ABSTRACT
Introduction: Nonadherence to medication is a challenge to effective treatment of many chronic diseases, including glaucoma, and persists even with interventions aimed at improving adherence. The reported rates of nonadherence to topical glaucoma medication vary widely from 16% to 67%, and it is estimated that less than a third of patients remained on their initial therapy after 12 months. Nonadherence can lead to disease progression and increased economic burden.
Areas covered: This review examines factors that contribute to nonadherence in patients with glaucoma, including the severity of the disease, complexity of treatment, lack of knowledge in patients, poor communications between physician and patient, difficulty with self-administration of drops, side-effects, and medication costs. We discuss the unique challenges in identifying nonadherence in glaucoma patients and investigate the current approaches to improving adherence.
Expert opinion: Strategies for improving adherence should combine new treatment methods with enhanced patient education. New pharmaceuticals may comprise multiple medications in a single bottle, making dosing regimens simpler. Novel drug delivery systems are in development, such as injectable products and implants releasing IOP-lowering medication without the need for self-administration. A comprehensive approach involving these new methods and more effective patient-physician communication may lead to improved adherence.
1. Introduction
1.1. Definition and history of adherence
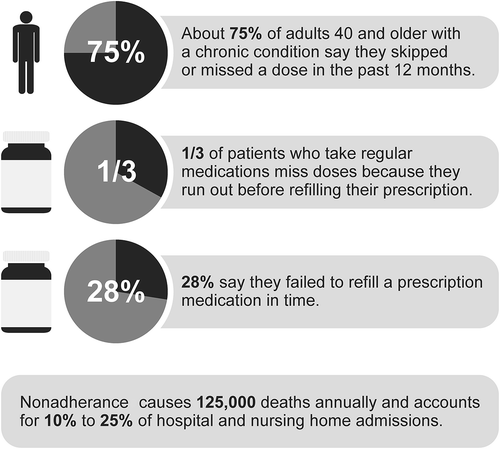
The challenge of nonadherence to therapeutic regimens or treatment plans remains a significant global healthcare concern. As former Surgeon General C. Everett Koop famously stated, ‘Drugs don’t work in patients who don’t take them’ [Citation1,Citation2]. In the United States, nonadherence has been estimated to cost between $100 and $300 billion each year and cause about 125,000 deaths per year [Citation3,Citation4].
Historically, there has been no consensus on the exact definition of adherence, as adherence has often been used interchangeably with compliance [Citation5]. The term ‘adherence’ is typically used to describe how well a patient takes the prescribed medication and ‘persistence’ assesses whether or not the patient stops taking the medication, whereas ‘compliance’ refers to whether or not patients follow their physician’s instructions [Citation6]. The medical community has been moving toward adopting the term adherence, which implies a partnership in which the patient is in agreement with healthcare provider recommendations, rather than compliance, which suggests simply following orders or regulatory guidelines [Citation5].
Adherence is a complicated multifactorial phenomenon that can be influenced by any number of variables including patient-related factors, therapy-related factors, condition-related factors and comorbidities, health system factors, and social and economic factors () [Citation5]. The World Health Organization (WHO) has defined adherence as ‘the extent to which a person’s behavior – taking medication, following a diet, or executing lifestyle changes, corresponds with agreed recommendations from a health care provider’ [Citation7]. In addition to the behaviors identified in the WHO definition, adherence can also encompass a patient’s overall healthcare utilization ().
Table 1. Adherence, compliance, and persistence.
Figure 1. Potential determinants of medication adherence. Adapted from Zullig and Hayden, 2017 [Citation8]. Available at: https://catalyst.nejm.org/optimize-patients-medication-adherence/. Accessed 1 November 2018.
![Figure 1. Potential determinants of medication adherence. Adapted from Zullig and Hayden, 2017 [Citation8]. Available at: https://catalyst.nejm.org/optimize-patients-medication-adherence/. Accessed 1 November 2018.](/cms/asset/2417de7e-d3c3-4d30-a23a-aa98e980a58e/ierl_a_1635456_f0001_b.gif)
Measurement of medication adherence is challenging at best; parameters need to be carefully defined and appropriate for the specific situation [Citation9]. Measures of patients’ adherence to medication may include dosing errors (the number of doses taken vs the number of doses recommended in a 24-h time period), coverage (the proportion of time covered according to the pharmacologic duration of the drug action), inter-dose interval (the interval between doses), or the percentage of doses taken (number of doses actually taken divided by the number of doses that were prescribed for the assessment period) [Citation10]. However, the Hawthorne effect could influence the outcomes of adherence assessments. The knowledge of being monitored could potentially alter the patient’s behavior, either increasing or decreasing adherence.
1.2. Nonadherence in chronic conditions
The problem of nonadherence is common and has been described and estimated in many healthcare settings and disease states, from dentistry (e.g., approximately 32% of Americans do not floss, and 37% do not floss every day) to cancer therapy (e.g., up to 48% of older patients with head and neck cancer were nonadherent to chemotherapy) [Citation11]. Nonadherence has been documented in chronic conditions in many countries [Citation7,Citation11–Citation15].
In the United States, the estimated prevalence of nonadherence in chronic conditions varies depending on the condition, the population studied, and the way in which adherence and nonadherence are both defined and measured. For example, the medication possession ratio, which is the percentage of days’ supply received by the patient divided by the period of time for which the medication was prescribed, has been used to assess nonadherence for chronic conditions (asthma, heart failure, coronary artery disease, depression, diabetes, hyperlipidemia, hypertension, and migraine) [Citation16]. Nonadherence ranged from 11% (any hypertension therapy) to 42% (inhaled corticosteroids for persistent pediatric asthma); 16% were nonadherent to any oral diabetes medications, and 21% were nonadherent to statin therapy. In contrast, a study of breast cancer survivors in the United States that also used medication possession ratios to assess adherence reported higher rates of nonadherence to antihypertensives (37%), diabetes medications (75%), and statins (39%) [Citation17]. A recent meta-analysis of 25 studies that used a validated tool to assess adherence to antihypertensive medications reported a higher rate of nonadherence (45%) among hypertensive patients [Citation13]. Rates of nonadherence to oral antidiabetic regimens ranged from 7% to 64% in retrospective and prospective studies, and more than one third (37%) of patients with diabetes were nonadherent to insulin therapy [Citation12].
Nonadherence remains relatively high even when multiple interventions are implemented in an attempt to improve adherence. Long-term adherence was not significantly improved in patients randomly assigned to lottery incentives and social support for medication adherence after an acute myocardial infarction (n = 1003 patients) compared with those receiving usual care (n = 506) [Citation18]. As a consequence, nonadherence can contribute not only to the morbidity associated with chronic and progressive diseases but also to the healthcare cost burden () [Citation5]. The costs associated with nonadherence result primarily from avoidable hospitalizations and also from direct costs related to the progression of a controllable disease [Citation3]. In the United States, nonadherence may be the source of an estimated $100 billion to $300 billion of avoidable healthcare costs annually [Citation3].
Figure 2. Costs and consequences of medication nonadherence. Adapted from the American Pharmacists Association Foundation. Available at: https://catalystrms.com/resources/news-articles/mymedtimes-vs-pill-reminder-and-medicine-apps/. Accessed 31 October 2017.
