute dacryocystitis is defined as medical urgency clinically characterized by rapid onset of pain, erythema, and swelling classically below the medial canthal tendon with or without pre-existing epiphora, mainly resulting from the acute infection of the lacrimal sac and surrounding tissues [Citation20]. The clinical course is often prolonged, with chances of relapse and possible progression to full-blown orbital cellulitis. The disadvantages of conservative management are the possibility of a cutaneous scar or a residual fistula, the possible need for repeated incision and drainage procedures and failure of subsequent DCR surgery due to scarring and fibrosis in the lacrimal sac [Citation21,Citation22]. It is however in this acute setting that the utility of En-DCR comes through as it can be performed even in the acute phase with decreased morbidity and faster recovery. Wu et al. [Citation22] reported that early endoscopic DCR had significantly higher success rate than delayed external DCR in cases of acute dacryocystitis. More recently, in a randomized clinical trial of 32 participants with acute dacryocystitis and lacrimal sac abscess, primary endoscopic DCR resulted in faster resolution without more recurrences compared with secondary treatment. No increased frequency of safety issues and operation time was identified, and anatomical and functional success appeared comparable between the two treatments [Citation23]. Kamal et al. [Citation21] reported anatomical success in 95% (19/20) and functional success in 90% (18/20) of the patients in their prospective interventional case series of 20 primary powered En-DCRs that were performed in patients presenting with acute dacryocystitis and lacrimal sac abscess and these benefits were maintained even at long-term clinical reviews [Citation24].
3.3. Bilateral primary acquired nasolacrimal duct obstruction (PANDO)
Simultaneous endoscopic DCR has medical and economic benefits, reduced morbidity, improved quality of life and prevents the stress of second surgery. General anesthesia is preferable when doing bilateral simultaneous endoscopic DCR [Citation25].
3.4. Revision DCR
The approach to the previously failed DCR is challenging. Either external or endoscopic approach can be performed for failed cases. As mentioned earlier, endoscopic revision DCR too, offers the advantage of avoiding another cutaneous incision (particularly if previous external scar is evident), but the most significant advantage of En-DCR is being able to identify the cause for failure and rectifying it. Without an endoscope, attempting a revision external DCR can be potentially unpredictable. This is because the cause for failure can be varying. Dave et al. [Citation26] reported the cause of failure of 100 consecutive failed DCRs that underwent revision. The most common causes of failure were inadequate osteotomy, followed by inadequate or inappropriate sac marsupialization and cicatricial closure of the ostium, ostium granulomas and paradoxical middle turbinate. These were identified pre-operatively or intraoperatively using an endoscope [Citation26]. The aim of revision DCR is to achieve mucosal approximation with possible primary intention healing, easy enlargement of ostium and managing sac pathologies [Citation23]. In summary, the endoscopic approach allow clear recognition of factors responsible for failure and thus their appropriate management because of better visualization which external DCR may not afford. In addition, the reported long-term outcomes of revision endoscopic DCRs are more encouraging when compared to the traditional external approach revisions [Citation27].
3.5. Pediatric endoscopic DCR
Persistent CNLDO in cases of complete absence of bony NLD, CNDLO with atonic sac or mucocele, repeated failed probing attempts are indications of DCR in children. Some of the challenges are anatomical factors: a low skull base, narrow nasal cavity and high occurrence of healing related issues such granuloma formation [Citation28,Citation29]. Currently there is inadequate literature on comparison of external and endoscopic approach in pediatric cases especially for persistent CNDLO cases, though the endoscopic approach has shown good success rates [Citation28,Citation30]. The advantages of endoscopic DCR in these cases may be mucosal preservation and approximation around ostium edges for primary intention healing, preservation of lacrimal pump and postoperative management of ostial granuloma.
3.6. Sinonasal anomalies
Many nasal conditions such as deviated nasal septum, concha bullosa, middle/inferior turbinate hypertrophy and nasal polyposis are thought to limit surgical access and influence the outcomes of En-DCR. Previously published literature shows varying rates of simultaneous endoscopic procedures ranging from 14% to as high as 78% [Citation11,Citation25,Citation31,Citation32]. Septoplasty is the most common adjunctive procedure required during endoscopic DCR [Citation11]. Creation of unrestricted access to the surgical site and adequate space for surgical maneuvering are the most common indications for correction of simultaneous nasal pathologies at the time of DCR. However, in addition to the endoscopic equipment, additional surgical skill sets are also required. Furthermore, lliterature suggests a higher frequency of simultaneous procedures among otorhinolaryngologists––since their skill and speed at performing endoscopic septoplasties does not increase the duration or morbidity of the overall procedure. It however, may be prudent for ophthalmologists performing En-DCR to be surgically adept at common procedures like septoplasty and polypectomy when and if they interfere with the lacrimal bypass procedure or its possible outcomes.
3.7. Post-operative evaluation
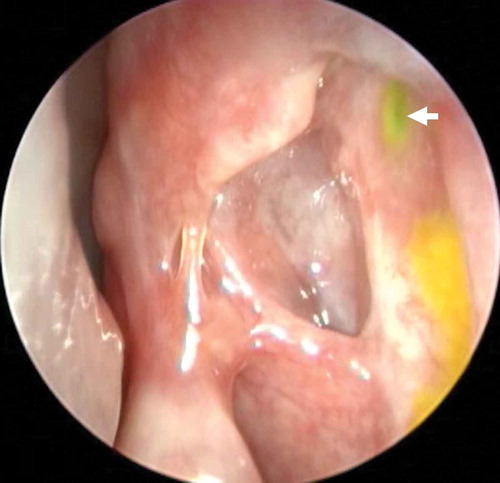
The utility of endoscopy is not restricted only to surgery but also extends post-operatively; periodic endoscopic assessment has allowed clinicians to understand how the DCR ostium behaves after surgery. There have been studies that have reported shrinkage of the ostium to be approximately 25% in the first 4 weeks following endoscopic DCR with adequate sized osteotomy, complete sac marsupialization and good mucosa to mucosa apposition [Citation33]. Ali et al. [Citation34] serially followed up patients who had undergone a powered endoscopic DCR beyond 4 weeks of surgery and reported that there was no statistically significant decrease in either the ostium size or the area for up to 2 years following surgery. The ostium achieved by the use of the powered endoscopic DCR technique remains stable in size from 4 weeks to 2 years post-surgery. Earlier it was understood that larger intraoperative osteotomy had a direct influence on the final ostium size () [Citation35]. However, studies on serial endoscopic assessments of the ostia and a better understanding of the importance of mucosa-to-mucosa approximation have led to the consensus that a large osteotomy size is not necessarily predictive of surgical success [Citation35]. It is not the size of the osteotomy but the complete exposure of the lacrimal sac that has a larger role in deciding the eventual size of the ostium [Citation33]. Ali et al. [Citation36] also proposed a DCR Ostium Scoring (DOS) system: based on the size, they proposed that at 4 weeks post-surgery, an ostium measuring ≥8 × 5 mm in size should be considered to be of a good, large size and an ostium ≤4 × 3 mm be considered a small one. However, in addition to size, the other parameters that were taken into consideration were location, shape, ostium cicatrization, synechiae, the internal common opening, stents (if present), functional endoscopic dye test results, ostial and periostial granulomas and other ostium pathologies. While still unvalidated, such scoring systems can pave the way for standardized objective methods for the assessment and comparison of physical and functional outcomes between different approaches and techniques. The same group of authors have also classified the DCR-related granulomas () based on their ostial locations and proposed their specific management strategies [Citation37].
Figure 3. Functional endoscopic dye test (FEDT): Fluorescein is instilled into inferior fornix and its flow observed with endoscope through the internal common opening (ICO) (white arrow). A well healed large ostium located anterior to axilla of middle turbinate, quick flow of dye through ICO, dynamic ICO without any granuloma are some of the features of overall good ostium.