The purpose of this review is to discuss adherence and related issues in patients with glaucoma or elevated IOP, with a focus on factors that contribute to nonadherence and strategies to address the problem.
2. Overview of adherence and persistence in glaucoma
Glaucoma is a chronic, progressive condition that is projected to affect approximately 76 million people worldwide in 2020, with the number expected to rise to almost 112 million in 2040 [Citation19]. Notably, glaucoma is the second leading cause of blindness worldwide and the leading cause of treatable blindness [Citation20,Citation21]. In 2010, the worldwide percentage of blindness due to glaucoma was 6.6%, and the contribution of glaucoma to blindness in adults aged ≥50 years was 8.5% as of 2015, with a global projection of >11 million cases of bilateral blindness by 2020 [Citation22–Citation24].
Most cases of glaucoma are primary open-angle glaucoma (POAG), with close to 53 million and 80 million people projected to have POAG in 2020 and 2040, respectively [Citation19]. Most patients with POAG have documented elevated intraocular pressure (IOP), although a subset has measured IOP that falls within normal ranges [Citation25,Citation26]. The degree of IOP elevation affects the risk of blindness and visual disability, and lowering IOP is the only proven way to reduce the risk of vision loss from glaucoma [Citation27].
Intraocular pressure can be lowered through the use of medication, with surgery, or laser therapy. Laser trabeculoplasty is often as effective as a single medication for lowering IOP and has been reported to have few adverse effects [Citation28]. Topically applied medications can be effective at lowering IOP, but their effectiveness is based on a patient’s ability to maintain their daily treatment plan [Citation29,Citation30]. Adherence to glaucoma medication requires the patient to consistently perform specific actions, including appropriately administering eyedrops (i.e., ability to instill a drop within the eye) and continuing to instill drops as prescribed. The ability to apply only a single drop becomes important because if more than one drop is instilled, the additional drops are wasted. If a patient runs out of drops prematurely, the inconvenience and expense of additional refills may lead to gaps in therapy. All of these elements of adherence are required for optimal outcomes in patients [Citation31–Citation33].
Poor adherence has been documented in patients with glaucoma in terms of both healthcare utilization and medication use. Almost 20% of patients do not visit their ophthalmologist in the 18 months after the diagnosis of glaucoma [Citation34], and only 10% of glaucoma patients continuously refilled their prescription within 12 months, suggesting that they are not taking their medications as prescribed [Citation35]. Reported rates of nonadherence with glaucoma medications range from 16% to 30% [Citation14,Citation36,Citation37]; considerable variability in reported rates may be related to how nonadherence was defined or measured in addition to the duration of the study.
Adherence to topical glaucoma medication regimens comprises a spectrum of patient behaviors. Patients must fill and refill their prescriptions and administer the proper dosage into the correct eye at the appropriate time every day [Citation38]. Specifically, adherence to medication consists of 4 steps: obtaining the medication, using the medication every day, appropriately timing the doses, and successfully instilling the medication into the eye [Citation6]. This means that if anything interferes with medication contacting the eye, such as not filling a prescription, missing a dose, or taking too long between doses, a patient is nonadherent [Citation10,Citation14,Citation39–Citation41]. Other patient-related variables that may negatively influence adherence include insufficient knowledge about the consequences of disease progression, socioeconomic barriers, poor health literacy, and physical limitations that make it difficult for the patient to administer eyedrops [Citation42]. In addition to patient-related factors, patient-provider communication, the nature of the disease, medication side effects, the environment, and obstacles in the healthcare system influence adherence [Citation38,Citation43]. The remainder of the review will focus on factors that influence adherence to glaucoma medications.
Persistence has been defined variously as ‘continuing the treatment for the prescribed duration’ or ‘time from initiation to discontinuation of therapy’ [Citation44]. In chronic diseases, including glaucoma, up to half of patients discontinue taking prescribed medications within the first few months of starting therapy [Citation7,Citation45–Citation49]. Although claims data do not provide insight into why a patient discontinues a medication, studies suggest that variability in persistence likely reflects differences in education, forgetfulness, tolerability, and costs [Citation48]. In newly diagnosed patients, a well-recognized reason for suboptimal persistence is that patients think the eyedrop bottle they are initially prescribed is actually the full course of treatment; once they have used the entire bottle, they stop treatment [Citation14].
Reported persistence rates in patients taking glaucoma medications vary depending on patient population (e.g., age, newly diagnosed), the time intervals over which patients are studied, the parameters used to describe discontinuation (e.g., discontinuation of therapy, switching of therapy), and the method of monitoring (e.g., electronic monitoring, prescription refills, medical charts, self-reported). In a systematic review, results from studies of glaucoma medication were stratified by adherence and persistence based on the measurement used (electronic monitoring, prescription fill/refill, medical chart review). Although the quantitative results varied, the studies consistently reported that a substantial number of patients did not remain on their recommended glaucoma regimen. Analysis of 14 studies that evaluated persistence using survival analysis based on prescription refills demonstrated that a mean (SD) of 31% (17%; range, 10–68%) of patients remained on their initial therapy at the end of 12 months [Citation46]. When persistence was described in terms of either discontinuation at 1 year or a change in therapy (12 studies using prescription refills), the persistence mean was 40% (19%; range, 14–67%) [Citation46]. Two of these studies followed the disposition of patients who had discontinued therapy during the first observation year. Although 55% of the total sample had been nonpersistent during the study, only 19% had completely discontinued all ocular hypotensive therapy without any evidence of restarting any therapy by the end of the year. In treatment-naive patients, of the 65% of patients who discontinued therapy at 180 days, 51% had failed to restart therapy by the end of the observation year [Citation46]. In 3 studies that used medication possession (i.e., patient’s supply of the medication based on prescription fill records) at 12 months to assess persistence, a mean of 51% (7%; range, 44–59%) of patients had a supply of the initially prescribed agent on hand at year’s end [Citation46]. Six studies that used the medication possession ratio or proportion of days covered reported a mean of 56% (19%; range, 37–92%). Five studies that reviewed medical charts reported mean persistence rates of 67% (7%; range, 62–78%) at 1 year based on discontinuation or change of therapy. Seven studies used medical monitoring devices to assess persistence; however, results were not compared across these studies because of variations in specific endpoints. Additionally, findings from studies that based persistence on conventional medical monitoring may not reflect long-term use in naturalistic settings because of short follow-up periods and designs that are more like clinical trials. Collectively, these findings underscore the need for interventions to improve adherence and persistence among patients with glaucoma. Additional challenges may be present in patients who receive multiple medications, where measuring adherence to only one medication may not provide the full picture and averaging adherence measurements for multiple medications may be difficult to interpret.
3. Challenges in identifying or assessing adherence in glaucoma
Although there are different methods to identify nonadherence (i.e., self-report, physician report, direct observation, electronic medication monitors), there exists no quantitative standard for measuring adherence to glaucoma medication [Citation6]. Additionally, detection of nonadherence in patients may be affected by the type of questions asked by the physician [Citation50].