4. Probing in CNLDO
Congenital nasolacrimal duct obstruction (CNLDO) is the most common cause of persistent epiphora in children, with incidence ranging from 1.2% to 6% [Citation38]. Most cases resolve spontaneously with conservative management in the form of sac compressions. The ones that persist, often require intervention. CNLDO has been broadly classified as simple or complex based on the findings during probing. Kushner described simple cases as those where there is lack of resistance in passing a probe through the NLD until a point of membranous obstruction that can be easily overcome [Citation39]. Jones and Wobig [Citation40] described different variations of the NLD that are typically associated with complex NLDO. Other conditions include buried probe, impacted inferior turbinate, a firm bony obstruction, non-development of the nasolacrimal duct and other craniofacial abnormalities [Citation38]. Complex CNLDO which is often seen in older children has lower reported success rates ranging from 33% to 73% [Citation38,Citation41,Citation42]. Ali et al. [Citation38] in their large retrospective chart review of all patients presenting with CNLDO have documented the different conditions that constitute ‘complex NLDO’. Apart from bony obstruction and craniofacial syndromes, the most common finding was buried probe. A buried probe was endoscopically defined as a condition when the entire NLD remained submucosally in the lateral wall of the nose up to the floor, without any opening into the inferior meatus. Other intraoperative endoscopic findings in cases of CNLDO can be dacryocele with and without intranasal cysts, anlage tracts, atonic sac and lateral nasal wall hypoplasia [Citation38]. The evaluation and classification of CNLDO cases would be possible only when the standard of care is an endoscopic-assisted probing. This underscores the increasing role of endoscopy and its additional role in the simultaneous correction of associated intranasal abnormalities.
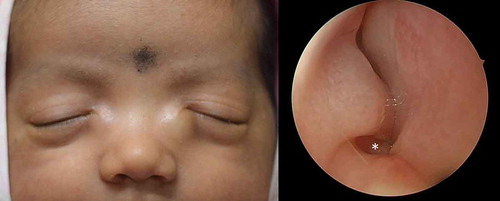
Another condition associated with CNLDO that requires prompt endoscopic intervention is a dacryocystocele. Typically, in a dacryocele or dacryocystocele, a distended lacrimal sac is noted. This is present since birth or appears shortly after birth. The appearance of this bluish cystic swelling at and below the medial canthal area often is accompanied by epiphora. The swelling is tense and can evolve into dacryocystitis and orbital cellulitis. If at initial presentation, compression does not lead to the resolution of dacryocystocele, probing should be performed as early as possible [Citation43]. A significant proportion of infants with dacryocystocele also present with an intranasal cyst (): some reports have pegged this figure to be as high as 100% [Citation44]. These children are at a high risk for developing respiratory distress. In such cases surgical management including lacrimal probing and endoscopic marsupialization is the treatment of choice. Long term outcomes of cruciate marsupialization of intranasal cysts has shown that it achieves good results in patients with congenital dacryocystoceles. Early diagnosis and management may prevent acute dacryocystitis in these patients [Citation45]. This highlights the importance of utilizing nasal endoscopy in the clinical evaluation to ensure proper diagnosis and management of dacryocystoceles.
5. Dacryoendoscopy
Dacryoendoscopy is the microendosocopy of lacrimal system, which allows direct internal visualization of the canaliculi, common canaliculus, lacrimal sac and nasolacrimal duct NLD [Citation46]. Dacryoendoscopic examination of the lacrimal drainage system has evolved over the past decade with the introduction of newer high-definition dacryoendoscopes with better resolution () [Citation47]. It has paved the way for the better understanding of canalicular pathologies like stenosis, obstructions, granulomas, and thereby facilitating their management [Citation48–Citation52]. Apart from its diagnostic role, it has also been utilized in understanding the physiology of lacrimal system [Citation53]. Its first use was published in 1997 for the diagnostic evaluation of patients planned for lacrimal intervention [Citation51]. Thereafter, it has been explored in innumerable conditions [Citation45]. A 0.6 mm microendoscope (Karl Storz, Tuttlingen, Germany) modified from the original sialoendoscope is commercially available. Recently, FiberTech, Japan introduced a high-definition dacryoendoscope specifically designed for the lacrimal system. This unit has a separate illumination system, an imaging system with an irrigation channel. Straight or curved tips with diameters varying from 0.7 to 0.9 mm are available. A specifically designed sleeve covering the microendoscope has been reported to provide better images with use of air insufflation rather than saline [Citation47]. Dacryoendoscope-assisted probing of complex CNLDO has shown higher success rates especially in cases with previous failed probings [Citation50]. Canalicular stenosis can be differentiated from total canalicular obstruction. Dacryoendoscopy-guided treatment of canalicular papillomas, removal of migrated punctal plugs, dacryoliths removal and recanalization of NLD have become possible only after advent of dacryoendoscopes [Citation48,Citation49,Citation54]. Dacryoendoscope guided recanalization of canaliculi and nasolacrimal duct with better outcomes will surely change the existing practice patterns. Its use especially in common canalicular pathologies and NLD will improve our understanding of their pathophysiology. Dacryoendoscopy potentially could be the ideal way to address the mucosal pathologies of lacrimal system.
6. Recent advances
Image guidance is employed routinely in neurosurgery, spinal surgeries, and endoscopic skull base procedures but very few reports on the use of navigation in lacrimal surgery. The term ‘image-guided dacryolocalization’ or IGDL was proposed by Ali et al. [Citation55] to include the use of stereotactic navigation for lacrimal disorders. The initial outcomes of image-guided surgery appear to be encouraging, especially in secondary acquired lacrimal duct obstructions, a major proportion of which are post-traumatic cases. Navigation is particularly useful when the sino-nasal anatomy is grossly distorted in cases such as post-traumatic SALDO, craniofacial anomalies, malpositioned lacrimal sacs, encephalocele in the vicinity, and post-maxillectomy cases. The distinct benefits of a navigation-enabled endoscopy include eliminating the need for multiple localizing instruments, uninterrupted radiological orientation, and sustained navigation guidance throughout the surgery [Citation56]. A new generation continuously variable endoscope, the EndoCameleon® (Karl Storz, Tuttlingen) has the ability to rotate from 15 to 90°. This can be used to perform DCRs and this has the potential to make surgery easier with the varied angulation it permits, to allow full visualization of the nasal cavity without physically moving the endoscope [Citation57].
The current standard endoscopes provide a two-dimensional (2D) view, and the major disadvantage with this is the lack of depth perception. Recently, new 3D nasal endoscopic units consisting of specialized 3D telescopes and a 3D monitor with multiple input and output options are commercially available which can be adapted for lacrimal surgery [Citation7]. The separation of images gives the observer an idea of the depth perception that can be achieved. There are limited data available on how this translates into surgeon performance and better outcomes. Ali et al. [Citation58] published their initial experience with the 3D platform and found that intraoperative tissue handling, and surgical maneuverability were more precise without depending on the spatial cues. Further, better anatomical delineation facilitated improved hand-eye coordination.
7. Expert commentary
The success rates of endoscopic lacrimal interventions now rival or in some instances better than those of conventional techniques. The utility for endoscopic lacrimal surgeries are gradually expanding beyond primary acquired NLDO to include other conditions such as failed DCRs, acute lacrimal sac abscesses, post-chemotherapy and radiation induced NLDO as well as common canalicular obstructions. Furthermore, newer adjuvants like mitomycin-C have shown to improve the success rates of endoscopic procedures [Citation59]. However, the utility of endoscopy is not restricted to DCR alone. The use of endoscopic equipment has changed the understanding of anatomy, physiology and the pathology and management of the entire spectrum of lacrimal diseases. In other lacrimal procedures such as probing for congenital NLDO, transcanalicular laser-assisted DCR and conjunctivodacryocystorhinostomy (C-DCR), endoscopic guidance can be very useful. With progress in the field of diode laser technology, transcanalicular diode laser-assisted DCR remains an option for PANDO with less operative times and potentially fewer complications [Citation60]. However, in the absence of unequivocal data comparing long-term outcomes of laser-assisted DCR with conventional external or Endo-DCR, the acceptance and practice of LA-DCR remains limited.
While En-DCRs are performed both by ENT specialists and ophthalmologists; given that disorders pertaining to the lacrimal system also fall within the domain of oculoplastic surgeons, we believe that En-DCR is a surgery that ophthalmic plastic surgeons should surgically adept in. Literature shows that En-DCR has excellent anatomical and functional success rate in the hands of trainee surgeons as well as expert surgeons [Citation8]. In terms of learning a DCR, there is evidence to show that a structured training program that involves supervision by experienced surgeons can result in efficient skill transfer. Training programs for oculoplastic surgery have begun incorporating training in endoscopic surgery as a part of the curriculum.