Studies show that doctors have trouble identifying patients who are nonadherent [Citation37,Citation51] and may tend to overestimate adherence in clinical trials [Citation30] and in clinical practice, which may be partially explained by patients over-reporting adherence and under-reporting nonadherence [Citation48]. In a study comparing self-reported adherence versus adherence assessed by Kali Drop (Kali Care, Santa Clara, CA), patients with glaucoma showed poor ability to accurately report their adherence [Citation52]. Additionally, physicians often do not know whether patients fill or refill their prescriptions, and they have few validated tools to help assess adherence in clinical practice; tools that assess adherence are used primarily in clinical trials.
3.1. White-coat adherence
One challenge in identifying adherence is the phenomenon known as ‘white-coat adherence.’ Similar to flossing before visiting the dentist, a patient’s adherence to treatment tends to improve during the time period shortly before a follow-up visit but subsequently declines after the visit [Citation30,Citation48]. A patient’s IOP may appear to be controlled based on assessment during an office visit; however, these measurements may not reflect IOP levels between office visits. Glaucoma patients typically increase adherence 24–48 h before a scheduled office visit [Citation45]. Here, adherence may be more of a cycling process, making it difficult to assess either adherence or long-term IOP levels [Citation45,Citation48,Citation50].
3.2. Lack of access to refill information
A general issue that limits a physician’s ability to assess adherence is lack of access to refill information. In most cases, physicians do not receive prescription refill data from pharmacies and must, therefore, rely on patient or caregiver reports. In this case, the physician may not realize that the patient is not adherent and may conclude instead that the patient is not responding to treatment. The physician may subsequently prescribe an unnecessary medication or an increasingly complex medication regimen, which can lead to yet more adherence issues [Citation14]. However, an increased number of prescribed medications is associated with reduced adherence [Citation10,Citation53].
Although refill information can contribute to the assessment of patients’ adherence, it cannot recognize whether the drops were properly instilled and would not be able to identify patients who took too many drops, ran out of medicine early, and refilled at the next available time. Additionally, in a closed pharmacy system (i.e., Veterans Affair pharmacies), physicians may routinely refill medications for patients at every visit, and if the pharmacy’s policy is to fill these orders without the patient’s request, medication possession ratios may be artificially high.
3.3. Limitations of the current assessment tool
More than 30 years ago, prototype electronic monitors were used within eyedrop bottles to highlight that nonadherence with topical medication was a problem in patients with glaucoma [Citation36,Citation37]. These were used with both twice-daily and four-times-daily topical IOP-lowering medications; there was no Hawthorne effect because patients were supplied bottles and were unaware of being monitored [Citation36,Citation37]. Since then, a variety of electronic tools have been developed and used to assess adherence to eyedrops [Citation54,Citation55]. Unfortunately, these tools are primarily used in research and are not widely available for use in clinical practice. Since accurate assessments of adherence using traditional tools (i.e., questionnaires) may be difficult, especially because self-reported adherence tends to be biased, direct measurements using electronic tools could be used both to help understand patients’ behavior and to help design interventions to improve adherence [Citation52].
A variety of clinical studies have used a Medication Event Monitoring Systems (MEMS) device. In these scenarios, the eyedrop bottle is placed with a MEMS device that tracks when a patient opens the outer bottle containing the eyedrop bottle [Citation10,Citation55]. One problem with this approach to date has been the short-term nature of the assessment; studies generally range from 1 to 3 months. Studies have measured adherence to a two- versus three-times-daily brimonidine regimen over 4 weeks; a once-daily prostaglandin eyedrop regimen over a 3-month period; and two different regimens (n = 31 taking 1 drug; n = 31 taking 2 drugs) using a MEMS device that recorded each bottle opening over a 60-day period [Citation10,Citation56].
Another method is to use a drop application monitor attached to the eyedrop bottle [Citation54]. Over a 3-day period, an 8-bit microprocessor was used for data acquisition and storage. The device was equipped with sensors that could measure the pressure applied to the bottle, temperature, and vertical position, confirmed by test subjects who manually noted each application [Citation54]. Although electronic monitoring tools may be more objective than patient self-report, they have limitations. For example, such tools can measure the frequency and timing of administration, but they cannot assess whether a drop is self-administered correctly. In addition, there is a possibility that a patient may intentionally empty the eyedrop without actually instilling any drops into the eye [Citation54].
4. Factors associated with nonadherence in glaucoma
Factors that influence adherence to glaucoma medications derive from multiple sources. Studies have consistently identified significant barriers to adherence, including poor communication between physicians and patients, patients’ lack of knowledge about the long-term effects of glaucoma, problems with reading instructions, difficulty with instillation of drops or poor technique, forgetting to take the medication, polypharmacy, healthcare/medication costs, and medication-related adverse effects [Citation10,Citation41,Citation43,Citation57–Citation63]. In children with glaucoma, poor parental health literacy has been associated with lower adherence [Citation64].
As many as 71 adherence barriers have been identified and classified into four categories: regimen, individual patient factors, medical provider issues, and situational factors [Citation43]. More recently, a questionnaire was administered to 185 patients with glaucoma (49 self-reported as nonadherent) assessing 11 barriers that had been identified in previous studies (). Among adherent respondents, 33% reported having no barriers to adherence compared with 14% of nonadherent respondents. A higher proportion of nonadherent respondents (80%) reported multiple barriers compared with adherent respondents (55%); only 6% of nonadherent respondents reported having one barrier to adherence versus 12% of adherent respondents. Overall, ≥23% of adherent respondents and ≥31% of those with poor adherence cited each of the 11 barriers as important [Citation38].
Table 2. Barriers to glaucoma medication adherence.
4.1. Issues with self-administration
A patient may purport to be adherent but is actually using a medication incorrectly or not taking any medication due to difficulty self-administering drops. Numerous studies in the United States and other countries have documented that poor technique is a considerable concern in nonadherence [Citation29,Citation65–Citation69].
Despite patient beliefs or self-reports that they are effectively administering eyedrops, studies suggest that is not always the case [Citation70,Citation71]. In a cross-sectional survey of 185 patients with glaucoma treated in a community setting, fewer than half (46%) reported they had no problems with eyedrop instillation [Citation38]. Almost 20% reported having difficulties administering medication, including difficulty with aim (24%); difficulty controlling the number of drops dispensed (18%); difficulty holding steady while squeezing the bottle (10%); flinching or blinking causing the drops not to enter the eye (10%); and difficulty squeezing the bottle (5%) [Citation38]. Over a 12-week study (n = 164), 42% of patients with glaucoma or ocular hypertension were observed to have difficulty with eyedrop administration. Although only 11% reported difficulty at study entry, 35% of those who reported difficulty at entry and 17% of those who denied difficulty at entry demonstrated difficulties at both study visits. Patients who reported difficulty at study entry were more likely to demonstrate difficulty at any visit and at the end of 12 weeks [Citation72]. Based on videotapes, fewer than one-third of patients (n = 173) could successfully instill a single drop without touching the bottle tip to the eye. Up to one-fourth of patients were unable to get a drop in their eye (20/116 [17%] and 16/64 [25%]) with 2.5-mL and 15-mL bottles, respectively [Citation70]. A study of patients with glaucoma (n = 205) and marked visual impairment (n = 204) that used video recording to evaluate patients’ self-administration found that both groups wasted drops, contaminated bottles, and had an inaccurate perception of their abilities. Approximately one-third of patients could not instill an eyedrop onto the surface of their eye; similarly, approximately one-third of patients contaminated the bottle tip [Citation71]. As was noted, patients with glaucoma are the most in need of adherence, yet their disease could progress because of poor eyedrop instillation [Citation71]. Additionally, it appears that patients with the most severe glaucoma may be the ones who have the greatest difficulty with adherence, often because of difficulty self-administering drops [Citation73–Citation75].