We recommend routine nasal endoscopic examination of all patients with NLDO regardless of the surgeon’s preferred route of surgery. This should be done pre-operatively to assess the nasal cavity as well as post-operatively––to study the size and condition of the ostium. In addition to routine cases, endoscopic DCR should be offered to patients in the setting of acute dacryocystitis as well. Looking ahead, promising areas of research include understanding the etiopathogenesis of PANDO and dacryoendoscopic recanalization of the obstructed lacrimal drainage pathways.
8. Five-year review
The future in lacrimal surgery is also dependent on the technological developments in the field of endoscopic hardware. Technological advances combined with breakthroughs on the clinical front have opened many directions for lacrimal surgery in the near future. Looking behind, lacrimal surgery has become lesser invasive––from external DCRs to endoscopic DCRs to dacryoendoscopic surgery. Some authors have made a strong case for endoscopic lacrimal duct recanalization using a microendoscope over conventional DCR [Citation61,Citation62]. However, the lack of long term outcomes and varying success rates have contributed in this treatment modality, not becoming a very popular procedure.
Recent studies in neurosurgery have deployed the use of ‘enhanced visualization’, also known as ‘augmented reality’. Augmented reality was developed to highlight vulnerable deeper structures overlaid on top of the surgical field in an attempt to make surgery safe. To achieve this final outcome, the preoperative image data are displayed with relevant anatomical structures being segmented and highlighted (in some cases by coloring). These processed data are then, anatomically superimposed onto the operative field to provide additional information regarding the surface anatomy for the surgeon. This has been used in visceral surgery, neurosurgery, and more recently in ENT surgery [Citation63]. New visualization techniques for endoscopic ENT surgeries have been developed, which show the target structures and instrument positions within the endoscopic video image [Citation64]. Furthermore, the surgeon can assess the distance to the target region intraoperatively as well. These ‘image-enhanced endoscopic systems’ are an exciting new development which can make surgery safer and potentially maximize outcomes. These technological breakthroughs are critical in ensuring that endoscopic lacrimal surgery become safer with better outcomes. With more ophthalmic plastic surgery fellowship programs incorporating structured training in endoscopic lacrimal surgery, the popularity and the safety of endoscopic lacrimal surgery by ophthalmologists is expected to increase [Citation7,Citation65,Citation66].
Key issues
The use of endoscopic equipment has changed the understanding of anatomy, physiology, and the pathology and management of the entire spectrum of lacrimal diseases. Endoscopic DCR has comparable outcomes to those of traditional external DCR without the concerns of an unsightly scar.
Sufficient evidence is now available to show endoscopic DCR can be performed in acute dacryocystitis/lacrimal abscesses with high success rates. This is yet another distinct advantage over external DCR.
While indications for endoscopic DCR are widening, some of the clinical challenges that persist include revision DCR cases and the complex issue of post-DCR functional epiphora,
Endoscopic assistance in other surgeries such as nasolacrimal duct probing in children and endoscopy guided C-DCR have improved our understanding of these pathologies.
Areas that require further research include techniques to address restenosis after canalicular or NLD recanalization and better techniques in endoscope-assisted NLD recanalization and development of dacryoendoscopes with better resolution.
Declaration of interest
MJ Ali receives royalties from Springer for his treatises, Principles and Practice of Lacrimal Surgery’ and ‘Atlas of Lacrimal Drainage Disorders’. MJ Ali’s research is also supported by the Alexander von Humboldt foundation, Germany. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer Disclosures
Peer reviewers on this manuscript have no relevant financial relationships or otherwise to disclose.
Additional information
Funding
References
- Hughes SM. The history of lacrimal surgery. Adv Ophthal Plast Reconstr Surg. 1986;5:139–168.
- Toti A. Nuovo metodo conservatoire di cura radicale delle suppurazioni croniche del sacco lacrimale (dacriocistorinostomia). Clin Mod Pisa. 1904;10:385–387.
- Ali MJ. Lacrimal disorders and surgery: historical perspectives. Int Ophthalmol. 2014;34(6):1309–1313.
- Caldwell GW. Two new operations for obstruction of the nasal duct with preservation of the canaliculi, and an incidental description of a new lachrymal probe. N Y Med J. 1893;57:581.