4.2. Gaps in patient knowledge/awareness
Inadequate knowledge about glaucoma, glaucoma treatment, and consequences of inadequate treatment may all contribute to nonadherence or nonpersistence. In the cross-sectional survey of glaucoma patients, 50% of nonadherent respondents cited knowledge about glaucoma as a barrier, which is consistent with findings from previous studies [Citation43,Citation68,Citation76–Citation78]. As with other asymptomatic chronic diseases such as systemic hypertension, patients with glaucoma do not have clear endpoints that tangibly signal improvement, and they may not fully understand what eye pressure is and what it affects. Physicians monitor loss of visual field and changes in IOP, amongst other measures to determine if glaucoma is worsening, but these measures may not be meaningful to patients. Worsening glaucoma is associated with changes in visual function, but these occur gradually, and patients may not be aware they are occurring (e.g., decreased reading speed, trouble with facial recognition, less activity, giving up driver’s license earlier). Some patients may be unconcerned about worsening glaucoma because of the lack of symptoms, while other patients may become fatalistic and give up, not care, and stop taking their medication. Both apathetic and fatalistic perceptions regarding the possibility of worsening glaucoma may lead to insufficient motivation to adhere to treatment regimens.
5. Approaches to improve adherence and persistence
Different strategies have been used to address the problem of nonadherence in other chronic diseases. For example, simplifying medication regimens, patient education about the disease and the importance of taking medications, and lowering cost are successful strategies for improving adherence in patients with systemic hypertension [Citation79].
5.1. Simplifying medical therapy
Nonadherence has been related to patients receiving multiple medications [Citation60]. Adherence among glaucoma patients decreased from 81% with one medication to 50% with four medications [Citation60]. Compared with the concomitant administration of two separate medications, fixed combinations have been associated with better treatment adherence [Citation80]. Electronic dosing aids were used to assess adherence to treatment with a fixed combination of two medications in a single bottle compared with separate containers of each medication in 161 patients with OAG. Throughout the 12 months of the study, the 1-bottle group was adherent on a greater percentage of days versus the 2-bottle group. In the first month of dosing, patients in the 1-bottle group were adherent with dosing a mean (SD) of 79% (26%) of days versus a mean of 67% (28%) in the 2-bottle group. Cumulatively, the 1-bottle group was adherent for a mean (SD) of 60% (28%) of days versus 43% (27%) and was consistently higher at 50% to 95% adherence thresholds [Citation80]. In a large retrospective study (N = 7883), adherence was significantly higher among patients who used a 1-bottle fixed combination (43%) compared with patients who used 2-bottle therapies (23% to 35%; P < 0.0001) [Citation81].
Simpler dosing requirements may reduce nonadherence because of the lower potential for improper administration of multiple drops. Similar to adherence, persistence also tends to be greater in patients who used 1-bottle (fixed-combination regimens) compared with 2-bottle regimens concomitantly [Citation81,Citation82]. Among patients with glaucoma, persistence declined as the number of medications increased. At 1 year, persistence was highest in patients using a 1-bottle therapy (35%), followed by a 2-bottle combination (27%; P < 0.0001 vs 1-bottle therapy), and finally a 3-bottle combination (24%; P < 0.0001 vs 1-bottle and 2-bottle therapy) [Citation82]. If fixed combinations in a single bottle are as effective as both of the ingredients given separately, that would be an adherence aid for many individuals. In the US, fixed-combination glaucoma medications may not be available to all patients through all health insurance plans and may cost more than their individual components.
5.2. Longer-acting therapies
Another option that could improve adherence is therapies that last longer, thereby minimizing a patient’s need for constant medication instillation [Citation83]. Novel drug delivery systems, including injectable products and implants that are currently in development, may offer sustained IOP lowering without the need for self-administration [Citation83]. Several of these devices are in phase 3 studies to establish the safety and efficacy profile relative to topical drops [Citation83]. In addition to reducing the need for daily self-administration, if these therapies can stabilize IOP with minor fluctuations, they may provide better outcomes for patients compared with regimens associated with large fluctuations.
A travoprost punctum plug (Ocular Therapeutic, Inc., Bedford, MA) is a sustained-release device that is placed in the vertical portion of the inferior or superior canaliculus; the active drug is slowly released over a 30-day period [Citation83]. A recent feasibility study found that sustained-release travoprost punctum plug reduced IOP in patients with ocular hypertension or OAG [Citation84].
Results from a phase 2 trial of a biodegradable particle formulation of travoprost for intracameral injection (Envisia Therapeutics, Research Triangle Park, NC) showed a clinically meaningful reduction in IOP for the 11-month period after 1dose. The device also demonstrated an IOP-lowering effect comparable to prestudy topical prostaglandin analogs and in-study topical timolol maleate daily eyedrops [Citation85].
A bimatoprost ring (ForSight, VISION5 [now Allergan], Menlo Park, CA) has been designed to rest on the surface of the eye in the conjunctival fornices. In phase 2 studies, IOP decreased 3.2 to 6.4 mmHg in patients using the ring and 4.2 to 6.4 mmHg for patients using twice-daily timolol maleate 0.5%. The product was well tolerated, with a safety profile similar to topical bimatoprost 0.03%, and the incidence of ocular hyperemia was slightly less (14% vs 25–45%, respectively) [Citation83].
An investigational biodegradable, sustained-release bimatoprost-coated rod can be implanted (Allergan) and is administered as an intracameral injection, remaining in the anterior chamber to deliver drug for 4 to 6 months [Citation83]. Interim results showed the device performed as effectively as topical bimatoprost in a 24-month phase 2 study, and no trial subjects needed an additional injection of the implant or a topical IOP-lowering rescue medication. Phase 3 studies are currently under way [Citation83].
In addition to simplifying medication regimens, interventions such as specialized technicians or health coaches have been used to address nonadherence in patients with diabetes, hyperlipidemia, and hypertension [Citation86]. In a randomized controlled trial, evaluating the impact of clinic-based medical assistant health coaching (training included education regarding medication adherence) versus usual care in patients with diabetes, hypertension, and hyperlipidemia, patients who received coaching achieved better glycemic and lipid control than usual-care patients. Health coaches may support patients’ self-management by addressing barriers related to time, resources, and cultural concordance [Citation86].
In another study, interactive voice response telemonitoring plus the engagement of a patient-designated support person (e.g., family member or friend) who was automatically updated with the telemonitoring data improved adherence among patients with diabetes [Citation87]. Patients who engaged with a support person showed significantly greater improvement in long-term adherence compared with patients who participated alone. Among the subset of distressed patients, there was a 25% reduction in the risk of weekly nonadherence for those with a support person (P= 0.030); the risk remained high for those who participated alone (P= 0.82) [Citation87].