- Rice DH. Endoscopic intranasal dacryocystorhinostomy. A cadaver study. Am J Rhinol. 1988;2:127.
- McDonogh M, Meiring JH. Endoscopic transnasal dacryocystorhinostomy. J Laryngol Otol. 1989;103:585–587.
- Ali MJ. Newer endoscopes and three-dimensional nasal endoscopy. In: Ali MJ, editor. Principles and practice of lacrimal surgery. Singapore: Springer; 2018. p. 97–101.
- Kamal S, Ali MJ, Nair AG. Outcomes of endoscopic dacryocystorhinostomy: experience of a fellowship trainee at a tertiary care center. Indian J Ophthalmol. 2016;64:648–653.
- Singh S, Alam MS, Ali MJ, et al. Endoscopic intranasal findings in unilateral primary acquired nasolacrimal duct obstruction. Saudi J Ophthalmol. 2017;31:128–130.
- Cheng AC, Wong AC, Sze AM, et al. Limited nasal septoplasty by ophthalmologists during endonasal dacryocystorhinostomy: is it safe? Ophthalmic Plast Reconstr Surg. 2009;25(4):293–295.
- Figueira E, Al Abbadi Z, Malhotra R. Frequency of simultaneous nasal procedures in endoscopic dacryocystorhinostomy. Ophthal Plast Reconstr Surg. 2014;30:40–43.
- Ali MJ, Singh S, Naik MN. Entire lacrimal sac within the ethmoid sinus: outcomes of powered endoscopic dacryocystorhinostomy. Clin Ophthalmol. 2016;10:1199–1203.
- Wormald PJ. Powered endoscopic dacryocystorhinostomy. Laryngoscope. 2002;112:69–72.
- Shun-Shin GA, Thurairajan G. External dacryocystorhinostomy - an end of an era? Br J Ophthalmol. 1997;81:716–717.
- Huang J, Malek J, Chin D, et al. Systematic review and meta-analysis on outcomes for endoscopic versus external dacryocystorhinostomy. Orbit. 2014;33:81–90.
- Lee DW, Chai CH, Loon SC. Primary external dacryocystorhinostomy versus primary endonasal dacryocystorhinostomy: a review. Clin Experiment Ophthalmol. 2010;38:418–426.
- Dolman PJ. Comparison of external dacryocystorhinostomy with nonlaser endonasal dacryocystorhinostomy. Ophthalmology. 2003;110:78–84.
- Grob SR, Campbell A, Lefebvre DR, et al. External Versus Endoscopic Endonasal Dacryocystorhinostomy. Int Ophthalmol Clin. 2015;55:51–62.
- Marcet MM, Kuk AK, Phelps PO. Evidence-based review of surgical practices in endoscopic endonasal dacryocystorhinostomy for primary acquired nasolacrimal duct obstruction and other new indications. Curr Opin Ophthalmol. 2014;25:443–448.
- Ali MJ, Joshi SD, Naik MN, et al. Clinical profile and management outcome of acute dacryocystitis: two decades of experience in a tertiary eye care center. Semin Ophthalmol. 2015;30:118–123.
- Kamal S, Ali MJ, Pujari A, et al. Primary powered endoscopic dacryocystorhinostomy in the setting of acute dacryocystitis and lacrimal abscess. Ophthal Plast Reconstr Surg. 2015;31:293–295.
- Wu W, Yan W, MacCallum JK, et al. Primary treatment of acute dacryocystitis by endoscopic dacryocystorhinostomy with silicone intubation guided by a soft probe. Ophthalmology. 2009;116:116–122.
- Li EY, Wong ES, Wong AC, et al. Primary vs secondary endoscopic dacryocystorhinostomy for acute dacryocystitis with lacrimal sac abscess formation: a randomized clinical trial. JAMA Ophthalmol. 2017;135:1361–1366.
- Chisty N, Singh M, Ali MJ, et al. Long-term outcomes of powered endoscopic dacryocystorhinostomy in acute dacryocystitis. Laryngoscope. 2016;126:551–553.
- Bayraktar C, Şimşek A. Increased concomitant nasal procedure frequency in bilateral endoscopic dacryocystorhinostomy. J Craniofac Surg. 2017;28:980–982.