In patients with comorbid diabetes and hypertension who were identified as nonadherent, a brief template-based telephone interview conducted by a pharmacist to identify barriers to adherence and provide appropriate education resulted in a significantly improved proportion of days covered (PDC) [Citation88]. While the PDC rates and discontinuation of angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers improved significantly among nonadherent elderly patients, overall PDC rates in the intervention and control groups were still lower than the recommended 80%. These data suggest that incorporating motivational interviewing (MI) with follow-up calls may better affect behavioral changes to lower adherence barriers [Citation88].
5.3. Patient education
Educating patients on glaucoma management may improve adherence to medication [Citation89,Citation90]. In a review of eight studies that focused on improving patient knowledge to improve adherence to glaucoma medications, five showed significant improvements in adherence after educational interventions, two showed nonsignificant improvements, and one showed no improvement in patients who had a relatively high baseline knowledge of glaucoma. Because the studies used very different interventions, it was difficult to determine which specific aspects of each educational intervention had the most impact on medication adherence [Citation89].
An assessment of videotaped provider/patient interactions found that patients who received positive reinforcement about taking their glaucoma medication had significantly better short-term improvements in measures of adherence [Citation90]. In addition, patients who received more education regarding glaucoma were more likely to take their glaucoma medication doses on time, consistent with the idea that better understanding of their disease may be associated with improved self-management [Citation89,Citation90].
Given that many glaucoma patients are unable to properly instill eyedrops, teaching proper eyedrop technique may reduce nonadherence related to drop instillation [Citation39]. In the Newman-Casey survey, respondents reported various difficulties administering eyedrops, including difficulty with aim (24%); difficulty controlling the number of drops dispensed (18%); difficulty holding steady while squeezing the bottle (10%); difficulty with flinching or blinking causing the drops not to enter the eye (10%); and difficulty squeezing the bottle (5%) [Citation38]. Data from a study in which patients were exposed to an instructional video about proper eyedrop instillation technique were encouraging [Citation91]. After patient instruction, instillation improved compared with before teaching, and patients felt more confident of their ability to self-administer eyedrops as their doctor prescribed because of the educational material [Citation91]. Because glaucoma is a chronic condition that patients manage at home, clinicians might consider including patient education tools (e.g., demonstrational videos or instructional handouts) and performing assessment follow-up as a part of healthcare services [Citation91].
5.4. Patient reminders
Although multiple factors contribute to nonadherence, forgetting to take medication is consistently identified as a key contributor [Citation38,Citation43,Citation53,Citation56,Citation77,Citation92]. For example, memory was significantly associated with taking medication among nonadherent patients compared with adherent patients (56% vs 19%, P < 0.001) [Citation77].
Automated reminders that directly target this barrier might improve adherence among patients who forget to take daily medications. A daily telecommunication-based reminder (phone call or text message, based on patient preference) linked to a patient’s health record was shown to increase adherence to once-daily glaucoma medication over a 3-month period [Citation56]. It is unclear, however, how long the improvement will persist. In a 12-month study following acute myocardial infarction in which both pill bottle reminders and incentives were given for good adherence, both of these interventions together made no difference in long-term adherence [Citation18].
5.5. Physician education
Physicians also need education on how to better communicate with patients to improve adherence and persistence. MI as a technique to increase medication adherence has been shown effective in diabetes and HIV. In this application, MI involves a 4-step framework: (1) initiating a conversation that employs empathy and reflective listening to support patients discussing factors that impact adherence; (2) steering the conversation to discrepancies in patients’ goals and their current behaviors, thoughts, or values in a nonjudgmental way; (3) responding with basic empathetic and reflective statements without arguing, giving advice, or confrontation; and (4) if patients have indicated that they are ready to change, support self-sufficiency and maintain optimism to support the patients’ confidence that they can make the change [Citation93–Citation95]. Physicians who used MI improved some patients’ engagement and adherence to treatment in chronic diseases according to a meta-analysis of 16 randomized controlled trials. The pooled MI intervention effect size was 0.12 (95% CI, 0.05–0.20, I2 = 1%). The most effective interventions were those based on MI only (β = 0.183; 95% CI, 0.004–0.362) or those in which interventionists were coached during intervention implementation (β = 0.465; 95% CI, 0.028–0.902). MI interventions that were delivered solely face to face were more effective than those delivered solely by phone (β = 0.270; 95% CI, 0.041–0.498) [Citation96,Citation97].
Even when providers are videotaped under ideal situations, they often do not provide enough education to patients [Citation98]. A study of videotaped physician-patient interactions revealed that ophthalmologists provided patients with incomplete education about glaucoma in 74% of visits; however, even among patients initiating glaucoma treatment, education regarding the relevance of IOP and goals of treatment occurred in only 57% of visits; education regarding the likelihood of long-term therapy occurred in only 24% of visits [Citation98].
Another strategy is a brief, skills-based educational program for physicians. In a prospective study using videotaped interactions, this type of program significantly increased physicians’ patient-centered communication skills and their detection of and engagement with patients’ nonadherence to glaucoma treatment [Citation99].
6. Conclusions
Nonadherence to medication is a common healthcare challenge for many chronic diseases that broadly contributes to clinical and economic burden. Nonadherence is multifactorial and cannot be addressed by a single strategy. Identifying and addressing nonadherence as well as nonpersistence among patients with glaucoma would likely improve patient outcomes. To improve adherence, physicians are encouraged to reduce the complexity of the medication regimen when possible, educate patients about the natural course of glaucoma and the need for lifelong treatment, address medication side effects, and discuss strategies for remembering to take drops at the same time every day. Hopefully, novel drug delivery modalities and longer-acting glaucoma medications will lessen the burden of nonadherence in the future, but clear communication between the patient and eye care provider will remain an important factor in the successful management of the disease.
7. Expert opinion
Although adherence has been a recognized issue in glaucoma for decades, it appears to be gaining traction and seems to cross all racial and socioeconomic strata. Until recently, it was thought that the only patients who were nonadherent were those receiving treatment from other physicians. We must first examine the doctor–patient relationship and see where doctors fall short in communicating with patients. As the cost of nonadherence is great and will become greater as the population ages, it becomes worthwhile to better educate both young and older physicians and their teams in how to better communicate with patients. Most studies in adherence for glaucoma are relatively short term (less than a year) but what is needed are studies looking at long-term strategies to address human nature, which will enable physicians to better understand nonadherence and communicate more effectively with patients. In the era of high tech and electronic medical records (EMRs), effective strategies must be undertaken to supplement physicians’ patient education. After addressing the issues affecting physicians, we must individualize patient care and determine first if a patient is adherent, and if not, which factor or factors are responsible.
There are several challenges to successful clinical management of glaucoma and ensuring adherence to medication. Currently, we as physicians are not able to accurately determine which patients are or are not adherent because there is no quantitative standard for measuring adherence. Often the only measure available to the physician is self-report from patients; however, self-report is notoriously inaccurate. Evidence suggests that over-estimation of adherence is more common than under-estimation, however. As such, physicians can assume that if a patient reports trouble with his or her drops, the problem is at least as large as reported. Visual changes often occur only gradually as glaucoma worsens, making the negative long-term impacts of nonadherence difficult for patients to recognize. Physicians and their team-members can spend time educating patients that glaucoma is the ‘silent thief of sight.’ Even with adequate education, many patients still find it difficult to adhere to regimens, especially those who require that multiple medications be administered several times per day. It is difficult enough for patients to comply with a 10-day course of an antibiotic, let alone a lifelong course required for a chronic disease like glaucoma. Simplifying drug regimens when possible, in terms of the number of bottles, frequency of dosing, and cost, is advisable. In those patients who are nonadherent, relatively safe long-term interventions are needed. Either subconjunctival/Sub-Tenon’s systems, intracameral drug delivery, or intra or extraocular depots might be employed. Laser trabeculoplasty may be an appropriate and potentially cost-effective intervention for patients who are unable to administer an eyedrop, cannot afford medications, or are on multiple medications (including systemic) that could interfere with adherence. Emerging sustained drug delivery modalities are promising, but not yet common in clinical practice. Patients’ adherence to scheduled clinic visits will remain a factor in long-term glaucoma outcomes, even with novel drug delivery methods. There is not a one-size-fits-all solution for poor adherence. Improving adherence will likely require a multifaceted approach by the patient and the healthcare team.
Although physicians have become more attuned to the issue of adherence, changing established practice patterns and implementing effective methods to address the problem remains challenging. Physicians will strive to take adherence out of patients’ hands, but the problem of follow-up will continue. There will be an emphasis on early surgical interventions with novel surgical techniques. Choices in glaucoma treatment will be analogous to a common scenario in proliferative diabetic retinopathy, where the choice between frequent antivascular endothelial growth factor injections with relatively excellent outcomes is compared to the more conventional pan-retinal photocoagulation. Within the next five years, research in glaucoma will focus on implantable devices and newer drug delivery systems. Devices that predict adherence and issues with glaucoma control that send messages to eye care providers such as the Medtronic Guardian Connect and Sugar IQ will emerge, which may help healthcare providers develop a better understanding of the underlying issues with adherence and, ideally, provide accurate assessments of adherence.
Article highlights
Nonadherence to therapeutic regimens is an important global healthcare concern and is especially challenging to quantify due to the complex underlying causes and lack of accurate measurement methods.
Glaucoma is a leading cause of treatable blindness and requires early diagnosis and management to halt disease progression. Early treatment is less expensive and may prevent visual disability. Today, successful treatment of the disease requires patients to administer eyedrops accurately and consistently.
Nonadherence to glaucoma medication results from the difficulty of maintaining the regimen, poor communication between healthcare providers and patients, medication costs, and patients’ lack of education about the long-term effects of glaucoma.
Accurate measurement of adherence is difficult because current assessment tools are limited by biased self-reporting, patients’ over-estimation of their ability to successfully administer eyedrops, and ‘white coat adherence’ in which patients increase adherence preceding a scheduled office visit.
Improved adherence may be achieved through the use of simpler treatment regimens, implantable medication-administering devices, and more effective communication between physicians and patients.
Declaration of interest
AL Robin is the executive vice president of the American Glaucoma Society, a consultant to Google Artificial Intelligence, and Versant Healthcare. KW Muir is on the advisory board for GrayBug Vision. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
The medical writing support was provided by Catherine DeBrosse and Natalia Zhukovskaya of Complete Healthcare Communications, LLC, North Wales, PA, and was funded by Novartis.
Additional information
Funding
References
- Brody JE The cost of not taking your medicine. New York Times. April 18; D7.
- Statistics: Patient Compliance and Medication Adherence. [cited 2017 Oct 30]. Available from: https://www.epill.com/statistics.html
- Iuga AO, McGuire MJ. Adherence and health care costs. Risk Manag Healthc Policy. 2014;7:35–44.
- Benjamin RM. Medication adherence: helping patients take their medicines as directed. 2012;127:2–3.
- Brown MT, Bussell JK. Medication adherence: WHO cares?. Mayo Clin Proc. 2011;86:304–314.
- Muir KW, Lee PP. Glaucoma medication adherence: room for improvement in both performance and measurement. Arch Ophthalmol. 2011;129:243–245.
- World Health Organization. Adherence to long-term therapies: evidence for action. Geneva, Switzerland: World Health Organization. 2003. ISBN 92 4 154599 2.
- Zullig LL, Bosworth H. Engaging patients to optimize medication adherence. NEJM Catal. 2017. Available from: https://catalyst.nejm.org/optimize-patients-medication-adherence/.
- Vitolins MZ, Rand CS, Rapp SR, et al. Measuring adherence to behavioral and medical interventions. Control Clin Trials. 2000;21:188S–194S.
- Robin AL, Novack GD, Covert DW, et al. Adherence in glaucoma: objective measurements of once-daily and adjunctive medication use. Am J Ophthalmol. 2007;144:533–540.
- Puts MT, Tu HA, Tourangeau A, et al. Factors influencing adherence to cancer treatment in older adults with cancer: a systematic review. Ann Oncol. 2014;25:564–577.
- Cramer JA. A systematic review of adherence with medications for diabetes. Diabetes Care. 2004;27:1218–1224.
- Abegaz TM, Shehab A, Gebreyohannes EA, et al. Nonadherence to antihypertensive drugs: A systematic review and meta-analysis. Medicine (Baltimore). 2017;96:e5641.
- Buller AJ, Connell B, Spencer AF. Compliance: clear communication’s critical. Br J Ophthalmol. 2005;89:1370.
- Thier SL, Yu-Isenberg KS, Leas BF, et al. In chronic disease, nationwide data show poor adherence by patients to medication and by physicians to guidelines. Manag Care. 2008;17:48–52, 55–47.
- Steiner JF, Koepsell TD, Fihn SD, et al. A general method of compliance assessment using centralized pharmacy records. Description and Validation Med Care. 1988;26:814–823.
- Calip GS, Elmore JG, Boudreau DM. Characteristics associated with nonadherence to medications for hypertension, diabetes, and dyslipidemia among breast cancer survivors. Breast Cancer Res Treat. 2017;161:161–172.
- Volpp KG, Troxel AB, Mehta SJ, et al. Effect of electronic reminders, financial incentives, and social support on outcomes after myocardial infarction: the HeartStrong randomized clinical trial. JAMA Intern Med. 2017;177:1093–1101.
- Tham YC, Li X, Wong TY, et al. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121:2081–2090.
- Weinreb RN, Khaw PT. Primary open-angle glaucoma. Lancet. 2004;363:1711–1720.
- Pascolini D, Mariotti SP. Global estimates of visual impairment: 2010. Br J Ophthalmol. 2012;96:614–618.
- Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–267.
- Bourne RR, Jonas JB, Flaxman SR, et al. Prevalence and causes of vision loss in high-income countries and in Eastern and Central Europe: 1990–2010. Br J Ophthalmol. 2014;98:629–638.
- Flaxman SR, Bourne RRA, Resnikoff S, et al. Global causes of blindness and distance vision impairment 1990–2020: a systematic review and meta-analysis. Lancet Glob Health. 2017;5:e1221–e1234.