- Dave TV, Mohammed FA, Ali MJ, et al. Etiologic analysis of 100 anatomically failed dacryocystorhinostomies. Clin Ophthalmol. 2016;10:1419–1422.
- Ali MJ, Psaltis AJ, Wormald PJ. Long‐term outcomes in revision powered endoscopic dacryocystorhinostomy. Int Forum Allergy Rhinol. 2014;4:1016–1019.
- Ali MJ. Pediatric dacryocystorhinostomy. Indian J Ophthalmol. 2017;65:1008–1009.
- Bothra N, Wani RM, Ganguly A, et al. Primary nonendoscopic endonasal versus external dacryocystorhinostomy in nasolacrimal duct obstruction in children. Indian J Ophthalmol. 2017;65:1004–1007.
- Bothra N, Naik MN, Ali MJ. Outcomes in pediatric powered endoscopic dacryocystorhinostomy: a single center experience. Orbit. 2018 May 22:1–5 Epub ahead of print. DOI:10.1080/01676830.2018.1477808.
- Ali MJ, Psaltis AJ, Wormald PJ. The frequency of concomitant adjunctive nasal procedures in powered endoscopic dacryocystorhinostomy. Orbit. 2015;34:142–145.
- Nussbaumer M, Schreiber S, Yung MW. Concomitant nasal procedures in endoscopic dacryocystorhinostomy. J Laryngol Otol. 2004;118:267–269.
- Mann BS, Wormald PJ. Endoscopic assessment of the DCR ostium after endoscopic surgery. Laryngoscope. 2006;116:1172–1174.
- Ali MJ, Psaltis AJ, Ali MH, et al. Endoscopic assessment of the dacryocystorhinostomy ostium after powered endoscopic surgery: behaviour beyond 4 weeks. Clin Exp Ophthalmol. 2015;43(2):152–155.
- Chan W, Selva D. Ostium shrinkage after endoscopic dacryocystorhinostomy. Ophthalmology. 2013;120:1693–1696.
- Ali MJ, Psaltis AJ, Wormald PJ. Dacryocystorhinostomy ostium: parameters to evaluate and DCR ostium scoring. Clin Ophthalmol. 2014;8:2491–2499.
- Ali MJ, Wormald PJ, Psaltis AJ. The dacryocystorhinostomy ostium granulomas: classification, indications for treatment, management modalities and outcomes. Orbit. 2015;34(3):146–151.
- Ali MJ, Kamal S, Gupta A, et al. Simple vs complex congenital nasolacrimal duct obstructions: etiology, management and outcomes. Int Forum Allergy Rhinol. 2015;5:174–177.
- Kushner BJ. The management of nasolacrimal duct obstruction in children aged between 18 months and 4 years. J AAPOS. 1998;2:57–60.
- Jones LT, Wobig JL. Surgery of the eyelids and lacrimal system. Birmingham, AL: Aesculapius Publishing Co.; 1976. p. 162–164.
- Kashkouli MB, Beigi B, Parvaresh MM. Late and very late initial probing for congenital nasolacrimal duct obstruction: what is the cause of failure? Br J Ophthalmol. 2003;87:1151–1153.
- Kashkouli MB, Kassaee A, Tabatabaee Z. Initial nasolacrimal duct probing in children under age 5: cure rate and factors affecting success. J AAPOS. 2002;6:360–363.
- Becker BB. The treatment of congenital dacryocystocele. Am J Ophthalmol. 2006;142:835–838.
- Levin AV, Wygnanski-Jaffe T, Forte V, et al. Nasal endoscopy in the treatment of congenital lacrimal sac mucoceles. Int J Pediatr Otorhinolaryngol. 2003;67:255–261.
- Ali MJ, Singh S, Naik MN. Long-term outcomes of cruciate marsupialization of intra-nasal cysts in patients with congenital dacryocele. Int J Pediatr Otorhinolaryngol. 2016;86:34–36.
- Ali MJ. Dacryoendoscopic examination of the lacrimal system. In: Ali MJ, editor. Principles and practice of lacrimal surgery. Singapore: Springer; 2018. p. 103–107.