- Fingeret M Optometric clinical practice guideline care of the patient with open angle glaucoma. [cited 2013 Nov 5]. Available from: http://www.aoa.org/documents/CPG-9.pdf
- Sommer A, Tielsch JM, Katz J, et al. Relationship between intraocular pressure and primary open angle glaucoma among white and black Americans. The Baltimore Eye Survey. Arch Ophthalmol. 1991;109:1090–1095.
- International Council of Ophthalmology. International council of ophthalmology guidelines for glaucoma eye care. [cited 2018 Feb 5]. Available from: http://www.icoph.org/downloads/ICOGlaucomaGuidelines.pdf
- The Glaucoma Laser Trial (GLT) and glaucoma laser trial follow-up study: 7. Results. Glaucoma Laser Trial Research Group. Am J Ophthalmol. 1995;120:718–731.
- Slota C, Sayner R, Vitko M, et al. Glaucoma patient expression of medication problems and nonadherence. Optom Vis Sci. 2015;92:537–543.
- Robin A, Grover DS. Compliance and adherence in glaucoma management. Indian J Ophthalmol. 2011;59:S93–96.
- Lee PP, Walt JG, Doyle JJ, et al. A multicenter, retrospective pilot study of resource use and costs associated with severity of disease in glaucoma. Arch Ophthalmol. 2006;124:12–19.
- Rossi GC, Pasinetti GM, Scudeller L, et al. Do adherence rates and glaucomatous visual field progression correlate?. Eur J Ophthalmol. 2011;21:410–414.
- Varma R, Lee PP, Goldberg I, et al. An assessment of the health and economic burdens of glaucoma. Am J Ophthalmol. 2011;152:515–522.
- Friedman DS, Nordstrom B, Mozaffari E, et al. Glaucoma management among individuals enrolled in a single comprehensive insurance plan. Ophthalmology. 2005;112:1500–1504.
- Friedman DS, Quigley HA, Gelb L. Using pharmacy claims data to study adherence to glaucoma medications: methodology and findings of the Glaucoma Adherence and Persistency Group (GAPS). Invest Ophthalmol Vis Sci. 2007;48:5052–5057.
- Kass MA, Gordon M, Morley RE Jr., et al. Compliance with topical timolol treatment. Am J Ophthalmol. 1987;103:188–193.
- Kass MA, Meltzer DW, Gordon M, et al. Compliance with topical pilocarpine treatment. Am J Ophthalmol. 1986;101:515–523.
- Newman-Casey PA, Robin AL, Blachley T, et al. The most common barriers to glaucoma medication adherence: a cross-sectional survey. Ophthalmology. 2015;122:1308–1316.
- Gupta R, Patil B, Shah BM, et al. Evaluating eye drop instillation technique in glaucoma patients. J Glaucoma. 2012;21:189–192.
- Hermann MM, Bron AM, Creuzot-Garcher CP, et al. Measurement of adherence to brimonidine therapy for glaucoma using electronic monitoring. J Glaucoma. 2011;20:502–508.
- Kholdebarin R, Campbell RJ, Jin YP, et al. Multicenter study of compliance and drop administration in glaucoma. Can J Ophthalmol. 2008;43:454–461.
- O’Hare F, Jeganathan VS, Rokahr CG, et al. Readability of prescription labels and medication recall in a population of tertiary referral glaucoma patients. Clin Exp Ophthalmol. 2009;37:849–854.
- Tsai JC, McClure CA, Ramos SE, et al. Compliance barriers in glaucoma: a systematic classification. J Glaucoma. 2003;12:393–398.
- Cramer JA, Roy A, Burrell A, et al. Medication compliance and persistence: terminology and definitions. Value Health. 2008;11:44–47.
- Vrijens B, Vincze G, Kristanto P, et al. Adherence to prescribed antihypertensive drug treatments: longitudinal study of electronically compiled dosing histories. BMJ. 2008;336:1114–1117.
- Reardon G, Kotak S, Schwartz GF. Objective assessment of compliance and persistence among patients treated for glaucoma and ocular hypertension: a systematic review. Patient Prefer Adherence. 2011;5:441–463.
- Skalicky SE, Goldberg I. Adherence and persistence: the challenges for glaucoma medical therapy. Asia Pac J Ophthalmol (Phila). 2013;2:356–361.
- Schwartz GF, Quigley HA. Adherence and persistence with glaucoma therapy. Surv Ophthalmol. 2008;53:S57–68.
- Haynes RB, McDonald HP, Garg AX. Helping patients follow prescribed treatment: clinical applications. JAMA. 2002;288:2880–2883.
- Schwartz GF Enhancing glaucoma patients’ adherence to prescribed medical therapy. [ cited 2018 Feb 5]. Available from: http://glaucomatoday.com/pdfs/gt0714_F_schwartz.pdf
- Okeke CO, Quigley HA, Jampel HD, et al. Adherence with topical glaucoma medication monitored electronically: the Travatan Dosing Aid study. Ophthalmology. 2009;116:191–199.
- Gatwood JD, Johnson J, Jerkins B. Comparisons of self-reported glaucoma medication adherence with a new wireless device: a pilot study. J Glaucoma. 2017;26:1056–1061.
- Patel SC, Spaeth GL. Compliance in patients prescribed eyedrops for glaucoma. Ophthalmic Surg. 1995;26:233–236.
- Hermann MM, Diestelhorst M. Microprocessor controlled compliance monitor for eye drop medication. Br J Ophthalmol. 2006;90:830–832.
- Sayner R, Carpenter DM, Blalock SJ, et al. Accuracy of patient-reported adherence to glaucoma medications on a visual analog scale compared with electronic monitors. Clin Ther. 2015;37:1975–1985.
- Boland MV, Chang DS, Frazier T, et al. Automated telecommunication-based reminders and adherence with once-daily glaucoma medication dosing: the automated dosing reminder study. JAMA Ophthalmol. 2014;132:845–850.
- Covert D, Robin AL, Novack GD. Systemic medications and glaucoma patients. Ophthalmology. 2005;112:1849.
- Robin AL, Covert D. Does adjunctive glaucoma therapy affect adherence to the initial primary therapy?. Ophthalmology. 2005;112:863–868.
- Denis P. Adverse effects, adherence and cost-benefits in glaucoma treatment. Eur Ophthalmic Rev. 2011;5:116–122.
- Djafari F, Lesk MR, Harasymowycz PJ, et al. Determinants of adherence to glaucoma medical therapy in a long-term patient population. J Glaucoma. 2009;18:238–243.
- Sleath B, Robin AL, Covert D, et al. Patient-reported behavior and problems in using glaucoma medications. Ophthalmology. 2006;113:431–436.
- Friedman DS, Okeke CO, Jampel HD, et al. Risk factors for poor adherence to eyedrops in electronically monitored patients with glaucoma. Ophthalmology. 2009;116:1097–1105.
- Sleath B, Blalock SJ, Carpenter DM, et al. Ophthalmologist-patient communication, self-efficacy, and glaucoma medication adherence. Ophthalmology. 2015;122:748–754.
- Freedman RB, Jones SK, Lin A, et al. Influence of parental health literacy and dosing responsibility on pediatric glaucoma medication adherence. Arch Ophthalmol. 2012;130:306–311.