- Sasaki T, Sounou T, Tsuji H, et al. Air-insufflated high-definition dacryoendoscopy yields significantly better image quality than conventional dacryoendoscopy. Clin Ophthalmol. 2017;11:1385–1391.
- Takahashi Y, Iwaki M, Nakamura Y, et al. Dacryoendoscopic findings of intracanalicular punctal plug migration with or without canaliculitis. Ophthalmic Plast Reconstr Surg. 2013;29(5):e1;28–30.
- Sasaki T, Nagata Y, Sugiyama K. Nasolacrimal duct obstruction classified by dacryoendoscopy and treated with inferior meatal dacryorhinotomy. Part II: inferior meatal dacryorhinotomy. Am J Ophthalmol. 2005;140:1070–1074.
- Fujimoto M, Ogino K, Matsuyama H, et al. Success rates of dacryoendoscopy-guided probing for recalcitrant congenital nasolacrimal duct obstruction. Jpn J Ophthalmol. 2016;60:274–279.
- Emmerich KH, Meyer-Rüsenberg HW, Simko P. Endoscopy of the lacrimal ducts. Ophthalmologe. 1997;94:732–735.
- Ali MJ, Singh S, Naik MN. High-definition dacryoendoscopic features of a canalicular squamous papilloma. Int Ophthalmol. 2017;37:1341–1343.
- Takahashi Y, Suzuki T, Kakizaki H. Lacrimal sac movement under intrasac pressure changes observed with dacryoendoscopy. Ophthalmic Plast Reconstr Surg. 2014;30:313–314.
- Ali MJ, Singh S, Ganguly A, et al. Dacryoendoscopy-guided transcanalicular intralesional interferon alpha 2b for canalicular squamous papillomas. Int Ophthalmol. 2018;38:1343–1346.
- Ali MJ, Naik MN. Image-guided dacryolocalization (IGDL) in traumatic secondary acquired lacrimal drainage obstructions (SALDO). Ophthal Plast Reconstr Surg. 2015;31:406–409.
- Saleh H, Choudhury N. Setup for nasal endoscopy and endoscopic surgery. In: Ali MJ, editor. Principles and practice of lacrimal surgery. Singapore: Springer; 2018. p. 83–89.
- Ali MJ, Singh S, Naik MN. The utility of continuously variable view rigid endoscope in lacrimal surgeries: first intraoperative experience. Ophthal Plast Reconstr Surg. 2016;32:477–480.
- Ali MJ, Naik MN. First intraoperative experience with three-dimensional (3D) high-definition (HD) nasal endoscopy for lacrimal system. Eur Arch Otorhinolaryngol. 2017;274:2161–2164.
- Nair AG, Ali MJ. Mitomycin-C in dacryocystorhinostomy: from experimentation to implementation and the road ahead: A review. Indian J Ophthalmol. 2015;63:335–339.
- Mor JM, Guo Y, Koch KR, et al. Transcanalicular diode laser-assisted dacryocystorhinostomy for the treatment of primary acquired nasolacrimal duct obstruction. J. Vis. Exp. 2017;(128):e55981. DOI:10.3791/55981
- Javate RM, Pamintuan FG, Cruz RT Jr. Efficacy of endoscopic lacrimal duct recanalization using microendoscope. Ophthalmic Plast Reconstr Surg. 2010;26:330–333.
- Chen D, Ge J, Wang L, et al. A simple and evolutional approach proven to recanalise the nasolacrimal duct obstruction. Br J Ophthalmol. 2009;93:1438–1443.
- Wilhelm D, Vogel T, Ostler D, et al. Enhanced visualization: from intraoperative tissue differentiation to augmented reality. Visc Med. 2018;34:52–59.
- Winne C, Khan W, Stopp F, et al. Overlay visualization in endoscopic ENT surgery. Int J CARS. 2011;6:401–406.
- Malhotra R, Norris JH, Sagili S, et al. The learning curve in endoscopic dacryocystorhinostomy: outcomes in surgery performed by trainee oculoplastic surgeons. Orbit. 2015;34:314‐9.
- Nair AG, Kamal S, Agarwal A. Indian survey on practice patterns of lacrimal & eyelid disorders (iSUPPLE): report 3 -cataract and nasolacrimal duct obstruction. Saudi J Ophthalmol. 2017;31:145–149.