- Winfield AJ, Jessiman D, Williams A, et al. A study of the causes of non-compliance by patients prescribed eyedrops. Br J Ophthalmol. 1990;74:477–480.
- Sleath BL, Krishnadas R, Cho M, et al. Patient-reported barriers to glaucoma medication access, use, and adherence in southern India. Indian J Ophthalmol. 2009;57:63–68.
- Sleath B, Ballinger R, Covert D, et al. Self-reported prevalence and factors associated with nonadherence with glaucoma medications in veteran outpatients. Am J Geriatr Pharmacother. 2009;7:67–73.
- Lacey J, Cate H, Broadway DC. Barriers to adherence with glaucoma medications: a qualitative research study. Eye (Lond). 2009;23:924–932.
- Olthoff CM, Hoevenaars JG, van Den Borne BW, et al. Prevalence and determinants of non-adherence to topical hypotensive treatment in Dutch glaucoma patients. Graefes Arch Clin Exp Ophthalmol. 2009;247:235–243.
- Stone JL, Robin AL, Novack GD, et al. An objective evaluation of eyedrop instillation in patients with glaucoma. Arch Ophthalmol. 2009;127:732–736.
- Hennessy AL, Katz J, Covert D, et al. A video study of drop instillation in both glaucoma and retina patients with visual impairment. Am J Ophthalmol. 2011;152:982–988.
- Schwartz GF, Hollander DA, Williams JM. Evaluation of eye drop administration technique in patients with glaucoma or ocular hypertension. Curr Med Res Opin. 2013;29:1515–1522.
- Aptel F, Masset H, Burillon C, et al. The influence of disease severity on quality of eye-drop administration in patients with glaucoma or ocular hypertension. Br J Ophthalmol. 2009;93:700–701.
- Sleath B, Blalock S, Covert D, et al. The relationship between glaucoma medication adherence, eye drop technique, and visual field defect severity. Ophthalmology. 2011;118:2398–2402.
- Tamrat L, Gessesse GW, Gelaw Y. Adherence to topical glaucoma medications in Ethiopian patients. Middle East Afr J Ophthalmol. 2015;22:59–63.
- Friedman DS, Hahn SR, Gelb L, et al. Doctor-patient communication, health-related beliefs, and adherence in glaucoma results from the Glaucoma Adherence and Persistency Study. Ophthalmology. 2008;115:1320–1327.
- Stryker JE, Beck AD, Primo SA, et al. An exploratory study of factors influencing glaucoma treatment adherence. J Glaucoma. 2010;19:66–72.
- Lunnela J, Kaariainen M, Kyngas H. The views of compliant glaucoma patients on counselling and social support. Scand J Caring Sci. 2010;24:490–498.
- Budenz DL. A clinician’s guide to the assessment and management of nonadherence in glaucoma. Ophthalmology. 2009;116:S43–47.
- Barnebey HS, Robin AL. Adherence to fixed-combination versus unfixed travoprost 0.004%/timolol 0.5% for glaucoma or ocular hypertension: a randomized trial. Am J Ophthalmol. 2017;176:61–69.
- Schwartz G, Burk C, Bennett T, et al. Adherence and persistence with glaucoma therapy: brimonidine/timolol versus dorzolamide/timolol and various two-bottle combinations. J Clin Exp Ophthalmol. 2012;3:1–6.
- Higginbotham EJ, Hansen J, Davis EJ, et al. Glaucoma medication persistence with a fixed combination versus multiple bottles. Curr Med Res Opin. 2009;25:2543–2547.
- Schehlein EM, Novack GD, Robin AL. New classes of glaucoma medications. Curr Opin Ophthalmol. 2017;28:161–168.
- Perera SA, Ting DS, Nongpiur ME, et al. Feasibility study of sustained-release travoprost punctum plug for intraocular pressure reduction in an Asian population. Clin Ophthalmol. 2016;10:757–764.
- Envisia Therapeutics releases interim ENV515 (travoprost XR) phase 2 data demonstrating 11-month duration-of-action after a single dose in patients with glaucoma. [ cited 2018 Feb 5]. Available from: http://www.envisiatherapeutics.com/envisia-therapeutics-releases-interim-env515-travoprost-xr-phase-2-data-demonstrating-11-month-duration-of-action-after-a-single-dose-in-patients-with-glaucoma/
- Willard-Grace R, Chen EH, Hessler D, et al. Health coaching by medical assistants to improve control of diabetes, hypertension, and hyperlipidemia in low-income patients: a randomized controlled trial. Ann Fam Med. 2015;13:130–138.
- Aikens JE, Trivedi R, Aron DC, et al. Integrating support persons into diabetes telemonitoring to improve self-management and medication adherence. J Gen Intern Med. 2015;30:319–326.
- Abughosh SM, Wang X, Serna O, et al. A pharmacist telephone intervention to identify adherence barriers and improve adherence among nonadherent patients with comorbid hypertension and diabetes in a Medicare Advantage plan. J Manag Care Spec Pharm. 2016;22:63–73.
- Newman-Casey PA, Weizer JS, Heisler M, et al. Systematic review of educational interventions to improve glaucoma medication adherence. Semin Ophthalmol. 2013;28:191–201.
- Sleath B, Carpenter DM, Blalock SJ, et al. Applying the resources and supports in self-management framework to examine ophthalmologist-patient communication and glaucoma medication adherence. Health Educ Res. 2015;30:693–705.
- Feng A, O’Neill J, Holt M, et al. Success of patient training in improving proficiency of eyedrop administration among various ophthalmic patient populations. Clin Ophthalmol. 2016;10:1505–1511.
- Taylor SA, Galbraith SM, Mills RP. Causes of non-compliance with drug regimens in glaucoma patients: a qualitative study. J Ocul Pharmacol Ther. 2002;18:401–409.
- Christie D, Channon S. The potential for motivational interviewing to improve outcomes in the management of diabetes and obesity in paediatric and adult populations: a clinical review. Diabetes Obes Metab. 2014;16:381–387.
- Powell PW, Hilliard ME, Anderson BJ. Motivational interviewing to promote adherence behaviors in pediatric type 1 diabetes. Curr Diab Rep. 2014;14:531.
- DiIorio C, McCarty F, Resnicow K, et al. Using motivational interviewing to promote adherence to antiretroviral medications: a randomized controlled study. AIDS Care. 2008;20:273–283.
- Zomahoun HTV, Guenette L, Gregoire JP, et al. Effectiveness of motivational interviewing interventions on medication adherence in adults with chronic diseases: a systematic review and meta-analysis. Int J Epidemiol. 2017;46:589–602.
- Lundahl B, Burke BL. The effectiveness and applicability of motivational interviewing: a practice-friendly review of four meta-analyses. J Clin Psychol. 2009;65:1232–1245.
- Sleath B, Blalock SJ, Carpenter DM, et al. Provider education about glaucoma and glaucoma medications during videotaped medical visits. J Ophthalmol. 2014;2014:238939.
- Hahn SR, Friedman DS, Quigley HA, et al. Effect of patient-centered communication training on discussion and detection of nonadherence in glaucoma. Ophthalmology. 2010;117:1339–1347 e1336.
